After many years without any new developments in this field, new diagnostic tools have recently been incorporated in both the latent phase and in the active infection phase. New methods were required in the study of the general population to improve the existing arsenal, and in the case of the renal patient, especially in advanced stages and in replacement therapy, the need for improvement was evident. As a result of the publication in this issue of the Journal on a study analysing the results of a comparison between the tuberculin skin test (TST) and new in vitro diagnostic methods for the detection of latent tuberculosis infection (LTBI), we will summarise the potential impact of these methods on the treatment of renal disease patients in these Editorial Comments.
TUBERCULOSIS DISEASE TODAY
According to data from the World Health Organization (WHO),1 one third of the world population currently has LTBI. In 2006, there were more than 9200 000 new cases of tuberculosis (TB) worldwide, with a prevalence of more than 14 million people and nearly 1.7 million deaths, which represents a mortality rate of 18%. The WHO believes that the global incidence rate reached its peak in 2002, with variations related to population changes. According to the latest data published by the Red de Vigilancia Epidemiológica de España (Spanish epidemiological surveillance network),2 6070 cases of TB were recorded in 2009. However, these figures should be viewed with caution because even though TB is a notifiable disease, it is estimated that at least one-third of the cases go unreported.
According to the Registry of the Spanish Society of Nephrology (S.E.N.), the incidence of patients receiving renal replacement therapy (RRT) in 2009 was 129 patients per million population (pmp), with the majority (85%) in haemodialysis (HD), 12% in peritoneal dialysis (PD) and 2.8% with pre-dialysis kidney transplant (KT). The prevalence was 1039.4 patients pmp, with 47.67% in HD, 4.8% in PD and 47.51% with functioning grafts. Although the incidence of patients receiving RRT has remained stable over the last ten years in Spain, there are differences between the different regions of the country. In Europe, the incidence varies from 94.6 to 263 patients pmp, and the prevalence between 64.9 and 1115.1 patients pmp, so we are in the average range compared to surrounding countries.3 It is clear that transplant patients, who are immunocompromised, as well as those undergoing dialysis, which causes uraemia-related alterations in the immune system, present a state of immunodeficiency that makes them more susceptible to infection. These alterations primarily affect cellular immunity,4 including the decreased proliferative response of the lymphocytes, Interleukin-2 deficit, peripheral B-lymphocyte deficiency and the increase in cellular apoptosis.5-7 As LTBI is characterised by a significant cellular immune response (in the absence of detectable mycobacteria), the alteration of this response could lead to an increase in the reactivation of TB in uraemic patients and a hyporesponse in the tests based on delayed hypersensitivity.
DIAGNOSIS OF TUBERCULOSIS INFECTION
The usual method to diagnose tuberculosis infection is the TST, which clearly shows, after injecting a purified protein derivative (PPD), a state of prior hypersensitivity in the body when confronted with this substance. The tuberculin used in Europe is the RT-23 PPD. In recent years, new diagnostic methods have been investigated and approved based on in vitro quantification of the cellular immune response. These methods, generically called by the acronym IGRA (Interferon-Gamma-Release Assays), detect the release of interferon gamma in response to specific TB antigens.8 Interferon-gamma is an indispensable molecule in the protective immune response against this microorganism. This cytokine, produced by CD4+ T lymphocytes, CD8+ T lymphocytes and NK cells, activates infected macrophages, with the consequent release of IL-1 and TNF-alpha which limit the growth and multiplication of the mycobacteria. Individuals with a deficit in the receptors or in genes that encode the synthesis of this molecule are likely to present mycobacterial infections more often and with greater severity. The same can be said for patients undergoing immunosuppressive treatment which interferes with these signalling pathways of the immune response.
