El síndrome hemolítico urémico (SHU) es una entidad clínica definida por la tríada anemia hemolítica no inmune, trombocitopenia e insuficiencia renal aguda, en la que las lesiones subyacentes están mediadas por un proceso de microangiopatía trombótica (MAT) sistémica. El SHU atípico (SHUa) es un subtipo de SHU en el que los fenómenos de MAT son consecuencia de la pérdida de regulación de la vía alternativa del complemento sobre las superficies celulares de causa genética. El SHUa es una enfermedad ultra-rara que, pese al tratamiento estándar con terapia plasmática, frecuentemente evoluciona a la insuficiencia renal crónica terminal, con elevada mortalidad. En los últimos años, se ha establecido el papel clave que desempeña el sistema del complemento en la inducción de daño endotelial en los pacientes con SHUa, mediante la caracterización de múltiples mutaciones y polimorfismos en los genes que codifican determinados factores del complemento. Eculizumab es un anticuerpo monoclonal que inhibe la fracción terminal del complemento bloqueando la formación del complejo de ataque de membrana. En estudios prospectivos en pacientes con SHUa su administración ha demostrado la interrupción rápida y sostenida del proceso de MAT, con mejorías significativas de la función renal a largo plazo y con una reducción importante de la necesidad de diálisis o terapia plasmática. En el presente documento se revisan y actualizan los diversos aspectos de interés de esta enfermedad, con especial atención a cómo los recientes avances diagnósticos y terapéuticos pueden modificar el tratamiento de los pacientes con SHUa.
Haemolytic uraemic syndrome (HUS) is a clinical entity defined as the triad of nonimmune haemolytic anaemia, thrombocytopenia, and acute renal failure, in which the underlying lesions are mediated by systemic thrombotic microangiopathy (TMA). Atypical HUS (aHUS) is a sub-type of HUS in which the TMA phenomena are the consequence of decreased regulation of the alternative complement pathway on cell surfaces due to a genetic cause. aHUS is an extremely rare disease that, despite the administration of standard treatment with plasma therapy, often progresses to terminal chronic renal failure with a high associated rate of mortality. In recent years, research has established the key role that the complement system plays in the induction of endothelial damage in patients with aHUS, through the characterisation of multiple mutations and polymorphisms in the genes that code for certain complement factors. Eculizumab is a monoclonal antibody that inhibits the terminal fraction of the complement protein, blocking the formation of a cell membrane attack complex. In prospective studies in patients with aHUS, administering eculizumab produces a rapid and sustained interruption in the TMA process, with significant improvements in long-term renal function and an important decrease in the need for dialysis or plasma therapy. In this document, we review and bring up to date the important aspects of this disease, with special emphasis on how recent advancements in diagnostic and therapeutic processes can modify the treatment of patients with aHUS.
INTRODUCTION
Haemolytic uraemic syndrome (HUS) is a clinical entity defined by the triad of non-immune haemolytic microangiopathic anaemia, thrombocytopenia, and acute renal failure.1 Histological damage from HUS is characterised by the appearance of systemic thrombotic microangiopathy (TMA), which primarily affects the renal blood vessels, producing thickening of the vessel wall, thrombosis, and obstruction of the vascular lumen. The majority of cases of HUS are caused by an enteric infection by shiga toxin-producing Escherichia coli (STEC) or other micro-organisms that produce verotoxin (VTEC), giving way to the so-called typical HUS or STEC (VTEC)-HUS. In a similar manner, TMA lesions and HUS can be secondary to other underlying diseases, drugs, certain types of organ transplants, or pregnancy. Rarely, HUS is produced as the consequence of deregulation of the alternative pathway for the complement system due to genetic abnormalities, which leads to endothelial damage and convey systemic TMA.2 This type of HUS is known as atypical HUS (aHUS), constituting a severe disease associated with a poor prognosis and elevated mortality rates. The prognosis for aHUS is sombre despite recommendations for intensive treatment regimens with plasma therapy (PT) and life support measures. More than 50% of patients with aHUS die from this disease, require dialysis, or develop permanent kidney damage during the year following diagnosis.3
Recent advances in the characterisation of the genetic component of aHUS (including the identification of multiple mutations and polymorphisms in the genes that code for certain complement proteins) have led to the conclusion that the endothelial damage produced by the complement system is the critical factor in the pathophysiology of this disease, and the hypothesis that inhibition of the complement system is a viable treatment option in these patients. In 2011, the governing regulatory agencies of the United States and Europe approved the indication for eculizumab (Soliris®; Alexion Pharmaceuticals, Connecticut, USA),4 a humanised monoclonal antibody that inhibits the activation of C5 and blocks the generation of the proinflammatory anaphylotoxin C5a and the lytic pathway of the complement system (which produces cell lysis), for the treatment of aHUS.5 In prospective studies of patients with aHUS, eculizumab has been shown to effectively interrupt the process of TMA and its consequences, associated with significant long-term improvements in haematological parameters and renal function.6-9
With the objective of elaborating updated diagnostic and treatment protocols for aHUS, a consensus conference was held on February 2 and 3 of 2012 in Barcelona, which brought together clinical and research experts in the field of TMA. Using the platform of the available scientific evidence and clinical experience, this conference discussed various aspects of interest of the disease, such as the aetiological classification of TMA, the pathophysiology of aHUS, and differential diagnosis, and recommendations were made for treating patients with aHUS. This article summarises the primary conclusions derived from this meeting.
