INTRODUCTION
Infection due to the hepatitis C virus (HCV) is relatively common in chronic renal failure (CRF) patients on renal replacement therapy with dialysis. Although there are geographic differences in prevalence, generally greater than 20% in Mediterranean countries, it is always higher than in the general population.1,2 There is strong evidence that kidney transplant recipients with chronic infection due to HCV have worse patient and graft survival than HCV-negative recipients.3-5 Increased mortality has been attributed to both liver disease progression, up to 40% have progression of fibrosis,6 and post-transplant extrahepatic complications, new onset diabetes, thrombotic microangiopathy and de novo or recurrent glomerulonephritis.
The insistence on treating dialysis patients who are infected by HCV and waiting for their inclusion on the kidney transplant list is based on the high risk of acute renal graft rejection induced by treatment with interferon (IFN). IFN has immunomodulatory effects, which include an increase in gene expression of human leukocyte antigens and cytokines, which promote the production of donor-specific alloantibodies that would induce acute humoral rejection.7 Due to all of the above, it is important to treat HCV infection in ESRD patients while they are on dialysis, before renal transplantation.
Most publications report results on IFN monotherapy efficiency as a result of past rejection of ribavirin (RBV) use in CRF patients, since renal clearance of the latter causes increased toxicity in these patients, which could trigger haemolytic anaemia, as well as aggravating anaemia resulting from chronic kidney disease. However, due to the efficacy of IFN and RBV in patients without CRF, we decided to combine the latter with treatment against HCV,8-10 and we found that, as with the general population, the sustained viral response (SVR) rate was greater with dual therapy (43%-50% versus 55%-66%).10,11
