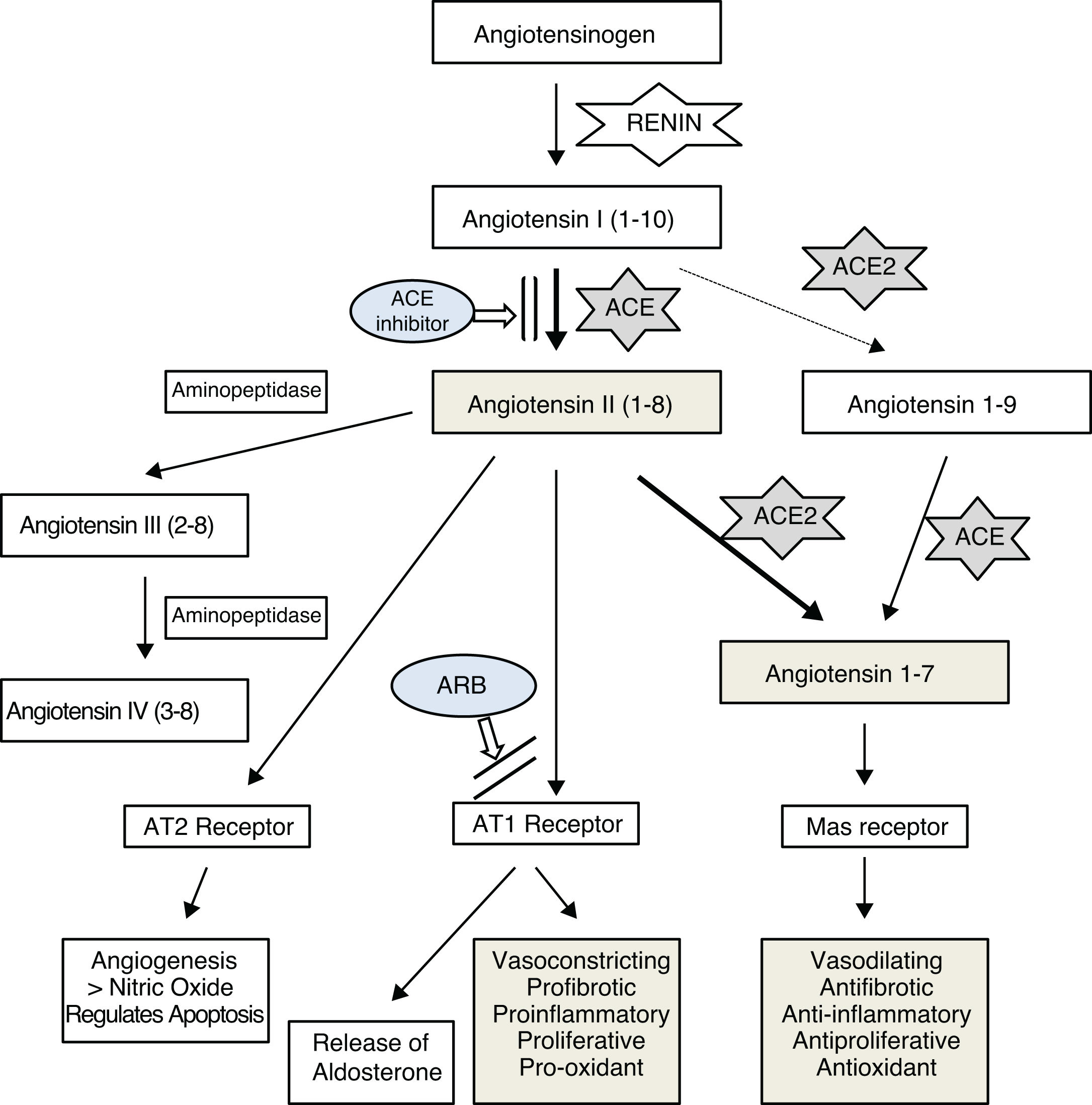
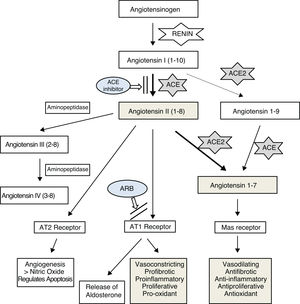
The renin–angiotensin system (RAS) is a known regulator of cardiovascular function, involved in hypertension, kidney disease, heart disease, arteriosclerosis, diabetes, and lung disease.1,2
It has been confirmed that the SARS-CoV-2 virus, like other coronaviruses, enters lung cells by binding to angiotensin-converting enzyme 2 (ACE2),3,4 which is an important element of the RAS. The main function of this enzyme, which is expressed in various human tissues,5 appears to be the maintenance of a balance between this vasoconstricting system, proinflammatory, proliferative, profibrotic, and oxidant effects and the other antagonizing effects through degradation and decreased production of angiotensin II and formation of angiotensin 1–76–8 (Fig. 1). It seems that the main mechanism by which ACE2 regulates angiotensin II is direct degradation, as its effect on angiotensin II has been estimated to be 400 times greater than its effect on angiotensin I.9
In recent years, various studies have been published on ACE2’s possible beneficial effects on the cardiovascular system, kidneys, and lungs,10–13 which have been linked to its angiotensin II-suppressing effect. Imai et al.13 found that angiotensin II worsened respiratory distress in different experimental models and that this poor course improved with angiotensin II receptor type 1 (AT1) blockade. They also found that the course worsened in the event of a reduction in ACE2 expression, as in coronavirus infections, and argued that this unfavorable course was due in part to a drop in angiotensin II regulation by this enzyme.
In this same line, Gurwitz14 and other authors15,16 have suggested that angiotensin II receptor blockers (ARBs) could have a beneficial effect on the course of lung disease in SARS-CoV-2 infection, either directly due to their capacity to increase ACE2 levels by counteracting the decrease in levels caused by the virus, or by blocking AT1 receptors and thus impeding the action of a possible excess of angiotensin II, the harmful effects of which in the lungs have been reported in cases of pneumonia due to the SARS-CoV-2 virus15 and the avian influenza A virus.17
In an experimental study in rats, Ferrario et al.18 found conflicting data on the effects of lisinopril, an ACE inhibitor, and losartan, an ARB, on mRNA expression of ACE2 and the activity of this enzyme, observing that both increase mRNA expression of ACE2, although only losartan, not lisinopril, increases its activity. Based on this study and on the fact that patients at higher cardiovascular risk infected with the SARS-CoV-2 virus seem to follow a worse course, Sommerstein and Gräni suggested that treatment with ACE inhibitors could have an unfavorable effect under these circumstances.19 By contrast, studies conducted in humans have not found any association between use of ACE inhibitors or ARBs and levels of ACE2.20