AETIOLOGICAL CLASSIFICATION OF THROMBOTIC MICROANGIOPATHY
The term TMA defines a histological lesion found in the arterioles and capillaries that is characterised by thickening and inflammation of the vascular wall, detachment of endothelial cells, subendothelial widening due to the accumulation of proteins and cellular debris, and platelet thrombi that occlude the vascular lumen (Figure 1).1 Two different clinical entities are characterised by primary TMA lesions, which have different causes and histopathological foundations: thrombocytopenic thrombotic purpura (TTP) and HUS.
Intravascular thrombosis in TTP is the consequence of a severe deficiency of metalloprotease activity of the ADAMTS13 (A Disintegrin And Metalloproteinase with a ThromboSpondin type 1 motif, member 13), a plasma enzyme responsible for fragmenting the ultra-long multimeres of the von Willebrand factor.10 This deficiency can be caused by genetic abnormalities or can be acquired through circulating IgG antibodies that block ADAMTS13 (especially in patients on treatment with anti-platelet medications).11
Approximately 90% of cases of HUS are caused by an enteric infection by STEC from contaminated foods (typical HUS/STEC[VTEC]-HUS).2 The shiga toxin exerts a direct damaging effect on the vascular endothelium, triggering multiple cellular and vascular phenomena that lead to the development of TMA.2 The disease appears clinically in the form of abdominal pain and diarrhoea, with acute renal failure appearing after 4-10 days. The prognosis tends to be good: mortality is <5% and a complete clinical recovery is achieved in 80% of patients.12,13
In contrast, aHUS is a disease in which the TMA phenomena are the consequence of a deregulation of the alternative complement pathway on cell surfaces. This alteration can be produced by mutations or polymorphisms that decrease the activity of complement regulating proteins, or that increase the function of activator proteins. In both cases, activation of the complement system (induced by a range of triggering factors) is not appropriately controlled, provoking endothelial damage and thrombosis. Of the more than 1000 patients with aHUS published in the medical literature, mutations have been detected in one or more complement proteins in 50%,14-21 although we cannot rule out that in the other cases there may have also been a genetic or environmental component involved that could have affected the abnormal complement system activity. In fact, it is notable that auto-antibodies against complement factor H (FH) are found in 5%-10% of patients with aHUS.22 In contrast to STEC-HUS, which tends to occur as a single event, aHUS is a chronic condition due to the genetic origins of the disease, and involves a poor prognosis. After the first episode of aHUS, mortality is 10%-15%, and 50% of patients will never recover renal function.3,14,15
In addition to infection by STEC or genetic deregulation of the complement system, there are many other clinical entities and triggering factors that can be associated with the development of HUS or TTP (secondary TMA). In children, some cases are associated with methylmalonic aciduria23 or, more frequently (5% of cases of HUS in children), with invasive infections by Streptococcus pneumoniae strains that produce the enzyme neuraminidase, which exposes the cryptoantigen T in the cell surface, thus producing TMA,24 or by H1N1 virus infection.25 In adults, cases of TMA have been described in association with human immunodeficiency virus (HIV) infection, certain types of cancer, drugs (chemotherapy agents, calcineurin inhibitors [cyclosporine and tacrolimus], mTOR [mammalian target of rapamycin; sirolimus, everolimus] inhibitors, vascular endothelial growth factor inhibitors, anti-platelet agents, and oral contraceptives, among others), malignant arterial hypertension, bone marrow and solid organ transplantation, pregnancy, and systemic diseases (systemic lupus erythematosus, scleroderma, and antiphospholipid syndrome).26
Table 1 displays the proposed aetiological classification of TMA. In certain patients more than one aetiological factor responsible for the TMA lesion may coexist, producing a variety of clinical presentations. Recently, it has been shown that as many as 25% of patients with STEC-HUS, and 86% of patients with HUS secondary to pregnancy, may have mutations in complement system gene sequences,27,28 which could signify that the underlying cause of these cases is in reality aHUS. In this context, Figure 2 represents the potential overlap that could occur between these two clinical entities. The primary motivation for discussion in this article is an update of aHUS mediated by altered complement regulation, and the following sections will refer exclusively to this entity.
