Renin-angiotensin system inhibitors (ACEI/ARB-II), diuretics and NSAIDs, a combination known as “Triple Whammy”, can result in decreased glomerular filtration rate (GFR) and acute kidney injury (AKI).
ObjectivesTo describe the incidence of AKI for each drug type and their combinations. To define the profile of patients admitted for drug-related AKI secondary to Triple Whammy drugs (AKITW), with an assessment of costs and mortality.
MethodsA retrospective observational 15-month study developed in three stages:
–First: a cross-sectional stage to identify and describe hospitalizations due to AKITW.
–Second: a follow-up stage of an outpatient cohort consuming these drugs (15,307 subjects).
–Third: a cohort stage to assess costs and mortality, which compared 62 hospitalized patients with AKITW and 62 without AKI, paired by medical specialty, sex, age and comorbidity according to their Clinical Risk Groups.
ResultsThere were 85 hospitalization episodes due to AKITW, and 78% of patients were over the age of 70. The incidence of AKITW in the population was 3.40 cases/1000 users/year (95% CI: 2.59–4.45). By categories, these were: NSAIDs + diuretics 8.99 (95% CI: 3.16–25.3);
Triple Whammy 8.82 (95% CI: 4.4–17.3); ACEI/ARB-II + diuretics 6.87 (95% CI: 4.81–9.82); and monotherapy with diuretics 3.31 (95% CI: 1.39–7.85). Mean hospital stay was 7.6 days (SD 6.4), and mean avoidable costs were estimated at €214,604/100,000 inhabitants/year. Mortality during hospitalization and at 12 months was 11.3% and 38.7% respectively, and there were no significant differences when compared with the control group.
ConclusionsTreatment with ACEI, ARB-II, diuretics and/or NSAIDs shows a high incidence of hospitalization episodes due to AKI; diuretics as monotherapy or dual and triple combination therapy cause the highest incidence. AKITW involves high health care costs and avoidable mortality.
Inhibidores del sistema renina-angiotensina (IECAS/ARA II), diuréticos y AINES, combinación conocida como “Triple Whammy”, pueden producir descenso de filtrado glomerular y fracaso renal agudo (FRA).
ObjetivosDescribir la incidencia de FRA para cada tipo de fármaco y sus combinaciones. Caracterizar el perfil de paciente que ingresa por FRA extrahospitalario secundario a fármacos de la Triple Whammy (FRAETW), evaluando costes y mortalidad.
Métodosestudio observacional retrospectivo realizado durante 15 meses y desarrollado en tres etapas:
–1° Etapa transversal de identificación y descripción de los ingresos hospitalarios por FRAETW.
–2° Etapa de seguimiento de una cohorte ambulatoria consumidora de estos fármacos (15.307 consumidores)
–3° Etapa de cohortes para evaluar costes y mortalidad, contrastando 62 pacientes ingresados con FRAETW, con 62 pacientes sin FRA, apareados por especialidad médica, sexo, edad y comorbilidad según Clinical Risk Groups.
Resultados85 ingresos por FRAETW, 78% mayores de 70 años. Incidencia poblacional de FRAETW: 3,40 casos/1.000 consumidores/año (IC95% 2,59-4,45). Por categorías: AINES + diuréticos 8,99(IC95% 3,16-25,3), la “Triple Whammy” 8,82(IC 95% 4,4-17,3), IECA/ARA II + diuréticos 6,87(IC95% 4,81-9,82) y la monoterapia con diuréticos 3,31(IC95% 1,39-7,85). Estancia media 7,6 días (DE 6,4), estimándose coste medio evitable de 214.604 €/100.000 habitantes/ año. Mortalidad del 11,3% durante el ingreso y del 38,7% a los 12 meses, sin diferencias significativas con los controles.
ConclusionesEl tratamiento con IECA, ARA II, diuréticos y/o AINES presenta elevada incidencia de ingreso por FRA, siendo los diuréticos en monoterapia, doble y triple terapia combinada los que ocasionan la mayor incidencia. El FRAETW supone elevados costes sanitarios y muertes evitables.
