Hemophagocytic Syndrome is a clinical condition characterized by the activation of either macrophages or histiocytes with a prominent hemophagocytosis feature in the bone marrow and other reticuloendothelial systems. It leads to the phagocytosis of erythrocytes, leukocytes, platelets and their precursors. The presence of hemophagocytosis can be associated to infections, malignancies, autoimmune diseases, drugs and a variety of other medical conditions. We report a case of a previously healthy 36 year-old woman that developed hemophagocytosis at the same time that fulfilled diagnostic criteria for systemic lupus erythematosus. Lupus related hemophagocytic syndrome is a rare and potentially fatal entity. It offers significant differential diagnosis challenges and requires urgent therapeutic intervention. There are only few cases reported in the literature. However, much is still needed in order to better understand its causes, all the immunopathogenic mechanisms, as well as its clinical and therapeutic aspects.
INTRODUCTION
Hemophagocytic Syndrome (HS) is a clinicopathologic entity characterized by increased proliferation and activation of benign macrophages with hemophagocytosis throughout the reticuloendothelial system.1 HS may develop as a rare but potentially fatal complication of several disorders including malignancies, drugs, infections and autoimmune disorders.
Patients usually present with an acute febrile illness, sometimes fulminant and lethal. Common manifestations include high fever, pancytopenia, hepatosplenomegaly, weight loss, lymphadenopathy, abnormal liver function tests (LFTs), hyperferritinemia and high blood triglyceride levels2. Disseminated intravascular coagulation and central nervous system dysfunction often ensue, and less frequently the lungs and cardiac tissues are involved.
Although the pathogenic mechanisms still remain unknown, overproduction of pro-inflammatory cytokines, uncontrolled activation of T cells and macrophages, associated with decreased natural killer cell and cytotoxic cell functions, seem to be the hallmarks of the immunologic abnormalities in HS3-5.
HS has been reported in patients with various connective tissue diseases, such as systemic juvenile idiopathic arthritis, adult-onset Still’s diseases, scleroderma, dermatomyositis, and systemic lupus erythematosus (SLE). HS developed in active SLE patients without evidences of other underlying causes of HS, such as infections or malignancies, has been called «acute lupus hemophagocytic syndrome (ALHS)»6. The occurrence of ALHS was closely related with the presence of high titers of antinuclear antibodies (ANA) and hypocomplementemia, indicating that the immune complex-mediated mechanisms might be responsible for the pathogenesis of ALHS7.
The first line therapy for the treatment of HS associated with autoimmune diseases includes high-dose corticosteroids and immunosuppressives, such as cyclophosphamide and cyclosporine8. High-dose intravenous immunoglobulin,9 anticancer drugs and TNF-α inhibitor (etanercept) have been used in the refractory cases10.
We report a case of SLE with an unusual presentation. The patient presented with acute febrile illness along with progressive pancytopenia. Concomitant polyarthritis, myositis, nephritis, high titer of antinuclear factor and positive test for anti-DNA antibody, made her fit the diagnostic criteria of SLE. No definitive evidence of associated infections was confirmed by the bacteriologic, serologic and viral studies. ALHS was diagnosed and the patient was treated with highdose steroids and cyclophosphamide with an excellent outcome.
The case is intriguing because ALHS is a rare and lifethreatening entity, challenging in differential diagnosis that requires urgent therapeutic intervention. Making a systematic review of the Medline database, we found only a few cases reported in the literature.
Possible pathogenetic mechanisms and currently applied therapeutic modalities are also briefly reviewed.
CASE REPORT
A 36-year-old, caucasian woman, with no previous serious illnesses, was admitted to our hospital because of continuous fever lasting for 4 weeks and thrombocytopenia. She had no family history of HS, and had been well until 2 months before admission, when she began increased muscle weakness, fever, weight loss, photosensitivity and symmetric polyarthritis involving the small joints of the hands and wrists.
On clinical examination, she appeared unwell, was febrile at 39 ºC, with a marked photosensitive rash on her face and an extensive confluent psoriasiform rash on both shoulder areas. A vasculitic rash most prominent on the tips of the fingers with periungual erythema and alopecia was present. The patient also had multiple painful oral lesions with necrotizing ulceration on the buccal mucosa, upper palate, and lips. No evidence of lymphadenopathy was observed and the rest of the examination was unremarkable.
Laboratory evaluation on admission revealed normochromic normocytic anemia (hematocrit 24.5%, haemoglobin 8,1 g/dL, red blood cell count 2,9 x 1012/L), leukopenia (white cell count 2,92 x 103/μL with normal differential) and low platelet count (PLT 21 x 103/μL). Biochemistry was abnormal for aspartate transaminase 84 U/L (normal range, 15-46 U/L), alanine transaminase 200 U/L (normal range, 10-66 U/L), gGT 144 U/L (normal range 12-58 U/L), lactic dehydrogenase 1,182 U/L (normal range 313-618 U/L) and albumin 21 g/L (normal range 35-50 g/L). Urea and creatinine were normal.