ATYPICAL HAEMOLYTIC URAEMIC SYNDROME: CLINICAL ENTITY
Epidemiology
aHUS is considered to be an extremely rare disease. Very few sources of data are available regarding the incidence and prevalence of this condition, severely limiting our understanding of its true epidemiology. In the United States, aHUS is estimated to have an annual incidence rate of ~1-2 cases/million inhabitants.29 In Europe, a recent international, multi-centre study reported an incidence of 0.11 cases/million inhabitants between the ages of 0 and 18 years. As regards prevalence, the EMA (European Medicines Agency) estimates approximately 3.3 patients/million inhabitants/year in individuals younger than 18 years of age, with lower rates in adults.
aHUS primarily affects children and young adults, although it can appear at any stage of life.14,15 The start of the disease is more common before the individual has reached 18 years of age (60% vs. 40%), and the sex distribution is similar (with a slight predominance in females when the disease appears later in life).14,16
Clinical manifestations
The initial onset of this disease can be abrupt, although it may occur progressively in approximately 20% of patients (a matter of weeks or months), with sub-clinical anaemia, fluctuating thrombocytopenia, and conserved renal function.14 Symptoms are characterised by the triad of non-immune microangiopathic haemolytic anaemia, thrombocytopenia, and acute renal failure.1 High levels of lactate dehydrogenase (LDH), undetectable haptoglobin levels, and schistocytes confirms the presence of intravascular haemolysis.3 Patients also develop haematuria, proteinuria, and/or acute renal failure (with or without oligoanuria). Arterial hypertension is a common finding, whether due to volume overload or vascular damage.1
Although aHUS predominantly affects the renal vessels, the diffuse character of TMA phenomena leads to involvement of the microvasculature of other organ systems (the brain, heart, intestines, pancreas, and lungs),1 which explains the common appearance of extra-renal symptoms.14,15 The most frequent of these symptoms are neurological (48%),30 which can include irritability, somnolence, confusion, convulsions, encephalopathy, cerebrovascular accidents, hemiparesia, hemiplexia, and coma.1,15,30,31 Myocardial infarction has also been described in as many as 3% of patients with aHUS, and can cause sudden death.15,32 Cardiomyopathy, heart failure, and peripheral ischaemic heart disease have also been described,22,30,33,34 in addition to diarrhoea (30%) and other gastrointestinal symptoms (colitis, nausea and vomiting, and abdominal pain).15,22,31,35 The variability in symptoms presented is an impediment to performing a differential diagnosis in comparison with other sources of TMA.
Pathophysiology
The complement system, which is composed of numerous proteins in plasma and in the cellular membranes, is essential in the defence against infection, the processing of immune complexes, the antibody responses, and the elimination of cell remnants from apoptosis. The activation of the complement system through any of the known pathways (classical/lectin and alternative pathways) leads to the formation of multi-protein complexes with C3-convertase activity that cleaves C3, generating C3b (Figure 3). This molecule can bind covalently to the cell surfaces responsible for complement activation, facilitating phagocytosis by polymorphonuclear leukocytes and macrophages, and initiating the cell membrane attack complex that induces cellular lysis. In addition, C3b exponentially amplifies the activation of the complement system, promoting the formation of more C3-convertase.36 In order to avoid complete consumption by the activation of the complement system as well as damage to untargeted tissues (C3b binds indiscriminately to both pathogenic cells and native somatic cells), a number regulatory proteins such as complement factor H (FH), membrane cofactor protein (MCP), and complement factor 1 (F1) act to dissociate the C3-convertase and induce the degradation of C3b. As a consequence, under normal conditions, C3b is maintained at low levels, and when the complement system is activated, its deposition is limited to those structures responsible for the activation.
Multiple studies have reported that 40%-60% of patients with aHUS carry of mutations in the complement system genes (complement factor H gene [CFH], membrane cofactor protein gene [MCP], complement factor B gene [CFB], and C3 gene [C3]),37-46 which are related to deregulation of the alternative pathway (Table 2). FH acts in the plasma controlling homeostasis of the complement system, as well as avoiding damage to self cell surfaces. Mutations in the FH gene associated with aHUS cluster at the C-terminal region, which results in a decreased FH-mediated protection of cell surfaces against accidental damage produced through activation of the complement system, but does not affect complement regulation in the plasma.47 One conclusion from the functional analyses performed with these FH mutants is that aHUS is the consequence of damage caused by the complement system due to the dysregulation of the complement activation on self cell surfaces. Functional analyses of mutations associated with aHUS found in other complement genes, such as MCP, CFI, CFB, and C3, have confirmed the hypotheses anticipated in the studies with FH mutants, demonstrating, in addition, that dysregulation of complement on cell surfaces in aHUS may be due to decreased activity of the regulatory proteins or to an abnormally high activity of the C3-convertase. In this context, whereas mutations in FH, MCP, FI, and thrombomodulin (THBD) incapacitate these proteins from being able to carry out their regulatory function, mutations in complement factor B (FB) or in C3 result in a more active C3-convertase.
Anti-FH auto-antibodies directed towards the C-terminal region are found in 5%-10% of all patients with aHUS, with similar consequences to those produced by FH mutations.48,49 The role of these mutations in the pathogenesis of aHUS is not completely understood, but it appears to be associated with both the onset and the recurrences of disease. Antibody titres decrease with time, and should be measured at the first indication of aHUS.