The combination of renin-angiotensin system inhibitor (ACEI or ARB-II) antihypertensive drugs with diuretics, which is often associated with analgesic treatments including non-steroidal anti-inflammatory drugs (NSAIDs) in senior patients, may cause acute kidney injury (AKI). These drug interactions have not been well studied in the literature.1-4
In 1996, the incidence of AKI in Spain was 209 cases/million adults/year5 according to a study by Liaño, et al., conducted in the Madrid Community. The 2002 study by L.M. Lou, et al.6, which was conducted at a Spanish district hospital with characteristics similar to ours, indicated that 79% of hospitalizations due to AKI originated outside the hospital. The cause was volume depletion in 34.2% of cases, a datum that highlighted the elevated incidence of antihypertensive treatments (primarily renin-angiotensin system inhibitors) and/ or diuretics as coadjuvant factors for AKI.
However, the study of drug-related acute kidney injury has received little attention in the literature; meanwhile, its implications in terms of increased mortality, days of hospitalization and costs are unknown. In 2000, MC Thomas7 coined the term “Triple Whammy” (TW) to refer to the adverse effects resulting from the combination of ACEI or ARB-II, diuretics and NSAIDs, particularly in seniors. Chertow, et al.8, observed that small creatinine increments of 0.3 or 0.4 mg/dL were associated with increased mortality, mean hospitalization days, and higher costs.
The objectives of our study were to describe the population prevalence of consumption and the incidence of outpatient AKI secondary to Triple Whammy drugs (AKITW): ACEI, ARB-II, diuretics and NSAIDs for each type of drug and their combinations, characterizing the profile of the admitted patients and analysing their mean hospital stay, hospital costs and mortality.
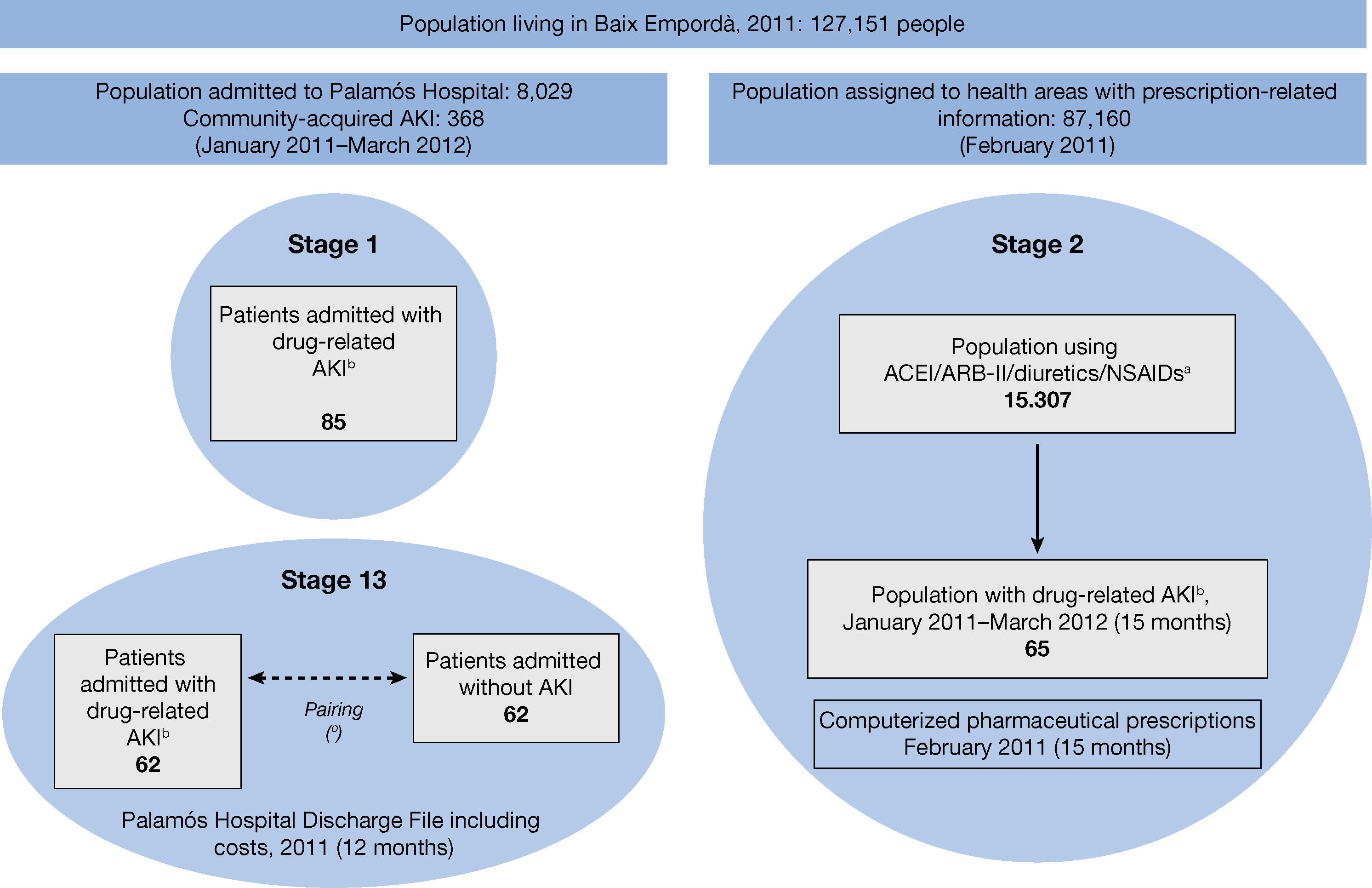
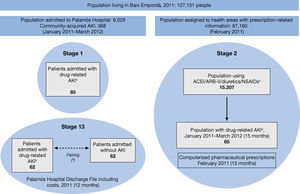
MethodsA retrospective observational study was conducted in three stages, with a mixed design, at Palamós Hospital (Baix Empordà district hospital), which is a reference hospital for 127,151 inhabitants with 130 beds (Figure 1).
– Study schedule. (a) Drugs according to ATC classification (NSAIDs not including ASA 100-300 mg, paracetamol or metamizole). (b) AKI associated with the use of ACEI/ARB-II/Diuretics/NSAIDs. (c) Randomized sample of controls paired by specialty, sex, age and comorbidity according to the Clinical Risk Groups (CRG). AKI: Acute kidney injury; ACEI: Angiotensin converting enzyme inhibitors; ARB II: Angiotensin-II receptor blockers; NSAID: Non-steroidal anti-inflammatory drugs.