In the last decade, as a result of the detrimental effects of HCV infection on renal graft and patient survival, active virus treatment policies have been proposed in dialysis units for patients who lose graft function. However, in the literature, there is experience of acute rejection in dialysis patients with non-functional grafts after treatment is started with IFN. It is necessary to know and provide an early diagnosis of acute graft rejection in a non-functional graft in dialysis patients who are going to receive these treatments, which means we must take a prior therapeutic approach (embolisation or transplantectomy). For this reason, we are reporting our experience of different cases with a different therapeutic management.
CASE 1
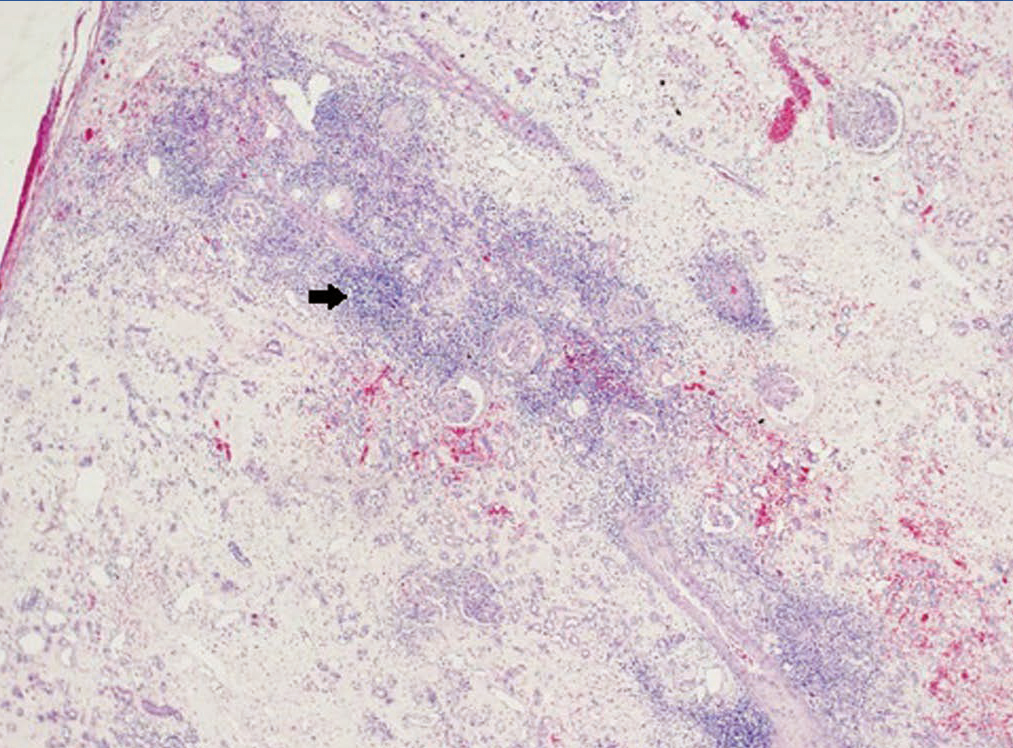
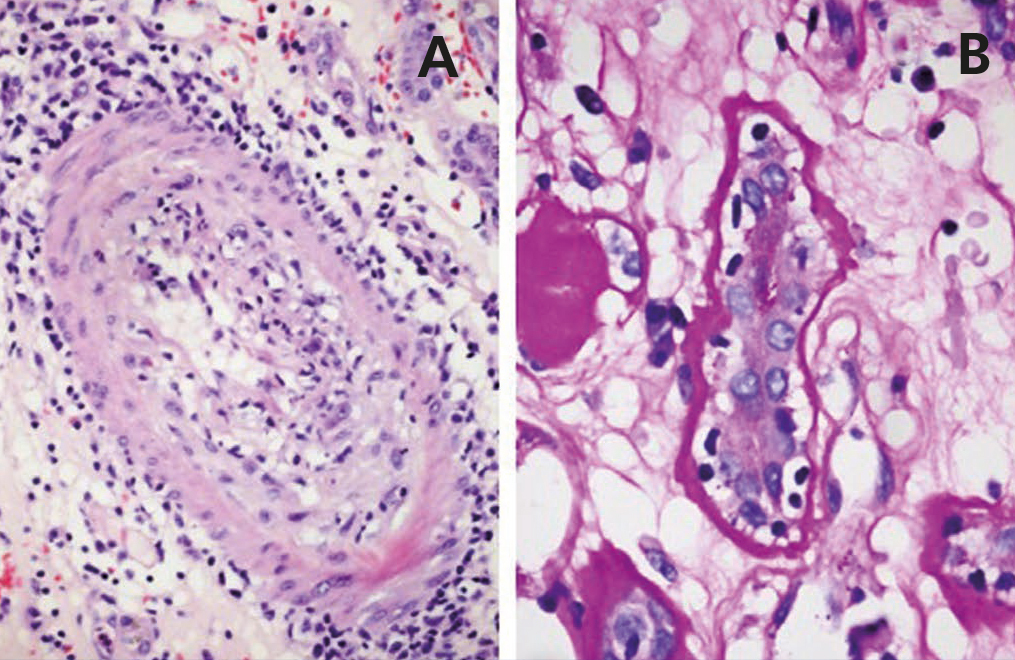
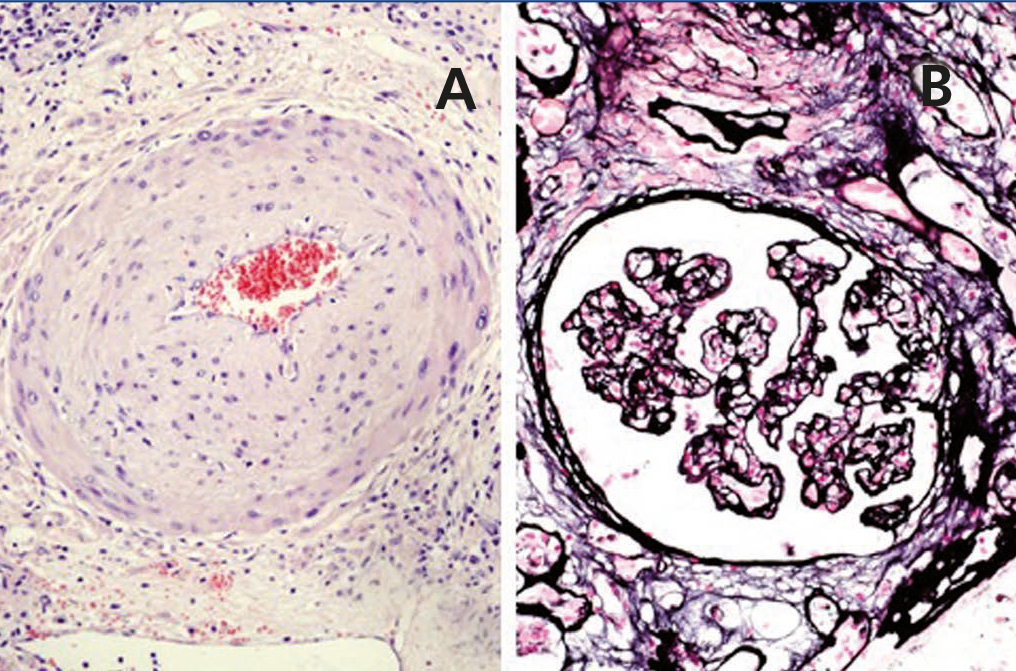
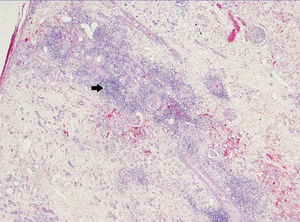
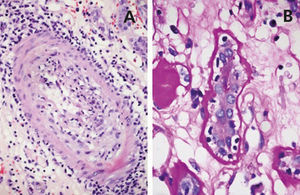
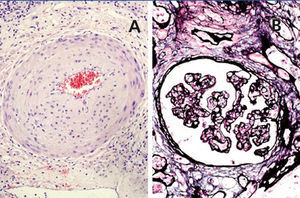
A 48-year-old Caucasian male, with CRF secondary to renal hypoplasia, began haemodialysis in 1984. That year, he received his first kidney transplant from a live donor, his father, which was functional until 1997, when he returned to haemodialysis due to chronic graft nephropathy and a transplantectomy was carried out. In 1999, he received a second renal transplantation from a deceased donor and returned to haemodialysis in November 2002 after developing membranoproliferative glomerulonephritis, secondary to HCV, on the renal graft. “Non-A, non-B” hepatitis was diagnosed in 1984 and a liver biopsy in 2000 showed chronic periportal hepatitis (P3L3F2) with positive HCV-RNA (genotype 3). After the return of the patient to the kidney transplant list, in March 2003, we started treatment with 3 million units of IFN-α three times a week. In April 2003, he experienced pain at renal graft level, haematuria and fever, and as such, IFN-α was discontinued and a scheduled transplantectomy was carried out. In the histology, we observed intense parenchymatous lesions of acute and chronic rejection (Figures 1, 2 and 3). In