Penetrance of aHUS in carriers of mutations in the complement genes is approximately 50%. It is common that in families with complement mutations only some carriers develop aHUS, with variable clinical presentations. There is also a wide range of clinical heterogeneity between unrelated patients with the same mutation. This suggests that additional genetic and environmental factors must exist that modulate the development and progression of this disease. The search for aditional mutations to genes in complement genes in patients with aHUS along with case-control association studies that using genetic polymorphisms in candidate genes or genetic markers distributed throughout the whole human genome have demonstrated that certain variants (polymorphisms) in the genes CFH and MCP modulate the presence and severity of this disease (Table 2).41,50,51 These observations, along with the fact that as many as 10% of patients with aHUS have mutations in more than one complement gene, indicates that the coincidence of different genetic risk factors is a determining factor in the development of aHUS (multiple hits theory).51
In addition to these genetic abnormalities, the onset of aHUS involves the participation of triggering environmental factors. The aforementioned mutations predispose the patient to disease, hampering adequate regulation of the complement system on cell surfaces in a situation that affects the microvasculature system. Infectious diseases can trigger aHUS in 50%-80% of patients,14,15,33 especially diseases of the upper respiratory tract (H1N1 virus). Diarrhoea from gastroenteritis may precede aHUS in as many as 30% of cases (including STEC diarrhoea14,15,22). In women, pregnancy can also be a triggering factor for aHUS.15,28
Prognosis
Table 3 displays the clinical evolution of patients with aHUS based on the type of mutation involved. In general, patients with FH and C3 mutations have a worse prognosis during the episode of aHUS and following months, with rates of mortality and terminal chronic renal failure (TCRF) or recurrence of 50%-70% and 50%, respectively. On the other hand, only 0.6% of patients with MCP mutations dies or develops TCRF, although the risk of recurrence is greater. Three out of four patients with FH, C3, or FB mutations die or develop TCRF.1
Recurrence of atypical haemolytic uraemic syndrome after kidney transplantation
The results of kidney transplants (KT) in patients with TCRF caused by aHUS have been limited historically by the high percentage of post-transplant recurrence of disease (~50%; graft loss rate: 80%-90%52,53), although results vary based on the type of mutation present. FH mutations are associated with a greater risk of recurrence or graft loss following KT (75%-90%), and high levels of risk are also associated with C3 and FI mutations (40%-80%; Table 3).15,35,40,54 Until now, very few transplants have been attempted in patients with FB mutations, but all cases that have been reported to date have involved recurrence of aHUS and graft loss.41,55 In general, the plasma complement factors involved in aHUS are primarily synthesised by the liver, and so patients with mutations to complement genes that code for these factors continue to be susceptible to aHUS after KT, since the dysfunctional factors continue to be produced. On the other hand, MCP is a trans-membrane protein primarily produced by the kidney, and so a KT may correct the deficit by producing unaltered MCP in the new kidney. More than 80% of patients with MCP mutations do not develop recurrent aHUS after KT, with a similar long-term survival rate to that of patients who receive transplants for other reasons.33,52,53 The risk of post-transplant recurrence in patients with THBD56 or anti-FH antibody mutations is not well understood, although in the case of FH antibodies, it appears that recurrence is related to persistently high antibody levels.22,57
DIAGNOSIS OF ATYPICAL HAEMOLYTIC URAEMIC SYNDROME
Figure 4 and Table 4 display the algorithm for the differential diagnosis of TMA and the biological tests recommended to be performed in patients with a suspected diagnosis of aHUS.2 Due to the rapid and severe progression of TMA, the diagnostic process must first involve an immediate phase (first 24 hours) that involves identifying the syndrome responsible and instating initial support measures, followed by a second phase of aetiological diagnosis.
In patients with TMA, laboratory results indicate the presence of thrombocytopenia (platelets <150 000mm3 or decrease of >25% since initial level3) and microangiopathic haemolysis (haemoglobin <10mg/dl with a negative direct Coombs test result [although some patients with HUS related to pneumococcal infection may have a positive direct Coombs test result],24 elevated LDH, decreased haptoglobin levels, reticulocytosis, and schystocytes3,56). Although it is possible to detect schistocytes in the majority of patients with kidney disease, preeclampsia, and mechanical valves, a number of schistocytes >1% is diagnostic of TMA when in the absence of another known cause.58
Elevated serum creatinine levels, low glomerular filtration rates (GFR), proteinuria, or haematuria would be indicative of renal dysfunction.3,14,54 In paediatric patients, a renal biopsy is not needed for diagnosis. A renal biopsy is usually indicated in adult patients in the case of acute renal failure in order to establish the aetiology of the disease, rule out other processes, and evaluate prognosis, although the indications for biopsy should be individually evaluated in patients suspected of TMA due to a risk of bleeding.
A complete clinical history is imperative, including family history, triggering factors, and an exhaustive physical examination. Contrary to medical opinion several years ago, it is currently held that the signs and symptoms for different types of TMA are not specific to each individual form, and so a differential diagnosis is not feasible.1 Traditionally, the distinction between HUS and TTP was based on clinical criteria, considering HUS to be an entity with predominately renal involvement, and TTP as primarily affecting the neurological system. However, it has been shown that 50% of patients with TTP have renal dysfunction, and 50% of patients with aHUS have neurological alterations.30,59 It is also impossible to use clinical symptoms to differentiate between STEC-HUS and aHUS, since as many as 30% of patients with aHUS first experience symptoms of gastroenteritis15 or diarrhoea,35 characteristic symptoms of STEC-HUS.