Erythrocyte sedimentation rate (ESR) was markedly elevated at 115 mm/ 1sth (normal range <12 mm/1sth) and C-reactive protein (CRP) was negative. Coagulation screen was normal. Ferritin and triglyceride blood levels were markedly elevated to 820 ng/ml and 7,3 mmol/L (normal ranges 9-120 ng/ml and <2,3 mmol/L respectively).
Urinalysis showed proteinuria and microscopic haematuria. Urine microscopy showed 10 erythrocytes and 2-4 granular casts per high power field. 24-hour urine protein excretion was 12 g.
The autoimmune profile revealed ANA >1,280, homogeneous, Anti-Ro/SSA antibodies and anti-RNP strongly positive. Serum complement C3 and C4 levels were both decreased to 450 mg/l and 5 mg/l (normal ranges 900-1,800 and 100-400 respectively). Direct Coombs test was positive. Circulating immune complexes were not detected, and other autoantibodies including anti-neutrophil antibody, antibodies against cyclic citrullinated peptide and rheumatoid factor were negative.
Repeated urine and blood cultures were negative. Sputum was negative for bacterial and fungal cultures. A thorough infection screen, including a viral panel for Herpes Simplex, Herpes Zoster, Epstein-Barr, Cytomegalovirus, Hepatitis B, Hepatitis C, HIV, Echo, Coxsackie and Parvo B19 viruses was negative. A malignancy survey excluded any significant pathology.
Chest X-ray, electrocardiogram, and echocardiography had no abnormal findings. Thoracoabdominal computed tomography showed no abnormalities other than mild hepatosplenomegaly.
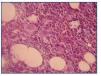
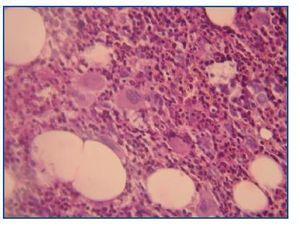
A bone marrow aspiration revealed hemophagocytosis with activated histiocytes (figure 1), normal hematopoiesis and no malignant cell invasion.
A percutaneous needle kidney biopsy was performed.
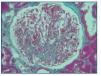
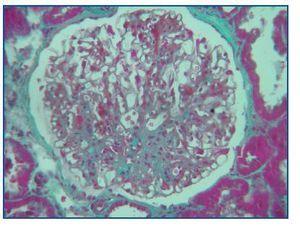
Histological examination at light microscopy showed sclerosis in 6 of the 9 glomeruli, modest mesangial proliferation, tubular atrophy and diffuse interstitial fibrosis (figure 2).
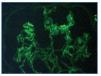
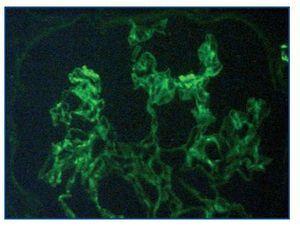
Immunofluorescence staining of 6 glomeruli revealed mesangial deposits of IgM and C3 (figure 3). The diagnosis of Focal Proliferative Lupus Nephritis (class III WHO) was established.
No causative disorder for HS could be detected other than active SLE. The diagnosis of acute lupus hemophagocytic syndrome was established.
The patient was treated with high-dose corticosteroid therapy with intravenous administration of methylprednisolone, 1 g/day for 3 days followed by 1 mg/Kg/day p.o. Because of a partial response, five days after the initiation of the therapeutic scheme, 1 g of intravenous cyclophosphamide was added to her treatment.
As the consequence of these treatments the patient improved clinically, the cytopenias reversed concomitantly with the suppression of hemophagocytic histiocytes, and the inflammatory indices, LFTs, and ferritin levels all gradually returned to normal. Two weeks later she was discharged in good condition on a tapered dose of oral steroids.
DISCUSSION
Hematologic involvement in SLE is a very common finding, but usually only one or 2 cell lines are affected. Pancytopenia occurs in fewer than 10% of patients and, in the absence of offending medication (ie, azathioprine, cyclophosphamide, and so on), is usually the result of peripheral destruction6,11.
In our patient, all laboratory findings pointed to central marrow suppression. The extremely high ferritin, with normal CRP could not be explained by any other factor (ie, infection) than HS, because our patient also did not have evidence of malignancy or hemochromatosis. Liver dysfunction and excessively high ferritin fit well in HS and are caused by high levels of circulating cytokine (IL-1, IL-6, interferon-gamma [INF-gamma], TNF α)12.
HS is a rare entity characterized by the proliferation of nonneoplastic cells of the histiocyte lineage that phagocytose the hematopoietic cells. It is usually aggressive, the outcome varies, and some cases are lethal2,6.