August of the same year, we confirmed the negativisation of HCV-RNA despite the patient had received only one month of antiviral treatment. However, in January 2004, he had a relapse, and as such, antiviral treatment was reintroduced and maintained for 12 months and a SVR was achieved without relapse. In February 2008, he received a third kidney transplant from a deceased donor. He currently has normal renal function and an undetectable viral load five years after the end of treatment.
CASE 2
A 50-year-old Caucasian female, with CRF secondary to polycystic kidney disease, started haemodialysis in 1987 and received her first kidney transplant from a deceased donor in 1988. As a result of transplant complications, she experienced acute rejection, which was treated with intense steroid therapy, anti-thymocyte globulin (ATG) and anti-CD3 monoclonal antibodies (OKT3). She returned to haemodialysis in June 2009 after loss of renal graft due to chronic nephropathy. “Non-A, non-B” hepatitis was diagnosed in 1987 in relation to multiple transfusions of blood products, with positive HCV-RNA (genotype 1a) subsequently being confirmed. In November 2009, five months after returning to dialysis, treatment was started with 135μg/week pegylated IFN α2a and 200mg RBV every 48 hours, with increasing doses of erythropoietin throughout the treatment. In February 2010, she displayed fever and haematuria, and consequently, the antiviral treatment dose was reduced and a graft embolisation was performed due to suspicion of acute rejection of the non-functional graft. Treatment with IFN and RBV was recommenced at the initial dose and had to be suspended in week 40 due to the occurrence of exudative erythema multiforme that did not respond to corticosteroid treatment. The patient had a quick viral response with an undetectable load in the fourth week of treatment and a subsequent SVR. In July 2011, she received a second kidney transplant from a deceased donor. Her renal function is currently normal and her viral load is negative.