Detection of shiga toxin or positive STEC cultures in patients with TMA is diagnostic of STEC-HUS,27 whereas the diagnosis of TTP requires a direct demonstration that plasma activity of ADAMTS13 is ≤5%.60 In all other cases, the diagnosis should be orientated towards aHUS,60 requiring additional tests to rule out secondary forms of TMA.
TREATMENT FOR ATYPICAL HAEMOLYTIC URAEMIC SYNDROME
Plasma therapy
The two modalities of plasma therapy are plasma infusion (PI) and plasma exchange (PE). PI involves viro-inactive, non-native, fresh frozen plasma (FFP) with functional complement regulators.61 In PE, the patient receives FFP to replace the native plasma with aHUS, which implies the administration of not only elevated levels of functional complement regulatory proteins, but also the elimination of dysfunctional endogenic soluble complement inhibitors, with a lower risk of volume overload. In addition, PE purifies the blood of anti-FH antibodies and the possible presence of inflammatory/thrombogenic factors that participate in producing endothelial damage and platelet hyper-aggregation. Traditionally, the treatment of choice recommended for episodes of aHUS consisted of early and intensive PE at high volumes and a variable frequency based on disease activity, whereas PI tends to be ineffective, except for in the few patients with complete deficit of FH.62 In general, PT is not considered to be effective in patients with MCP mutations, since this is a non-circulating protein anchored in the cell membrane, and virtually all of these patients go into remission after the episode of aHUS, regardless of the use of PT.15
Although information from prospective clinical trials is not available, PT has been empirically the treatment of choice for aHUS for several years, after observing more than three decades ago that this treatment decreases mortality rates in patients with HUS-TTP. Table 5 presents the results of the largest international registry of PT in patients with aHUS (International Registry of Recurrent and Familial HUS/TTP), which included 273 patients diagnosed between 1996 and 2007.15 In this registry, the rates of complete haematological and renal recovery in patients treated with PT were generally lower than 50% (with the exception of patients with THBD and MCP mutations), and these rates were especially low in patients with FH and FI mutations (5% and 12.5%).15 Mortality and progression of TCRF are globally high, occurring in 3 out of 4 patients with FI mutations. Certain observations indicate that early and intensive PE is essential for salvaging patients from aHUS, and maintenance of this type of treatment can prevent disease recurrence and TCRF,14,61 but we still have yet to establish the most effective treatment regimen, nor do we fully understand the long-term impacts of this type of therapy on renal function.
In patients with anti-FH antibodies, it has been shown that concomitant immunosuppressant treatment along with PT can improve results.22,63,64 In these cases, an elevated antibody titre is correlated with a greater risk of recurrence and renal sequelae.22
The appearance of anaphylactic reactions to FFP, hypervolemia, hypertension, heart failure, and hyperproteinaemia are potential complications of PI. The primary complications that arise due to PE are venous access obstruction (6%), hypotension (5%), and allergy (4%),65 and these are most common in paediatric patients.65
Eculizumab
Eculizumab is an IgG2/4κ humanised monoclonal antibody that binds to complement C5 proteins with great affinity, blocking its excision into C5a and C5b and impeding the production of the C5b-9 terminal complement complex (membrane attack complex) (Figure 3).5 In aHUS, deregulation of the alternative complement pathway leads to uncontrolled activation of this process, provoking damage to endogenous structures through the formation of membrane attack complexes. In this sense, blocking the terminal complement complex with eculizumab quickly and sustainably diminishes this activity, and in many cases of patients with aHUS that receive this treatment, a good clinical response to the drug has been reported (Table 6).31,35,54,66-84
The efficacy and safety of eculizumab as a treatment for aHUS was evaluated recently in two phase II prospective, multi-centre, controlled, open clinical studies of 26 weeks duration, carried out in patients ≥12 years of age and with primary or recurrent (post-KT) disease.85,86 One included 17 patients resistant to PT (≥4 sessions/week) (Study C08-002)85 and one included 20 patients on chronic plasma treatment (≥8 weeks) (study C08-003).86 The most common mutations in this sample were related to FH (15 patients) and FI.8 Three patients had MCP mutations, three had C3, and one had FB. We found anti-FH antibodies in 4 patients and no genetic abnormalities were observed in 10 patients. In both studies, the response to eculizumab was similar between patients with mutations and/or ant-FH antibodies and those with no genetic abnormalities. The response was also similar regardless of the type of mutation identified. After 6 months of treatment with eculizumab, rates of haematological normalisation (≥2 consecutive normal measurements of platelets and LDH) reached 76% in resistant cases and 90% of chronic cases. The rates of patients free from TMA events (no reduction in platelets >25%; with no need for PT or new dialysis sessions) were 88% and 80%, accompanied by a significant reduction in the rate of daily interventions for TMA (PE or PI sessions or new dialysis sessions) from baseline values of 6 and 1.5, respectively, to 0 (P<.0001). At the end of the follow-up period, GFR had increased significantly for both patient groups (resistant patients: +31ml/min/1.73m2; P=.0001; chronic patients: +6.1ml/min.1.73m2; P=.0003), and 80% of resistant patients who were on dialysis at the start of treatment (4 out of 5) were able to exit dialysis by the end of the treatment period. We also observed that patients who started treatment with eculizumab during the first 10 days following the diagnosis of aHUS exhibited a greater increase in GFR than those who started treatment after 2-4 months (+59ml/min/1.73m2 vs. +7ml/min/1.73m2; P=.03). In extended studies (62-64 weeks mean follow-up period), the improvement in renal function was maintained.6-9 After one year of eculizumab treatment, proteinuria levels were significantly lower than baseline values (P=.05). Adverse side effects were observed in 19 patients, including 4 severe adverse effects (peritonitis, influenza infection, venous disorder, and severe hypertension). Due to its mechanism of action, eculizumab increases the risk of infection by Neisseria meningitidis, which prompted to vaccination of all patients against Neisseria (tetravalent vaccine) before starting treatment or antibiotic prophylaxis, with no cases of meningitis produced. Survival was 100% in both studies.
