Kidney transplantation is the treatment of choice in the paediatric population with end-stage renal disease (ESRD).1 There are few studies evaluating the long-term effectiveness and safety of alemtuzumab for kidney transplantation in the paediatric population.
This is a descriptive study conducted at the Hospital Pablo Tobón Uribe (HPTU). All kidney transplant patients under 18 years of age from 2005 to 2012 who received alemtuzumab as induction therapy were included.
The immunosuppression protocol used included administering alemtuzumab and a triple maintenance therapy, with a calcineurin inhibitor (tacrolimus or cyclosporine), antimetabolite (azathioprine or mycophenolate), and steroids. This study was approved by the Hospital Pablo Tobón Uribe ethics committee.
During 2005–2012, 21 paediatric kidney transplants were performed with alemtuzumab received as the induction therapy; 57.1% were boys, and the median age was 13 years (p25–75: 9–15); malformations of the urinary tract were the most common cause of chronic kidney disease (42.9%). The serological status for cytomegalovirus was recipient negative/donor positive in 23.8%, recipient positive/donor positive in 71.4%, and recipient positive/donor negative in 4.8%. The median cold ischaemia time was 18h (p25–75: 12–20).
The 6-, 12-, 24-, 36-, and 60-month patient survival rate after the kidney transplant was 100%, 100%, 95.2%, 95.2%, and 95.2%, respectively. One patient died during the study. Post-transplant 6-, 12-, 24-, 36-, and 60-month kidney graft survival were 95.2%, 95.2%, 90.5%, 85.7%, and 85.7%, respectively.
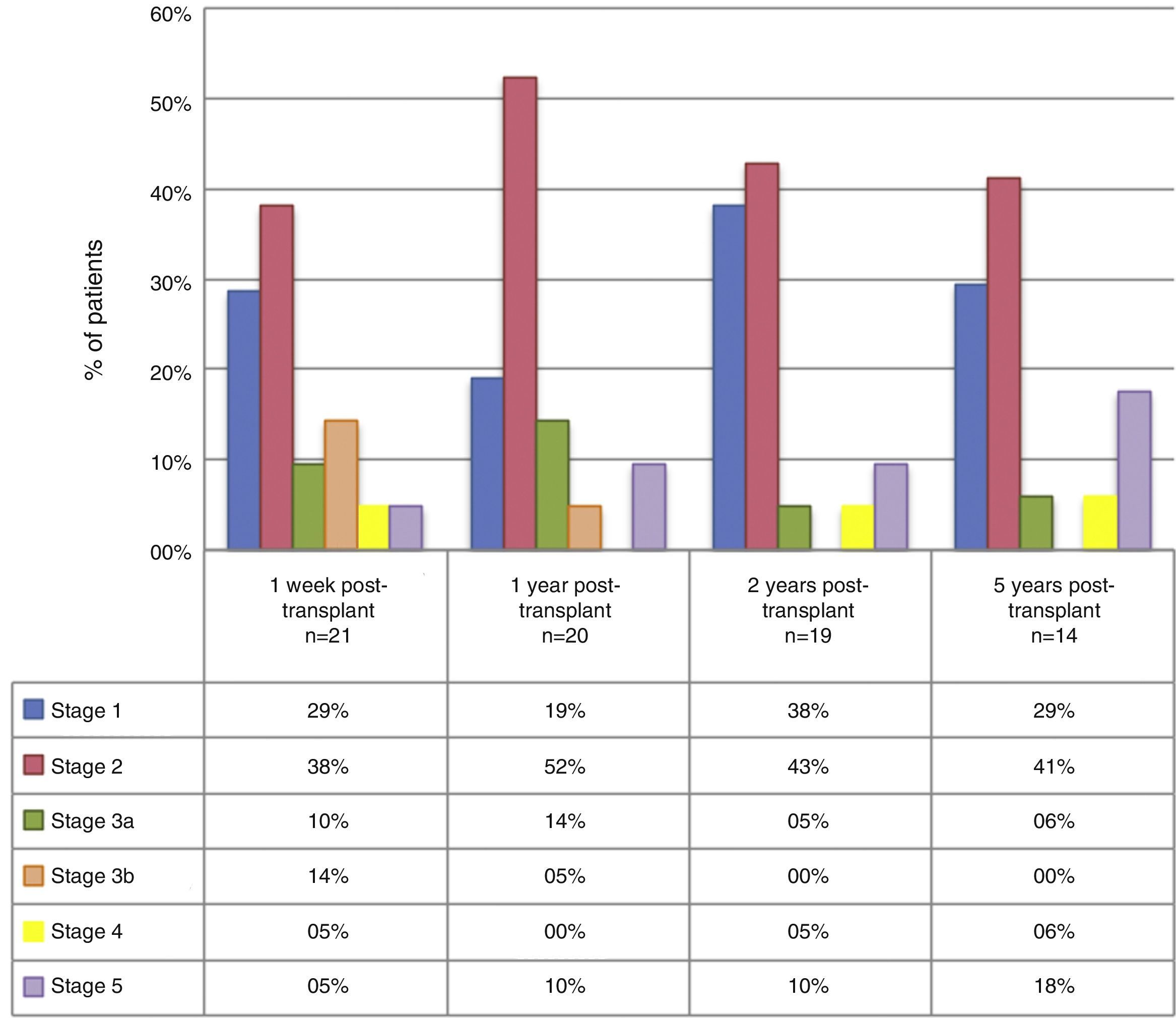
The median GFR 1 week, and 1, 2, and 5 years after the transplant were: 72.5ml/min (p25–75: 45–94; n=21), 70.5ml/min (p25–75: 61–88.5; n=20), 89ml/min (p25–75: 66–108; n=19), and 76ml/min (p25–75: 61–101.5; n=14), respectively. At 1, 2, and 5 years of follow-up, 28.6%, 19.1%, and 29.4% of patients, respectively, presented a GFR under 60ml/min/1.73m2. By grouping the patients according to CKD stage, we found that, during the 5-year follow-up, a high percentage of patients were in stage 1 and 2 (Fig. 1). Table 1 describes the main complications found.