CASE 3
48-year-old Caucasian male, with CRF secondary to chronic interstitial nephropathy within a vesicoureteral reflux, started haemodialysis in 1990. He received his first kidney transplant from a deceased donor in 1991, returning to dialysis in 1997 due to chronic graft nephropathy. In 1999, he received a second kidney transplant with an urgent transplantectomy five months later due to kidney rupture caused by acute rejection. In 2005, he received a third kidney transplant from a deceased donor, returning to dialysis in January 2010 after loss of renal graft function due to chronic nephropathy. Again, as a result of many transfusions received, he was diagnosed with “non-A, non-B” hepatitis in 1990, with the positivity of HCV-RNA (genotype 1a) subsequently being confirmed. Six months following his return to dialysis, we performed an embolisation on the third renal graft, given this patient’s immunological risk due to a history of acute rejection, hyperimmunisation and three previous transplantations. In September 2012, after determining IL28B C/C polymorphism (responder genotype), we began antiviral treatment of chronic viral hepatitis, with mild fibrosis appearing in the fibroscan, with 135μg/week pegylated IFN α2a, 200mg RBV every 48 hours and an increase in the dose of erythropoietin throughout treatment. We observed negativisation of the viral load in the third month (early viral response). A year after the start of treatment, it remained undetectable and there was no acute rejection of the non-functional renal graft.
DISCUSSION
In patients with a positive PCR (Polymerase Chain Reaction) for HCV and a non-functional graft, there are no reliable data about IFN-α efficacy. Nevertheless, most of them do not have immunosuppressive treatment, or many receive low doses of corticosteroids, and as such, their response to antiviral treatment, IFN monotherapy or IFN and RBV should not be different to that presented by haemodialysis patients who have never received a kidney transplant. However, in terms of the normal adverse effects of this treatment in patients with ESRD, pancytopenia, flu-like syndrome, anorexia and infections, we should include acute failure of the non-functional graft, since due to its immunomodulatory effect, it stimulates alloantibody formation.
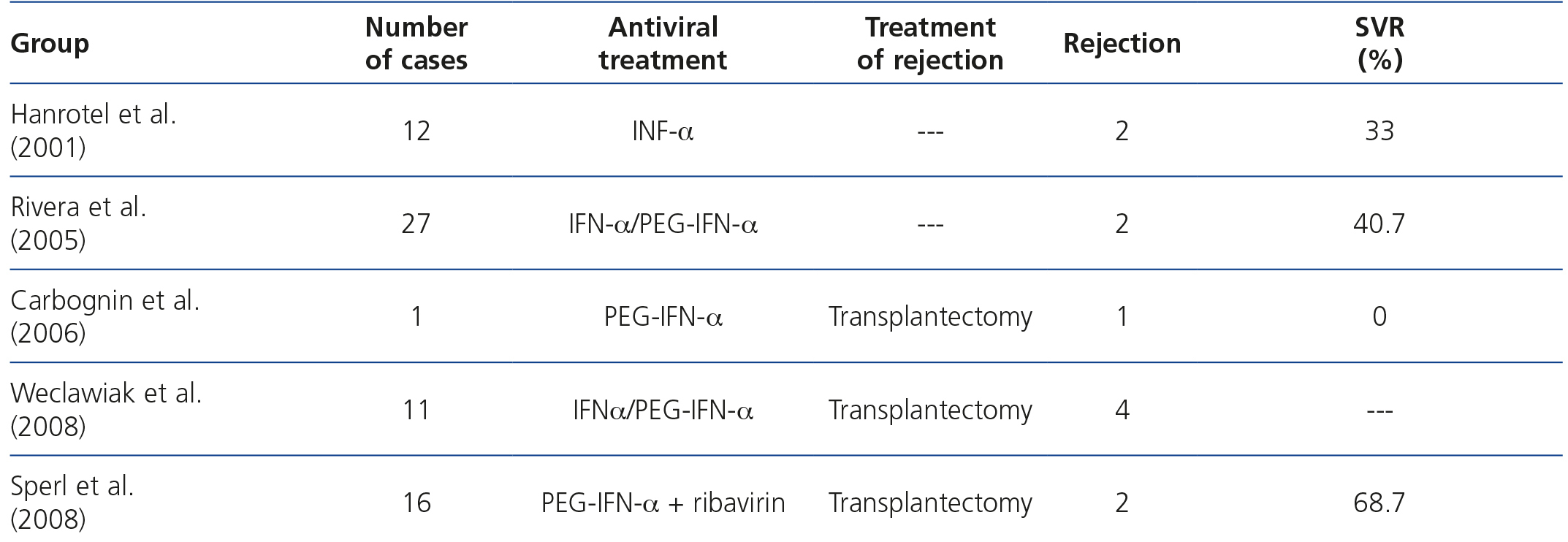
In 2001, Hanrotel et al.12 published a study about the response to IFN-α in 12 haemodialysis patients with chronic infection due to HCV. In this group, 2 patients had non-functional kidney transplants and 8 others had received a transplant on which a transplantectomy had been performed before the start of antiviral treatment. These patients were on dialysis for 24 months, with suspension of immunosuppressant treatment 6 months before the start of HCV eradication therapy. The two patients who had non-functional grafts displayed acute graft rejection. In 2005, Rivera et al.11 analysed the data of 27 dialysis patients, in whom HCV was treated, for their inclusion on the kidney transplant list after a mean period of 161±93 months on the aforementioned