Hyponatremia is the most prevalent hydroelectrolytic disorder in clinical practice, and is linked to higher rates of morbidity and mortality. Coexistence of hyponatremia and COVID-19 infection (an emerging respiratory disease caused by the novel SARS-CoV-2 coronavirus) has been reported in recent studies, but without knowledge of the possible underlying pathophysiological mechanisms.1,2 We present the case of a patient with severe hyponatremia and COVID-19 infection.
A 59-year-old man had a history of hypertension being managed with a combination of angiotensin-converting enzyme inhibitors and hydrochlorothiazide, which had been suspended four days before admission. He sought care due to signs and symptoms for 10 days consisting of a dry cough, slight difficulty breathing, and fever. Three days earlier, he also developed abdominal pain, nausea, and vomiting, and 24 h earlier he also experienced headache and drowsiness. During examination he showed confusion, bradypsychia, and clinical signs of mild dehydration of the skin and mucosae. Laboratory testing revealed severe hyponatremia (102 mEq/L) in the absence of azotemia as well as C-reactive protein 5.05 mg/dL (0.02−0.05 mg/dL), ferritin 252 ng/mL (30−400 ng/mL), D-dimer 174 ng/mL (0−500 ng/mL), and lymphocytes 1.35 × 103/µL. All other laboratory values are shown in Table 1. A chest X-ray showed a bilateral alveolar-interstitial pattern. PCR for SARS-CoV-2 yielded a positive result.
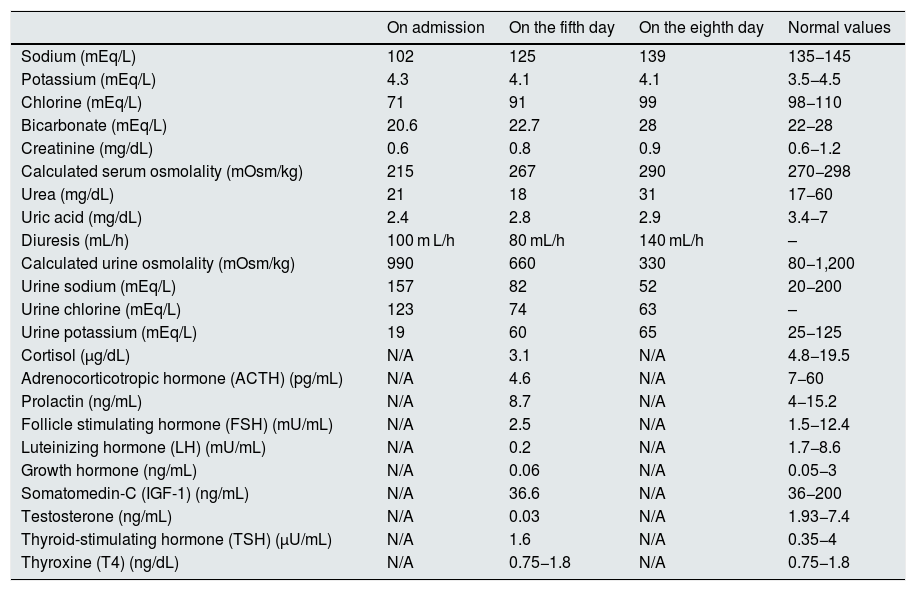
Course of laboratory values during admission and pituitary hormone testing.
| On admission | On the fifth day | On the eighth day | Normal values | |
|---|---|---|---|---|
| Sodium (mEq/L) | 102 | 125 | 139 | 135−145 |
| Potassium (mEq/L) | 4.3 | 4.1 | 4.1 | 3.5−4.5 |
| Chlorine (mEq/L) | 71 | 91 | 99 | 98−110 |
| Bicarbonate (mEq/L) | 20.6 | 22.7 | 28 | 22−28 |
| Creatinine (mg/dL) | 0.6 | 0.8 | 0.9 | 0.6−1.2 |
| Calculated serum osmolality (mOsm/kg) | 215 | 267 | 290 | 270−298 |
| Urea (mg/dL) | 21 | 18 | 31 | 17−60 |
| Uric acid (mg/dL) | 2.4 | 2.8 | 2.9 | 3.4−7 |
| Diuresis (mL/h) | 100 m L/h | 80 mL/h | 140 mL/h | – |
| Calculated urine osmolality (mOsm/kg) | 990 | 660 | 330 | 80−1,200 |
| Urine sodium (mEq/L) | 157 | 82 | 52 | 20−200 |
| Urine chlorine (mEq/L) | 123 | 74 | 63 | – |
| Urine potassium (mEq/L) | 19 | 60 | 65 | 25−125 |
| Cortisol (µg/dL) | N/A | 3.1 | N/A | 4.8−19.5 |
| Adrenocorticotropic hormone (ACTH) (pg/mL) | N/A | 4.6 | N/A | 7−60 |
| Prolactin (ng/mL) | N/A | 8.7 | N/A | 4−15.2 |
| Follicle stimulating hormone (FSH) (mU/mL) | N/A | 2.5 | N/A | 1.5−12.4 |
| Luteinizing hormone (LH) (mU/mL) | N/A | 0.2 | N/A | 1.7−8.6 |
| Growth hormone (ng/mL) | N/A | 0.06 | N/A | 0.05−3 |
| Somatomedin-C (IGF-1) (ng/mL) | N/A | 36.6 | N/A | 36−200 |
| Testosterone (ng/mL) | N/A | 0.03 | N/A | 1.93−7.4 |
| Thyroid-stimulating hormone (TSH) (µU/mL) | N/A | 1.6 | N/A | 0.35−4 |
| Thyroxine (T4) (ng/dL) | N/A | 0.75−1.8 | N/A | 0.75−1.8 |
N/A: not applicable.