In line with Imai et al.13, the worse course in infection with the SARS-CoV-2 virus in patients at higher cardiovascular risk (hypertensive, diabetic, and elderly patients as well as those with greater cardiac morbidity)21 could also be interpreted to be due to them having higher levels of angiotensin II and, consequently, higher levels of ACE2 to degrade it and thus reduce its harmful effects on the lungs. Also supporting the idea of the presence of excess angiotensin II in this process is the fact that both patients who have SARS-CoV-2 infection and patients who have survived SARS-CoV-2 infection have a higher incidence of hypertension, cardiovascular problems, and glucose metabolism disorders.22
According to this hypothesis, blocking angiotensin II production with ACE inhibitors could be an alternative for decreasing its levels and its inflammatory potential in the lungs and, consequently, decreasing ACE2 expression. This would render the virus less infectious in the early phases of the disease and in contacts, as it would decrease the receptors available for the SARS-CoV-2 virus to enter cells. Experimental studies have found that ACE2 knockout mice are not infected by these viruses.13 It has also been suggested that ACE inhibitors could partly alter the structure of the enzyme due to their similarity to ACE, which would also impede the binding of the virus.5
With respect to more severe cases, the data are more contradictory, as it has been reported that the drop in ACE2 activity caused by the virus could increase lung injury, although under these circumstances the possible benefit of ACE2 could be due to the fact that it increases angiotensin II degradation, which would be elevated by a defect in its regulation and an increase in renin activity stimulated by hypoxia. Such conditions, which are seen in 5%–10% of patients with SARS-CoV-2 infection, are characterized by devastating acute inflammation with predominant pulmonary expression (pneumonia, hypoxia, and severe respiratory distress) and are probably tied to different expressions of interferon, patterns of cytokines, and other unknown molecular mechanisms, which would explain the different clinical presentations, independent of patient age.23
It is interesting to note that there are differences between individuals in terms of the structure of ACE and its accompanying effects. Different studies have shown the pharmacogenomic significance of the three variants of endothelial nitric oxide synthases (eNOS) with regard to ACE inhibition responses. Genetic variability in eNOS genes and in the genes that contribute to their activation could affect the ACE inhibitor response. One of these studies focused on patients with hypertension treated with enalapril, an ACE inhibitor.24
The beneficial effects of ACE inhibition in the lungs have been demonstrated in various studies, both experimentally (enalapril was found to exert an anti-inflammatory effect in an animal model of induced pulmonary toxicity,25 and perindopril, another ACE inhibitor, was found to have the same effect on lung impairment secondary to sepsis26) and clinically (through a meta-analysis that found ACE inhibitors to possess a protective effect with respect to pneumonia risk).27