IN VITRO TRIALS BASED ON INTERFERON PRODUCTION (IGRA)
There are two techniques on the market for in vitro diagnosis of TB infection: QuantiFERON-TB-Gold In Tube (Cellestis®, Victoria, Australia)9 and T-SPOT.TB (Oxford Immunotec®, Oxford, United Kingdom)10. First generation QuantiFERON TB, approved by the Food and Drug Administration (FDA) of the United States in 2001, detected the release of interferon-gamma in response to the TST. In 2004, the FDA approved the second generation of this diagnostic test called QuantiFERON-TB Gold, which unlike the first generation, does not use the mycobacterial antigens of the TST, but rather synthetic peptides which simulate more specific antigens such as the Early Secreted Antigenic Target-6 (ESAT-6) and the Culture Filtrate Protein-10 (CFP-10). These two molecules are encoded by the RD-1 region of the Mycobacterium tuberculosis genome and significantly increase the specificity compared to the TST. These antigens are absent in M. bovis and in the majority of non-tuberculous mycobacteria (with the exception of M. kansasii, M. marinum or M. szulgai). At present, the third generation of this test, called QuantiFERON-TB Gold In Tube (QFT-GIT), is already on the market and includes a third mycobacterial antigen: the TB 7.7 and tubes specifically designed to collect blood samples for this test.
VALIDITY OF QTF-GIT AND T.SPOT.TB IN PREDICTING THE DEVELOPMENT OF TUBERCULOSIS DISEASE
The risk of developing active TB in a person with a positive TST is estimated at 5%-10%.11,12 However, there are few longitudinal studies that allow us to conclude the ability of IGRA to predict the risk of developing active TB.
A study was conducted in Germany on 601 close contacts of people who had an acid-fast bacilli smear and were culture-positive for M. tuberculosis. The QFT-GIT yielded better performance in predicting active TB13 than the TST, using a cut-off point of 5mm. Five (2.3%) out of the 219 close contacts with an induration of >5mm developed TB, whereas six (14.6%) of the 41 close contacts with positive results from the QFT-GIT developed the disease. However, 59% of the close contacts had an induration (TST) of 5-9mm. The percentage of those considered TST-positive with a cut-off point of 10mm and developed active TB (5 out of 90 [5.6%]) was similar to the percentage who were QFT-GIT-positive (6 out of 41 [14.6%]). Furthermore, only 2 out of 6 close contacts that were QFT-GIT-positive and developed active TB were microbiologically confirmed. In another study, sensitivity to predict subsequent active TB did not show any difference between the two tests.14
The outcome of another study on 339 immigrants in the Netherlands demonstrated that the TST and the QFT-GIT had similar validity in predicting active TB.15 Follow-up was carried out for 2 years on those close contacts with a TST>5 between 0 and 3 months after the diagnosis of the index patient. Nine (3.1%) out of 288 close contacts with a TST>10mm developed active TB, and seven (3.8%) out of 184 with a TST>15mm, five (2.8%) out of 178 with a positive QFT-GIT, and six (3.3%) out of 181 with a positive T-SPOT.TB also developed the disease. Sensitivity to detect the development of active TB in the follow-up period was 100% for the TST with a cut-off point of 10mm, 88% for a TST with a cut-off of 15mm, 63% for the QFT-GIT and 75% for the T-SPOT.TB. Although the TST with a cut-off point of 10mm detected the greatest number of close contacts who developed active TB (100%) and the QFT-GIT identified the least number of close contacts who developed active TB (5/[63%]), the sensitivity of both tests were not any different. In view of everything published, IGRA do not seem to bring any added advantage in predicting the development of tuberculosis disease compared to the TST.
USE OF QTF-GIT AND T.SPOT.TB IN CONTACT TRACING
So far, there have been numerous studies carried out based on TB contact tracing. It was initially based on the TST, but since the introduction of IGRA, the latter have often been the subject of research in this group.16, 7 In two papers, it was observed that more recent exposure (longer exposure or a greater number of alcohol-resistant bacilli in the sputum) is associated with more positive IGRA than positive TST, which suggests that IGRA could be more effective in the detection of recent infection.
SENSITIVITY OF SCREENING TESTS
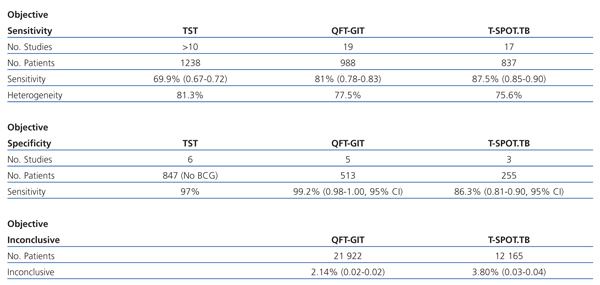
There are two meta-analyses18, 9 that summarise the results obtained so far on IGRA (Table 1).