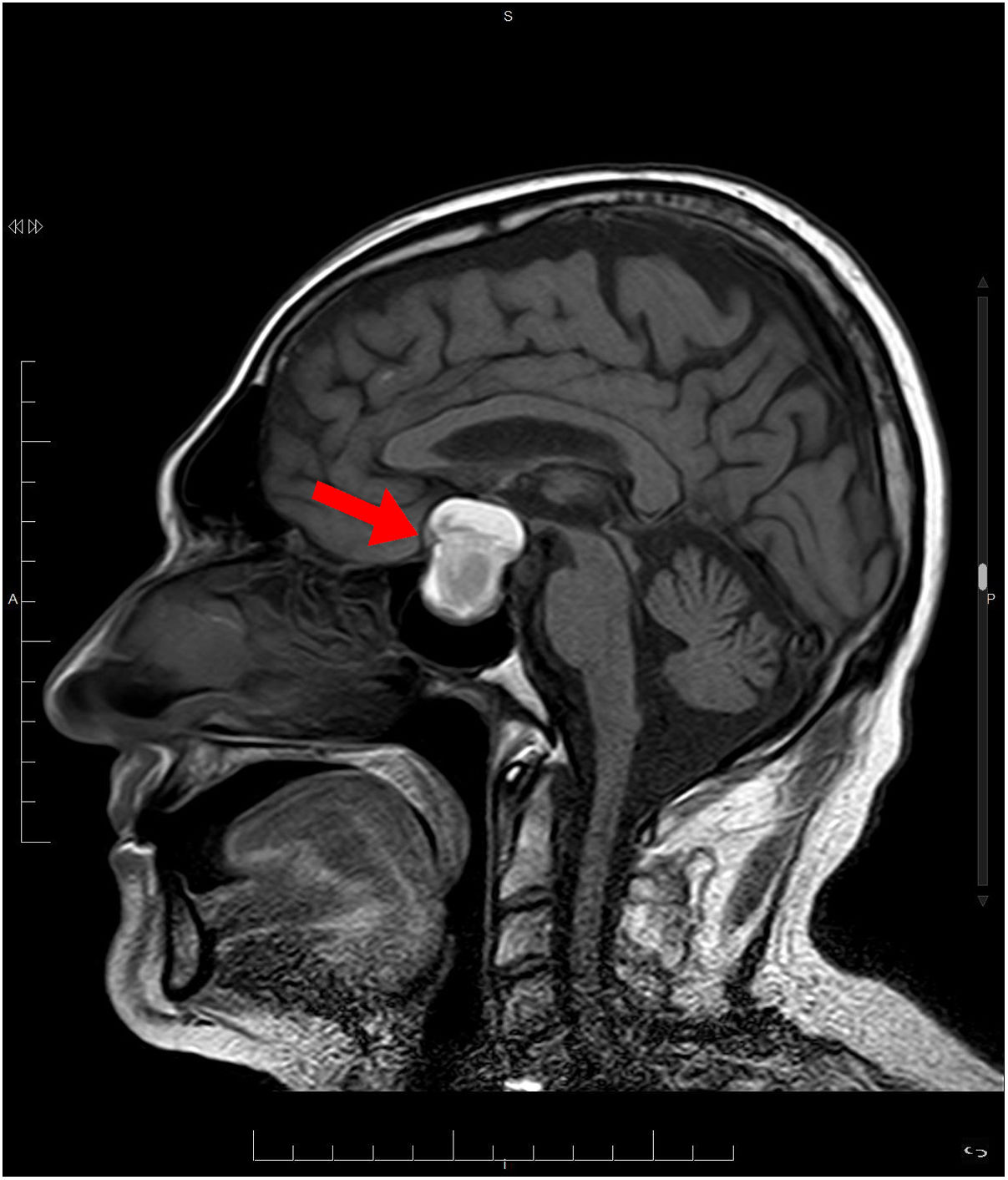
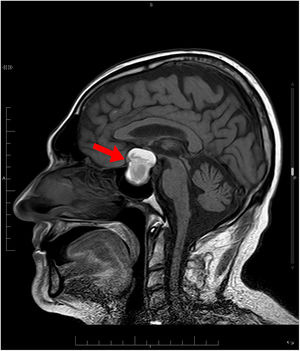
Treatment was started with hypertonic saline 3%, correcting the patient’s natremia to 125 mEq/L, as well as azithromycin with hydroxychloroquine for the patient's COVID-19 infection. Initially, syndrome of inappropriate antidiuretic hormone secretion (SIADH) was suspected. Despite treatment with restriction of fluid, salt, and urea, on the fifth day, the patient had not yet achieved natremia values >125 mEq/L. The patient was found to have low levels of adrenocorticotropic hormone (ACTH) and cortisol (Table 1). Magnetic resonance imaging (MRI) showed a pituitary macroadenoma, with signs of intralesional bleeding (Fig. 1). On the sixth day, he was prescribed intravenous Actocortina [hydrocortisone] at an initial dose of 100 mg every/12 h and subsequently a maintenance dose of 100 mg/24 h. The three following days, his natremia was 139 mEq/L. Campimetry revealed the presence of bitemporal hemianopia. The final diagnosis was severe hyponatremia caused by adrenal insufficiency (AI) secondary to hypopituitarism (HPT) due to a pituitary macroadenoma with radiological signs of subacute pituitary apoplexy in a patient with COVID-19 infection. With this diagnosis, the patient underwent transsphenoidal surgical decompression of the lesion.
Our patient showed signs and symptoms of severe hyponatremia difficult to account for with only his vomiting and/or diuretic treatment, with a striking discrepancy between his clinical and laboratory findings, i.e. dehydration but no azotemia. Hyponatremia is a known form of presentation of HPT, having been reported in various clinical situations, but uncommonly as an initial sign of a pituitary tumor in the context of a respiratory infection due to COVID-193, which probably exacerbated its presentation. Lippi et al.4, following an electronic search on MEDLINE (PubMed), Scopus, and Web of Science, using the keywords sodium, potassium, chlorine, and calcium in patients with COVID-19 disease, identified five studies with a total of 1,415 patients. Sodium was significantly lower in patients with severe disease compared to patients with mild disease due to COVID-19 (weighted mean difference: −0.91 mmol/L, 95% CI: −1.33−0.5 mmol/L). However, it is not yet known whether there is a greater risk of hyponatremia or other electrolyte abnormalities in patients with COVID-19, nor is the mechanism that would cause it understood.