The study project complied with the principles of the World Medical Association Declaration of Helsinki and was approved by the Serveis de Salut Integrats Baix Empordà (SSIBE) [Baix Empordà Comprehensive Health Service] Clinical Research Committee. Given the study methodology, which was based on a retrospective review of clinical-administrative records, informed consent was not requested. Data collection was performed by an authorized SSIBE professional and data were managed anonymously.
Design and patientsFirst stage of the study: A cross-sectional analysis was made of the 8,029 hospital admissions in the study period (January 2011 to March 2012) in order to identify drug-related AKI cases secondary to ACEI/ARB-II, diuretics and/or NSAIDs, and to describe the clinical characteristics of the admitted patients and their previous medications. Drug-related acute kidney injury secondary to the use of Triple Whammy drugs (AKITW) was defined as hospital admission with a discharge code of AKI (ICD-9-CM 584.9) in a patient who had been receiving any of those drugs prior to admission and where, following an audit of the medical record by a nephrologist, the possible cause of AKI is attributed to the use of ACEI/ARB-II, diuretics and/or NSAIDs, either alone or in combination (a prerenal situation may be involved based on the presence of fever, vomiting or diarrhea). In addition, the patient's baseline creatinine value was 1.3 mg/dL or lower at least one month before admission, which increased to more than 1.4 mg/dL on admission.
Excluded from the study were cases of hospital-acquired AKI (defined as patients who are admitted with a creatinine value below 1.3 mg/dL that increases to more than 1.4 mg/dL during hospitalization), obstructive AKI, glomerulonephritis, hepatorenal syndrome, decompensated heart failure, multiple organ failure, infection, ischemia, poisoning, end-stage cancer, exposure to radiocontrast agents or known nephrotoxic drugs, and multifactorial cases
Second stage of the study: Four basic health care areas (BHA) share an information system of computerized medical files with our hospital. As the prescription records of the 87,160 patients belonging to the BHA were available to us, we followed up a cohort of the 15,307 outpatients who had been prescribed one or more ACEI/ARB-II, diuretics or NSAIDs in February 2011. Our goal was to analyze the prevalence of use and incidence of drug-related AKI of this population for each type of drug and their combinations. The data from this cohort were cross-referenced with the data from hospital admissions due to AKITW that had been identified in the first stage. Out of the 85 cases of AKITW detected in stage 1 (15 months of follow-up), we selected the 65 cases belonging to the population of the four BHA with available computerized medical records and drug prescription data.