INVALID RESULTS FOR BOTH TESTS
Inconclusive results for QTF-GIT
Out of a total of 21 922 patients, 469 (2.14%) presented inconclusive results (CI 95%, 0.02-0.023).
Inconclusive results for T-SPOT.TB
Out of a total of 12 165 patients, 462 (3.80%) had inconclusive results (CI 95%, 0.035-0.042). If 80 cases were added in which there was not a sufficient number of cells available for testing, the number of inconclusive results would rise to 4.46 (CI 95%, 0.041-0.048).
The difference in the percentage of inconclusive results between the two IGRA is greater for the QTF-GIT.
Inconclusive results in immunocompromised patients
In cases where the QTF-GIT increases up to 4.42% and in cases where the T.SPOT.TB is up to 6.12%.
ADVANTAGES OF TECHNIQUES FOR IGRA TESTING VERSUS TUBERCULIN SKIN TEST
IGRA techniques offer important advantages over the TST: 1) They do not interfere with the BCG vaccine; 2) They avoid subjectivity in interpreting and the reading visits, and 3) They include a positive control that provides valuable information when interpreting a presumably false negative test as a true negative or inconclusive (technical errors or immunosuppression).
Experience with dialysis patients
Due to the alterations in the immune system, dialysis patients are particularly likely to develop active TB, which could reach a TB incidence of up to eight times greater than in the general population.20 Moreover, it is associated with a higher mortality, which is why the detection of LTBI is an important issue in this population. For decades, the TST has yielded poor results in detecting latent tuberculosis in these patients and an anergy rate that could reach 44%.21-24 Although they are beginning to publish studies on the validity of IGRA in the detection of LTBI, comparing them occasionally with tuberculin, we have little data on sensitivity and the rate of inconclusive results in patients with renal disease in general, and specifically on treatment with replacement techniques.
To date, there are no similar studies available on the PD population. In the study published in this issue, the validity of IGRA versus TST was analysed for the detection of LTBI in 54 PD patients, and it revealed promising results. As in the study carried out on HD patients, there is a substantial percentage of inconclusive results. Therefore, it is vital to accumulate new series to strengthen and clarify the results. In that study, an assessment by an expert pulmonologist is used as the gold standard to detect LTBI and the authors concluded that IGRA could complement the tuberculin skin test, but there is still not sufficient evidence.
Kidney transplant patients
The prevalence of TB among kidney transplant recipients has been widely published. The incidence of LTBI among these patients is estimated at 20-70 times higher than in the general population.25 In these patients, TB contributes to graft dysfunction through direct effects on the graft as well as drug interactions. Furthermore, it increases mortality.26 Reducing the risk of TB is an important priority in organ transplants, especially in countries where the disease is endemic. Anti-tuberculosis treatment is complicated, particularly due to the anti-TB drugs that induce cytochrome P-450 (rifampicin)27 and liver dysfunction caused by Isoniazid.28 The results on IGRA in solid-organ transplant are controversial, while in some studies the sensitivity is similar to the TST, in others, it is higher.25,29
Utility of IGRA in the future
With current knowledge, the question is whether or not IGRA could replace TST to rule out tuberculosis infection in immunocompromised patients. Pending further studies about this subject, we can state that: 1) Currently, there is not enough data available on the long-term development of TB that enables us to make the decision whether or not to treat based solely on the results of IGRA in patients receiving RRT; 2) The theoretical basis of IGRA indicates that these techniques measure a different type of immune response than that which occurs in the delayed hypersensitivity to the TST; 3) Unlike what occurs in the contact tracing study, in immunocompromised patients, both recent and remote tuberculosis infections are just as important; 4) There seems to be no sufficient evidence as of yet that shows that IGRA can replace the TST, and 5) We can conclude that IGRA are supplemental assays to the TST as performing both tests simultaneously increases the likelihood of diagnosing TB. In any event, IGRA represent a significant advancement in the diagnosis of tuberculosis infection.
Table 1. Results of the study comparing different diagnostic methods for detecting confirmed tuberculosis