Our patient met nearly all the criteria for SIADH, except for the presence of hormonal abnormalities in thyroid, adrenal, and pituitary function.5 In the differential diagnosis, we ruled out cerebral salt-wasting syndrome (CSWS) given the absence of polyuria and the correction of natremia following volume and sodium replacement, which are fundamental factors in CSWS. By contrast, secondary AI is caused by insufficient hypothalamic-pituitary stimulation, with deficiencies in ACTH and glucocorticoids, but proper mineralocorticoid function and an intact renin–angiotensin–aldosterone axis.6 This explains why our patient did not show classic AI symptoms, as mineralocorticoid deficiency is only present in primary AI.7 Our patient’s severe hyponatremia presented after he developed gastrointestinal signs and symptoms and respiratory infection. We do not know whether stress-induced glucocorticoid decompensation was triggered by this infectious condition.
Endogenous cortisol exerts a tonic inhibitory effect on ADH secretion. Glucocorticoid deficiency features ADH release that cannot be suppressed despite existing hyposmolality.8 Glucocorticoids cause a negative feedback loop in both corticotropin release and ADH release.9 This corrects the hydroelectrolytic abnormality (which would not occur in CSWS) and normalizes ADH levels and renal aquaporin-2 mRNA expression.10
In conclusion, in patients diagnosed with severe hyponatremia, unusual causes should be considered among the possible diagnoses. In our case, we believe that COVID-19 infection may have played a role in the severity of the patient's hyponatremia.
Please cite this article as: De La Flor Merino JC, Mola Reyes L, Linares Gravalos T, Roel Conde A, Rodeles del Pozo M. Inusual caso de hiponatremia aguda grave en paciente con infección por COVID-19. Nefrologia. 2020;40:356–358.