Another retrospective study involved 19 paediatric patients treated in clinical practice with eculizumab for a mean 28 weeks.4,11 In this study, 89% of patients obtained normal platelet levels and 68% were free of TMA events. The rate of interventions for TMA was reduced from ~3/patient/week to 0. Mean GFR increased by ≥15ml/min/1.73m2 in 47% of patients, and the need for dialysis was eliminated in 50% of patients. The most common adverse effects of treatment were pyrexia (47%), diarrhoea (32%), and upper respiratory tract infections (32%).
Based on these results, the FDA (Food and Drug Administration) and the EMA approved in the United States and Europe, respectively, the indication for eculizumab in the treatment of aHUS.4 The recent availability of this drug in Spain has offered the possibility of substantially improving the management of patients with aHUS, since the approved indication authorises its use as a primary treatment. Here, we present the recommendations for treating aHUS as compiled by the authors of this document based on the available evidence and pertinent clinical experience.
RECOMMENDATIONS FOR THE MANAGEMENT OF ATYPICAL HAEMOLYTIC URAEMIC SYNDROME
Treatment recommendations
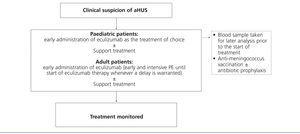
Considering the technical difficulties involved in administering PT to paediatric patients (due to body size), as well as the potential complications that arise from this strategy, the early use of eculizumab in this population is especially highly indicated, thus avoiding the need for PE. As such, given a suspicion of aHUS in a paediatric patient, it is recommended to provide early treatment with eculizumab as the first choice (Figure 5). In adult patients with the suspicion of aHUS, early eculizumab is also recommended. In the case that the start of this treatment might be delayed, early and intensive PE should be administered until eculizumab is an available option.62
Prior to this point, it is necessary to first vaccinate all patients against Neisseria meningitidis (preferably with conjugated tetravalent vaccines against serotypes A, C, Y, and W135). In the case that treatment with eculizumab cannot be delayed until obtaining proper vaccination, treatment can still be started along with antibiotic prophylaxis4 against Neisseria meningitidis based on the protocol at each hospital. Considering the more elevated rate of invasive infection by meningococci in paediatric patients, as well as the absence of protection against serotype B infections (which is the most prevalent type due to the systematic vaccination of the other serotypes), these patients should be maintained on antibiotic prophylaxis with penicillin or amoxicillin to accompany eculizumab.2 In adult patients, antibiotic prophylaxis should be maintained throughout the duration of treatment with eculizumab as based on the attending physician’s judgement and patient characteristics. In paediatric patients, vaccination against Haemophilus influenzae and pneumococci is also necessary, along with strict adherence to local guidelines regarding obligatory vaccinations for each age group.
When patients exhibit a positive response to eculizumab, treatment should be extended (chronic) as advised by the drug technical data sheet.4 It is currently impossible to provide recommendations regarding the most appropriate duration of treatment, although as our experience with this drug continues to develop, we may be able to evaluate the possibility of treatment removal in certain low-risk patients on an individual basis. In patients with clinical indications requiring the removal of treatment, subsequent care must include close monitoring for at least 12 weeks in order to detect possible abnormalities suggestive of TMA.4 In these cases, patients should be evaluated for reintroduction to eculizumab and/or treatment with PE.4
When considering the application of PT in patients with aHUS, PE with FFP should be preferentially evaluated (1.5 per volume plasma [60-75ml/kg] per session). Sessions should continue until platelet levels have normalised, haemolysis has stopped, and a sustained improvement in renal function has been observed for several days. Later, 5 sessions per week should be administered during the following 2 weeks, followed by another two weeks of 3 sessions per week and an individualised assessment for considering the appropriateness of continued treatment.2,62
Immunosuppression therapy should be administered as a concomitant treatment in patients with aHUS and anti-FH antibodies on PT.22,57,63,64 The response to treatment in these cases should be monitored through the evolution of antibody titres.57
Whenever necessary, support measures can be put into place to control hypertension and volemia. For the treatment of anaemia, red blood cell transfusions and/or erythropoietin stimulating factors can be administered. Platelet transfusions should be limited to cases of severe platelet depletion (<30 000mm3) or in rare cases of severe haemorrhage or in anticipation of invasive procedures with a risk of major blood loss, since this could worsen the TMA phenomena. Another priority is the identification and treatment of possible triggering agents of aHUS. Paediatric patients with aHUS should be transferred to specialised paediatric nephrology centres for treatment from experienced personnel with the availability of a paediatric intensive care unit in order to guarantee appropriate care.