The clinical effect of ACE inhibitors and ARBs in patients with COVID-19 who require hospital admission is being evaluated in a study in progress at King's College Hospital and Princess Royal University Hospital in London.28 Its preliminary results do not find a worse course in patients treated with ACE inhibitors compared to patients not taking these drugs; it is even noted that they seem to follow a more favorable course. Regarding ARBs, it is not yet possible to draw conclusions given the limited number of cases analyzed.
Regardless of other more specific treatment options — antivirals, direct ACE2 blockade, treatment with recombinant ACE2 in an attempt to saturate the capacity for binding of the virus to the same, vaccines, and others29,30 — the option proposed should be considered because it is a well-known, accessible, and safe treatment, if administered with proper management and monitoring, which could aid in reducing the spread and effects of the infection.
In an initial phase, it would be necessary, as other authors have remarked,13,18 to analyze as soon as possible the epidemiological data available for infected patients, with respect to their course and history of taking antihypertensive drugs; at the same time, it would be appropriate to propose randomized prospective studies in initial infections in which one of the arms includes not only the treatment considered most suitable for each case but also an ACE inhibitor, with strict clinical management of these patients, in line with those already started by other researchers.
It is necessary to continue conducting research aimed at improving knowledge of the correlation between angiotensin II levels and ACE2 activity in different tissues — lung, heart, and kidney tissues — since it seems that the degree of activity of the components of the RAS may vary by organ and in accordance with plasma levels. In addition, it must be clarified whether the possible beneficial effect reported with higher ACE2 levels is due to greater degradation of angiotensin II and increased angiotensin 1–7 or to an action through other metabolic pathways.
We also believe that it is very important to have, as soon as possible, results from regulated and systematized studies on the effects of blockade of angiotensin II and other components of the RAS in SARS-CoV-2 infection, since the lack of scientific evidence in this regard is resulting in a proliferation of scientific literature with highly conflicting opinions on the possible effects of the drugs that interfere with this system, based only on experimental studies with many limitations, such as some of those mentioned, and on subjective interpretations.31,32 Indeed, there have even been formal — and, we believe, hasty — proposals of actions contrary to the positioning of scientific associations and the European Medicines Agency on the use of these medicines in patients with COVID-19.33
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr. Sara Lamas-Alvarez for her useful suggestions and contributions in improving and clarifying the content of the manuscript.
Please cite this article as: Lamas-Barreiro JM, Alonso-Suareza M, Fernández-Martín JJ, Saavedra-Alonso JA. Supresión de angiotensina II en la infección por el virus SARS-CoV-2: una propuesta terapéutica. Nefrologia. 2020;40:213–216.