renal replacement therapy. Two had acute non-functional graft rejection. No more data were provided on time on immunosuppression, treatment that they received after diagnosis or whether they had a SVR. In 2006, Carbognin et al.13 reported a patient with chronic infection due to HCV, who 6 months after starting dialysis and 5 since immunosuppression was discontinued, started antiviral treatment. He also had acute graft rejection. In 2008, Weclawiak et al.14 reported 4 cases of dialysis patients who, after the loss of graft function, maintained low doses of prednisone for 6 months and started antiviral treatment against HCV 2, 9, 15 and 23 months after starting haemodialysis, presenting a clinical profile consistent with acute non-functional graft rejection, which was confirmed in the transplantectomy specimen. In 2008, Sperl et al.15 reported 2 new cases of acute non-functional graft rejection in 2 patients who 4 and 8 months after starting haemodialysis due to loss of graft function started treatment with pegylated IFN α2a. Four other patients in this study, who also had non-functional kidney transplants did not present acute rejection, although the time of starting antiviral therapy was not specified (Table 1).
In all of the abovementioned cases, the acute rejection treatment of choice was transplantectomy. In our case 2, graft embolisation was sufficient, thus avoiding the risks of exposure to a general anaesthetic, open surgery and a postoperative period. In case 3, due to prior experience in previous cases, a renal graft embolisation was chosen before antiviral therapy was begun. Performing an embolisation on a high immunological risk patient was sufficient for avoiding acute rejection of the non-functional graft, which would very likely have occurred after starting anti-viral therapy. In addition to the immunological risk, the time from starting dialysis and discontinuing immunosuppression to the start of antiviral therapy has an important role in the development of this entity; nevertheless, this has not been demonstrated due to the lack of studies in this regard. However, despite more than 12 stable months on dialysis, Rivera et al., Hanrotel et al. and Weclawiak et al. published cases of acute rejection of a non-functional renal graft after treatment was started with IFN.
In summary, nephrologists should be aware of the adverse effects of IFN treatment in patients with an infection due to HCV with a non-functional graft, in order to be able to diagnose acute rejection and act quickly, since there is a risk of graft rupture and therefore a risk to the patient’s life. We recommend performing a preemptive embolisation of the graft in high immunological risk patients (history of acute graft rejection, hyperimmunisation, several previous transplantations), since, despite being an invasive technique, the risks are minimal when is performed by an expert team.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Previous experience.
Figure 1. Overview of acute and chronic rejection.
Figure 2. Acute rejection.
Figure 3. Chronic rejection.