Recommendations for transplantation in patients with atypical haemolytic uraemic syndrome
Prior evaluation
In patients with TCRF and aHUS who are candidates for KT, an exhaustive analysis is first required to determine plasma levels of C3, C4, FH, FI, and FB, as well as MCP expression in peripheral leukocytes.35 A complete genetic analysis should also be performed in order to detect known complement mutations (and polymorphisms associated with risk factors), along with a screening process for anti-FH antibodies.35 Based on these analyses, the patient should be evaluated on an individual basis for the indications for KT and its concomitant treatment, based on the risk of recurrence of aHUS.
Clinically, aHUS is considered as a contraindication for live kidney donation (especially in patients with high-risk mutations) due to the elevated rates of recurrence and graft loss, as well as the risk that the donor could have undetected complement mutations that would facilitate the development of aHUS.1,53,87 Currently, the advancements in genetic diagnostics and the use of eculizumab could allow for considering these patients for live kidney donation through an individualised evaluation of select cases. In the case of related donors, a complete genetic-molecular analysis is also required. As a general rule, the transplant should only be considered as a viable option if the recipient mutations known to be associated with aHUS have already been identified, and these mutations have been ruled out in the potential donor.
Treatment and prophylaxis for the recurrence of atypical haemolytic uraemic syndrome following transplantation
Treatment for recurrence of disease in patients who receive kidney transplants for aHUS should be provided under the same framework as for aHUS in native kidneys, based on early administration of eculizumab,6-9,35,54,70,71,75,88 while taking into account the usefulness and convenience of applying PE. Figure 6 displays a management algorithm for the primary causes of TMA in KT.
Before the availability of eculizumab, it was impossible to recommend single kidney transplants in patients with TCRF secondary to aHUS on dialysis with a high risk of recurrence of disease following transplantation (patients with high-risk mutations and/or episodes of recurrence of aHUS). In recent years, several initial pilot studies have been reported with positive experience with the use of eculizumab as prophylaxis in paediatric patients with FH mutations prior to KT from cadaveric donors,72-74,89 suggesting that KT along with preventative treatment with eculizumab could be the indicated treatment option for these patients. Recently, a study administered prophylactic eculizumab to 9 patients in order to prevent the recurrence of aHUS following KT.88 This study involved 6 paediatric patients and 3 adults with different mutations to the alternative complement pathway (5 FH mutations, 1 C3, and 3 patients with non-homologous genomic reorganisations between CFH and CFHR1 genes). Two patients received post-KT PE and were then transferred to treatment with eculizumab, 2 received eculizumab starting at the week prior to transplantation (live unrelated donor, urgent transplant from cadaveric donor), and the remaining 5 received eculizumab immediately following transplantation. Except for one case involving an early thrombosis that led to graft loss, the other 8 cases had favourable evolution with no recurrences after a mean follow-up period of 14.5 months (mean creatinine: 71.6±44.8µmol). In this context, the recent guidelines from an aHUS research group in France recommended prophylaxis with eculizumab in patients with TCRF secondary to aHUS who are candidates for KT.90
In children with aHUS that have mutations to genes that code for complement factors synthesised in the liver (FH, FB, or FI), liver/kidney and liver transplants have been performed in order to correct the consequences of the genetic defect and prevent disease recurrence. This strategy, combined with perioperative administration of plasma (in order to provide sufficient functional complement factors until the liver graft recovers its ability for synthesis) or eculizumab, has produced successful results in several cases, yielding good liver function and no aHUS recurrence to date.91-95 However, it is essential to evaluate the risk and the morbidity/mortality associated with these procedures, as well as the possible secondary side effects from the long-term immunosuppression required by transplant recipients. It is also unclear whether the minimal extra-hepatic synthesis of FH (in adipose tissue, renal tissue, and monocytes) could induce recurrence of aHUS in the post-transplant period.2
Taking into account that calcineurin inhibitors can be associated with post-transplant TMA due to their nephrotoxic effect, these drugs must be used with caution in patients with aHUS who have received transplants. To this end, a treatment regimen free of calcineurin inhibitors based on belatacept and/or mTOR inhibitors could be a possibility, taking into account the immunological risk of the patient, although no conclusive data are available regarding the most appropriate immunosuppression regimen for the at-risk population.
CONCLUSIONS
- aHUS is caused by a deregulation of genetic origin of the activation of the alternative complement system pathway on cell surfaces, leading to the development of systemic TMA. In recent years, several mutations and polymorphisms have been identified in the genes of certain complement factors that have been related to this deregulation.
