Dear Editor,
Anti-glomerular basement membrane (GBM) antibody disease accounts for 20% of all rapidly progressive glomerulonephritis.1 Occasionally it has been diagnosed in patients with normal renal functions and those patients have favorable renal prognosis. Here, we report a patient with anti-GBM antibody disease who presented with nephrotic range proteinuria, normal renal function and lack of pulmonary symptoms. Contrary to the previous data in literature; he developed end stage renal disease (ESRD) within three years despite appropriate treatment.
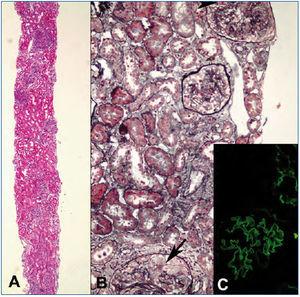
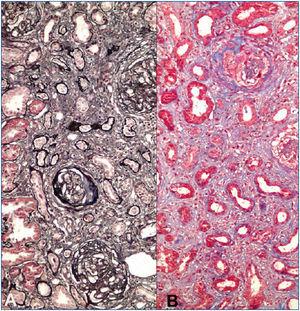
A 23-years-old non-smoker university student male patient presented with minimal bilateral edema in lower extremities that was present for two weeks. He had no gross hematuria, hemoptysis or other pulmonary symptoms. His past medical history was unremarkable. Laboratory evaluation revealed 7200mg/day proteinuria with normal renal functions (serum creatinine: 1mg/dL). Serum albumin level was 4g/dL. Urinalysis showed 3+ proteinuria with microscopic hematuria. Anti-nuclear and anti-double-stranded DNA antibodies, HIV, hepatitis B and hepatitis C serologies were negative. Complement levels were normal. Renal ultrasonography was normal. Renal biopsy was consistent with anti-GBM antibody disease with diffuse linear IgG staining along the GBM, diffuse endocapillary proliferation and cellular/fibrocellular crescent formation in 40% of glomeruli (Figure 1). Numerous glomeruli showed segmental scarring. As soon as the diagnosis was confirmed with positive anti-GBM antibody in serum, plasmapheresis and immunosuppressive treatment was started. Fourteen plasmapheresis sessions were performed until antibodies disappeared. After three days of intravenous pulse methylprednisolone treatment (500mg/day), he was maintained on oral prednisolone (started with 1mg/kg/day and tapered slowly) and monthly intravenous 750mg cyclophosphamide infusions. After twelve intravenous cyclophosphamide treatments, serum creatinine was 1.3mg/dL, albumin was 3.1g/dL and 24-h protein excretion was 4g/day. Thereafter he was maintained on low dose prednisolone (5mg/day) and azathioprine (100mg/day) combination. However under this treatment his renal functions deteriorated and a second biopsy had to be performed after 18 months when creatinine level increased to 2mg/dL and proteinuria to 6g/day. Although serum anti-GBM antibody and ANCA were negative at that time, histomorphologic examination demonstrated ongoing active disease with crescents, linear immunofluorescent staining for IgG on GBM’s and significant chronic injury (Figure 2). Pulse methylprednisolone followed by oral prednisolone, cyclosporine and mycophenolate mofetil could not prevent further deterioration of renal functions. Furthermore he suffered from a herpes-zoster infection and had to struggle with intracranial abscess caused by actinomyces. Immunosuppressive treatment was stopped and regular hemodialysis treatment was started on the 27th month after first diagnosis.
This patient with anti-GBM antibody disease presented with nephrotic range proteinuria with normal renal functions. In spite of normal renal function at presentation, ESRD could not be prevented with intensive treatment.
The first interesting point about the patient is the clinical and laboratory data at the time of diagnosis. He had an unusual presentation with normal renal function and absence of pulmonary symptoms, and the indication of the renal biopsy was nephrotic range proteinuria. Isolated nephrotic syndrome is not a classical feature of anti-GBM antibody disease although it may occasionally accompany disturbed renal function. The cause of nephrotic syndrome in these patients may be a co-existing glomerulopathy which is membranous glomerulonephritis in most of the cases.2 Minimal change disease,3 IgA nephropathy4 or membranoproliferative glomerulonephritis5 may also be associated with anti-GBM antibody disease. There was no accompanying glomerular pathology in this patient based on light microscopic and immunofluorescence studies. Although electron microscopy could not be performed, unresponsiveness of the proteinuria to the steroid and cyclophosphamide decreases the possibility of accompanying minimal change disease.
Another interesting point of this case is the progressive course of the disease despite normal renal function at the beginning. It is known that prognosis of this disease is intimately dependent on the initial creatinine level.6 Patients with anti-GBM antibody disease with normal renal function at presentation uniformly showed good renal prognosis.7 This patient had progressive deterioration in renal functions despite intensive immunosuppressive treatment.
Unusual presentation and course in this patient is difficult to explain. Several hypotheses have been proposed for atypical presentations in anti-GBM antibody disease. The disease is classically characterized by circulating autoantibodies against non-collageneous domain of alpha-3 chain of type-IV collagen.8 It has been suggested that presence of antibodies against non-collagenous domains of alpha-1 and alpha-4 chains of type-IV collagen may result in differing presentations of anti-GBM antibody disease.9 Another possible mechanism to explain the atypical presentation of anti-GBM antibody disease is involvement by the different IgG subclasses. It was shown that anti-GBM antibody is most likely IgG1 or IgG4, and only IgG1 can activate complement.10
In conclusion anti-GBM antibody disease may present with normal renal functions and nephrotic range proteinuria and appropriate treatment may not prevent ESRD in these patients.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. First renal biopsy from the patient.
Figure 2. Second kidney biopsy from the patient.