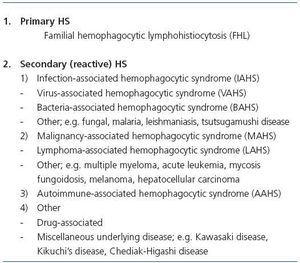
HS can be associated with infection, malignancies, autoimmune diseases, drugs and immunosuppression. The infective agents include bacteria, viruses, most commonly Epstein-Barr virus and cytomegalovirus, or fungi. Causative malignancies are usually hematologic, especially lymphomas, but solid organ tumors have also been implicated. Many autoimmune disorders are related with HS, including SLE, Sjögren syndrome, mixed connective tissue disease, progressive systemic sclerosis, rheumatoid arthritis, Crohn disease, vasculitis and sarcoidosis2. According to historical and current findings, HS is now classified as shown in table 1.
HS can complicate both active and inactive SLE. When HS is diagnosed in inactive SLE, the cause can be an infection, usually a viral one, or the immunosuppressive treatment. In 1991, Wong, et al reported patients with active SLE who demonstrated reactive hemophagocytosis in bone marrow6. The occurrence of hemophagocytosis was associated with activity of SLE itself, and they proposed the disease entity of acute lupus hemophagocytic syndrome (ALHS). In 1995 and 1997, Kumakura, et al reported cases of reactive HS, which were associated with autoimmune diseases other than SLE, and proposed a new disease entity, autoimmune associate hemophagocytic syndrome (AAHS)13,14. Diagnostic criteria for AAHS have been proposed by Kumakura, et al (table 2)15. To diagnose secondary HS, it is necessary to detect the underlying disorder causing HS.
In our patient the clinical and biochemical findings met the American College of Rheumatology diagnostic criteria for SLE, and the kidney biopsy established the diagnosis of Lupus Nephritis class III. At the same time she fulfilled diagnostic criteria for AAHS. An extensive study showed no evidence of infection, drugs or malignancy, but she currently had symptoms, signs and serologic findings of active SLE. To the best of our knowledge, our patient had no family history suggestive of familial hemophagocytic lymphohistiocytosis (FHL). Therefore, no causative disorder for HS could be detected other than active SLE, and the diagnosis of ALHS was established.
The proposed pathogenetic mechanisms of HS in SLE, which are not mutually exclusive, include: 1) autoantibodymediated phagocytosis of hematopoietic cells, 2) deposition of immune complexes on the bone marrow hematopoietic cells, and 3) oversecretion of cytokines (ie, IL-1, IL-6, INF-gamma, TNF) by primary uncontrolled T-cell activation2,6. Autoantibodies and/or immune-complexes account for the sensitization of marrow hematopoietic cells to macrophages, which are then engaged into uncontrolled phagocytosis12. Cytokines secreted by T cells involved in the previously mentioned process or acting independently enhance the inappropriate activation of macrophages. Central to the uncontrolled phagocytosis in lupus-related HS is the intrinsic lupus-related macrophage dysfunction per se accentuated by auto-reactive lymphocytes or concurrent infection2,6.
Another interesting point is that our patient presented high titers of ANA and hypocomplementemia, indicating that immune complex-mediated mechanisms might be responsible for the pathogenesis of ALHS. In our patient triglyceride blood levels were markedly elevated, which may be explained by the decrease in lipolytic activity induced by IL-1 and TNF-α cytokines16.
The strategies of treatment for secondary HS include therapy for the underlying disease and that for hypercytokinemia, which plays a major role and causes hemophagocytosis.
When the trigger event is disease flare, pulse steroids and cyclophosphamide are indicated2,6,12.
In one case report tacrolimus was considered beneficial17.
IVIG has also been used extensively as an immunomodulator, both in infection related and in lupus related2,17-20. Because the pathogenetic mechanisms are not elucidated, therapeutic interventions are not clearcut either; immunosuppressants have been used to counteract the activation of macrophages, whereas IVIG has been given in infection-related HS, presumably acting by binding cytokines and inhibiting their synthesis2.
Plasmapheresis has been reported as a therapeutic option in a few cases only17,21,22. However, this procedure alone has not been shown to benefit, probably because of resultant antibody rebound23. Takahashi, et al reported a case of ALHS who was refractory to high-dose corticosteroids, cyclosporine, and IVIG, but was successfully treated with additional administration of TNF-α inhibitor, etanercept10.
In conclusion, we describe a rare case of ALHS that responded well to pulse intravenous steroids and pulse cyclophosphamide. In febrile patients with SLE pancytopenia and unduly high ferritin levels, infections should be readily exclude and a bone marrow aspiration, which is crucial for diagnosis, carried out promptly. If hemophagocytosis is confirmed, early treatment should be initiated.
Figure 1.
Figure 2.
Figure 3.
Table 1. Classification of HS
Table 2. Diagnostic criteria for AAHS proposed by Kumakura