- This syndrome is characterised clinically by the triad of non-immune microangiopathic haemolytic anaemia, thrombocytopenia, and acute renal dysfunction, commonly associated with extra-renal manifestations. The prognosis varies based on the type of mutation present, although in general, the syndrome is associated with high mortality rates and occurrence of TCRF, despite standard treatment with PT. There is also an elevated rate of recurrence of aHUS following KT.
- Given clinical signs and symptoms suggestive of TMA, the diagnosis must be orientated towards aHUS if the shiga toxin/STEC test is negative, plasma activity of ADAMTS13 is >5%, and secondary forms of HUS have been ruled out.
- Eculizumab is a monoclonal antibody that inhibits the activation of C5 and the formation of the cell membrane attack complex, which is responsible for the damage produced to native cell structures in aHUS. In prospective studies involving patients with aHUS, eculizumab effectively interrupted the process of TMA, associated in the long-term with significant haematological improvements and recovery of renal function.
- The FDA, the EMA, and the Spanish Agency of Medicines have approved the use of eculizumab for the treatment of aHUS, authorising its use as a first line of treatment. The authors of this document recommend its early use in patients with a clinical suspicion of aHUS in native kidneys, as well as in patients with recurrent aHUS following KT.
APPENDIX 1. RECOMMENDATIONS FOR THE TREATMENT OF SECONDARY FORMS OF HAEMOLYTIC URAEMIC SYNDROME
The management of secondary forms of HUS should be based on the primary aetiology of the disease and the administration of PE. In the case of resistance to treatment, eculizumab should be administered (probably on a temporary basis) as a salvage therapy in selected severe cases,96-98 although little data is available to establish a concrete recommendation in this context.
APPENDIX 2. RECOMMENDATIONS OF INTEREST FOR THE DIAGNOSIS OF GENETIC ABNORMALITIES
Although not necessary for the clinical diagnosis of aHUS, a complete evaluation of the complement system is recommended, including plasma levels of all complement factors and a complete genetic analysis of affected patients. Samples should be taken prior to the start of treatment and sent to a reference laboratory (Table 7). Taking into account that complement mutations have been identified for STEC-HUS and secondary HUS, it is also recommended that these patients undergo an individualised genetic analysis in order to examine the possible correlation with complement abnormalities/aHUS.2
However, a genetic diagnosis of abnormal complement genes would allow for assessing prognosis on an individual bases, as well as the risk of recurrence of disease, making this a recommended step for all patients. In patients who are potential candidates for KT, the genetic analysis is indispensable.
An online database of complement mutations in patients with aHUS is currently available in Spain to facilitate data collection and future studies: https://www.tmaddd.es.
We also recommend taking graft samples from patients who receive kidney transplants due to aHUS for future studies.
Sources of funding and acknowledgements
The research carried out by Dr Rodriguez de Cordoba is financed by the Ministry of Economy and Competition (SAF2010-26583), the Autonomous Community of Madrid (S2010/BMD-2316), and the Fundacion Renal Inigo Alvarez de Toledo.
The authors would like to thank Alexion Pharmaceuticals for their logistic support in carrying out the consensus meetings, as well as editorial support through Ogilvy Healthworld.
Conflicts of interest
Dr Ariceta, Dr Blasco, Dr Campistol, Dr Praga, Dr Rodríguez de Córdoba, and Dr Vilalta have provided consultation and teaching services to Alexion Pharmaceuticals. Dr Torra has formed part of expert consensus committees for aHUS sponsored by Alexion Pharmaceuticals. Dr Espinosa also has participated in clinical studies sponsored by Alexion Pharmaceuticals.
None of the aforementioned activities have influenced the elaboration or interpretation of this manuscript, and the authors are responsible for all content.
Approval
This document has been approved by the Spanish Transplant Society (S.E.T.) and the Spanish Society of Nephrology (S.E.N.) and Spanish Association of Pediatric Nephrology (A.E.N.P.).
Table 1. Aetiological classification of thrombotic microangiopathies
Table 2. Risk factors for atypical haemolytic uraemic syndrome
Table 4. Tests recommended for the diagnosis of atypical haemolytic uraemic syndrome
Table 5. Prognosis of patients with atypical haemolytic uraemic syndrome treated with plasma infusion or plasma exchange
Table 6. Cases published of patients with atypical haemolytic uraemic syndrome who received eculizumab
Table 7. Protocol for sample collection for complement analyses in patients with atypical haemolytic uraemic syndrome
Figure 1. Renal histopathological lesions from haemolytic uraemic syndrome
Figure 2. Representation of the clinical overlap of the different types of haemolytic uraemic syndrome
Figure 3. Complement dysregulation in atypical haemolytic uraemic syndrome
Figure 4. Algorithm for the differential diagnosis of primary thrombotic microangiopathy
Figure 5. Treatment for atypical haemolytic uraemic syndrome
Figure 6. Diagnosis and treatment of thrombotic microangiopathy in kidney transplants
Table 3. Clinical characteristics of patients with atypical haemolytic uraemic syndrome based on complement abnormality