Third stage of the study: A cohort study was used to assess the impact of AKITW on hospital stays, costs and mortality. The study population was limited to hospitalization episodes in 2011 (12 months) of residents of the four BHA for whom prescription data pharmacy costs were available. All the hospitalization episodes due to AKITW belonging to the 4 BHA were considered cases (62 patients). The control group was made up of 62 randomly selected hospital admissions of patients without AKI, paired by medical specialty, sex, age and comorbidities according to Clinical Risk Group (CRG).9,10
Variables and data sourcesData for the selection and analysis of the hospitalizations were obtained from the Palamós Hospital computerized hospital admission files. The medical records were reviewed thoroughly by a nephrologist, who compiled detailed information about patient characteristics and progression. The variables collected on hospitalization episodes for AKITW were: age, sex, use of ACEI/ARB-II, diuretics and NSAIDs before admission except for ASA 100-300 mg (drug, mean daily dose and treatment duration in weeks), history of hypertension (HTN) (defined by antihypertensive medication use), chronic kidney disease (CKD) prior to admission (defined as any patient admitted under code ICD-9: 585), chronic heart failure (CHF) (defined as any patient admitted under code ICD-9: 428), prior rheumatic disease (previous diagnosis of rheumatic disease with follow-up by a rheumatologist), associated prerenal cause (depletion due to fever, vomiting or diarrhea), signs of dehydration (admitting physician recorded dehydration on the ER report, or patient was admitted with hypotension and the examination revealed dry skin or mucosae), edema, pain and cause of pain, need for dialysis, CKD as a post-dialysis sequela (considered as patients with ICD-9 code: 585 who did not have CKD before admission), creatinine (baseline, peak on admission, on discharge and at six months following admission), albuminemia on admission, K on admission, hospital stay (days), death (date). Renal function was measured with creatinine analyzed by Jaffe's method, alkaline picrate and kinetic reading; traceability was guaranteed with the isotopic dilution mass spectrometry (IDMS). Glomerular filtration (GF) was calculated with MDRD4 IDMS.
Exacerbated CKD was considered as a patient previously diagnosed with CKD who had a creatinine level of more than 0.3 mg/dL over their baseline creatinine value, which had been measured at least one month before admission.
The pharmacy data of the population were obtained from the computerized prescription files of the primary health care services. Drugs of interest were classified by groups based on ATC codes (World Health Organization Anatomical, Therapeutic, Chemical classification system): ACEI, ARB-II, diuretics and NSAIDs (not including acetylsalicylic acid 100-300 mg, paracetamol or metamizole). The ACEI and ARB-II groups were considered a single drug type because of their similar mechanism of action. The use of one, two or three diuretics and NSAIDs were considered separately. Drugs prescribed during hospitalization were not considered.
Data for hospitalization costs, which are calculated yearly with the full-costing system, were obtained from the Serveis de Salut Integrats (SSIBE) costs file. This system follows the methodology and recommendations from the analytical accounting workgroup of the Servei Català de la Salut [Catalan Health Service] Central Financial Office11,12 and the Red Española de Costes Hospitalarios (RECH) [Spanish Hospital Costs Network]13,14. The cost associated with each hospitalization episode was calculated individually using the clinical and activity information recorded from admission to discharge. Thus, the cost of each hospitalization episode was obtained based on the number of hospitalization days, minutes in the operating room, dialysis sessions, single medication doses, laboratory tests, imaging tests and other tests performed from admission to discharge. In the 2011 fiscal year, the mean hospital stay cost at Palamós Hospital was €279.63, one minute in the operating room was €10.39 and each dialysis session was €146.45. The cost of single medication doses was assigned directly to the episode at the weighted mean price. Finally, the costs for laboratory tests, diagnostic imaging tests and the remaining tests was input according to the mean cost of each technique performed based on each department's catalogue of services.
Statistical analysisIn order to identify the hospitalizations due to AKITW in association with these drugs, we conducted a univariate statistical analysis with frequency, mean and standard deviation, according to the characteristics of each variable.
Drug combinations were analyzed as exclusive categories. To measure the impact of drug use, the population incidence was measured for each category with its 95% confidence interval.