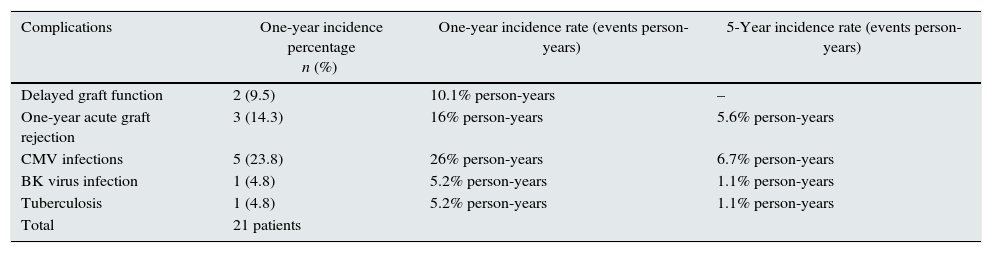
Complications one year after kidney transplantation.
| Complications | One-year incidence percentage n (%) | One-year incidence rate (events person-years) | 5-Year incidence rate (events person-years) |
|---|---|---|---|
| Delayed graft function | 2 (9.5) | 10.1% person-years | – |
| One-year acute graft rejection | 3 (14.3) | 16% person-years | 5.6% person-years |
| CMV infections | 5 (23.8) | 26% person-years | 6.7% person-years |
| BK virus infection | 1 (4.8) | 5.2% person-years | 1.1% person-years |
| Tuberculosis | 1 (4.8) | 5.2% person-years | 1.1% person-years |
| Total | 21 patients |
CMV: cytomegalovirus.
This study describes the clinical outcomes of paediatric kidney transplant patients who received alemtuzumab as induction therapy. Among the most important findings, a good kidney graft survival stands out, with a low incidence of rejection and few complications. One patient who lost the graft 18 months after transplantation died. The patient was admitted, placed on haemodialysis and died due to decompensated heart failure. The other three patients who lost the kidney graft were secondary to acute rejection, due to poor adherence to the immunosuppressant therapy.
Other benefits that we found with the use of this therapy was GFR stability over time. Furthermore, in our study, only 28.6%, 19.1%, and 29.4% of the patients had a GFR under 60ml/min/1.73m2 at 1, 2, and 5 years. According to this, most of the study population remained in stage 1 and 2 chronic kidney disease during the follow-up period, which involves a low future risk of kidney disease-related complications.
The cumulative 1-, 2-, and 5-year incidence of acute rejection in our patients was 14.3%, 21.1%, and 35.7%, respectively. Poor adherence to the immunosuppressant treatment was documented in all these patients, and 3 of them subsequently lost the kidney graft. Only one of the rejections was classified as antibody-mediated.
One of the main fears with the use of alemtuzumab has been its safety profile. In this study, the incidence of CMV infection was found to be 23.8% in the year after transplant, which is very high in comparison with other studies, and which can be explained because we did not use universal prophylaxis against this virus.2–9 As for other infectious complications such as BK virus and tuberculosis, both infections only presented in one patient throughout the entire follow-up period (incidence of 1.1% person-years).
During the follow-up period, which had a median of 6 years, no cases of PTLD was documented in the study population, which is consistent with the literature10; in addition, 23.8% of the patients in this cohort were seronegative for the Epstein–Barr virus. None of the studies with which we compared our outcomes found cases of PTLD.5,7–9,11 One of the possible explanations why alemtuzumab has a low rate of PTLD is its T and B lymphocyte depletion effect, which arguably prevents abnormal lymphocyte clone proliferation, as the clones cause this type of lymphoproliferative disorder.
In conclusion, induction immunosuppression therapy with alemtuzumab, in paediatric kidney transplant recipients, is effective in preventing acute rejection, provides a suitable short- and long-term safety profile, encourages an adequate glomerular filtration rate, and good patient and kidney graft survival.
Please cite this article as: Vélez-Echeverri C, Guerrero-Tinoco GA, Villafañe-Bermúdez DR, Nieto-Ríos JF, Serna-Higuita LM, Serna-Campuzano A, et al. Alemtuzumab en trasplante renal pediátrico: experiencia de 5 años en el Hospital Pablo Tobón Uribe de Medellín, Colombia. Nefrologia. 2016;36:709–711.