To compare groups in the case-control analysis, a bivariate statistical analysis was conducted with Student's t-test or chisquare test, depending on the variable's characteristics. Fisher's exact test was used when applicable. P < 0.05 was considered statistically significant. The IBM SPSS Statistics 20.0 program was used.
Results1. First stage of the study: Identification and characterization of drug-related AKIOut of the 8,029 hospital admissions during the study period, 415 cases of AKI were identified, of which 368 were drug-related AKI and 85 were attributed directly to the use of ACEI/ ARB-II, diuretics and/or NSAIDs (AKITW) (Figure 1).
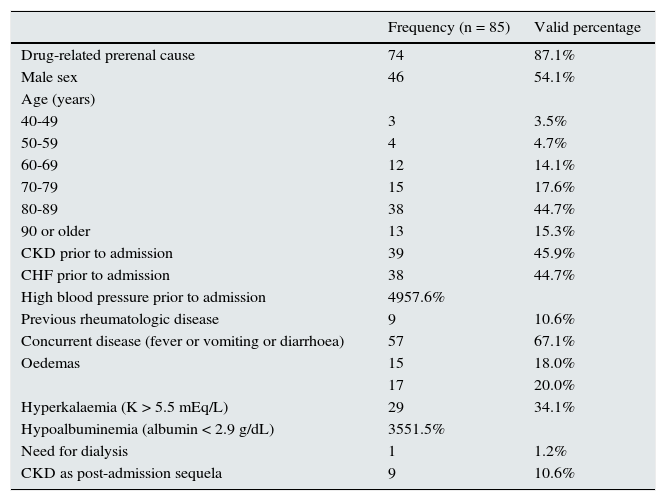
These 85 cases had a similar distribution between both sexes and mean age was 78.72 years (SD 11.69). When analyzed by age groups, the case distribution clearly shifted to the older age ranges (Table 1).
– Clinical characteristics of hospitalization episodes due to acute kidney injury associated with ACEI, ARB-II, diuretics and/or NSAIDs (Hospital de Palamós, January 2011–March 2012).
| Frequency (n = 85) | Valid percentage | |
|---|---|---|
| Drug-related prerenal cause | 74 | 87.1% |
| Male sex | 46 | 54.1% |
| Age (years) | ||
| 40-49 | 3 | 3.5% |
| 50-59 | 4 | 4.7% |
| 60-69 | 12 | 14.1% |
| 70-79 | 15 | 17.6% |
| 80-89 | 38 | 44.7% |
| 90 or older | 13 | 15.3% |
| CKD prior to admission | 39 | 45.9% |
| CHF prior to admission | 38 | 44.7% |
| High blood pressure prior to admission | 4957.6% | |
| Previous rheumatologic disease | 9 | 10.6% |
| Concurrent disease (fever or vomiting or diarrhoea) | 57 | 67.1% |
| Oedemas | 15 | 18.0% |
| 17 | 20.0% | |
| Hyperkalaemia (K > 5.5 mEq/L) | 29 | 34.1% |
| Hypoalbuminemia (albumin < 2.9 g/dL) | 3551.5% | |
| Need for dialysis | 1 | 1.2% |
| CKD as post-admission sequela | 9 | 10.6% |
Practically half of the cases had a history of CKD or CHF prior to admission. In two-thirds of cases, there was a prerenal cause associated with ACEI/ARB-II, diuretics and/or NSAIDs (history of fever, vomiting or diarrhea in the days before admission). In four out of five cases, the medical records did not specify a cause of pain justifying the chronic use of NSAIDs; in 59% of cases where the cause was specified, it was chronic joint pain.
The mean baseline creatinine value prior to admission was 1.32 mg/dL (SD 0.50) and the mean creatinine increase from baseline to admission was 1.77 mg/dL (SD 1.51). In 34% of patients, peak creatinine doubled baseline creatinine levels. Six months after discharge, mean creatinine had an increase of 13.89% from the pre-admission baseline creatinine. The mean baseline GFR was 57.3 mL/min/1.73m2, and at six months it was 52.77 mL/min/1.73 m2, which was a decrease of 4.6%.
More than half the patients had hypoalbuminemia, which was serious (< 2.5 g/dL) in 19% of cases. One-third were admitted with hyperkalemia, and three cases were serious enough to cause death after admission. One patient required dialysis and more than 10% of patients had CKD as a sequela for six months after admission.
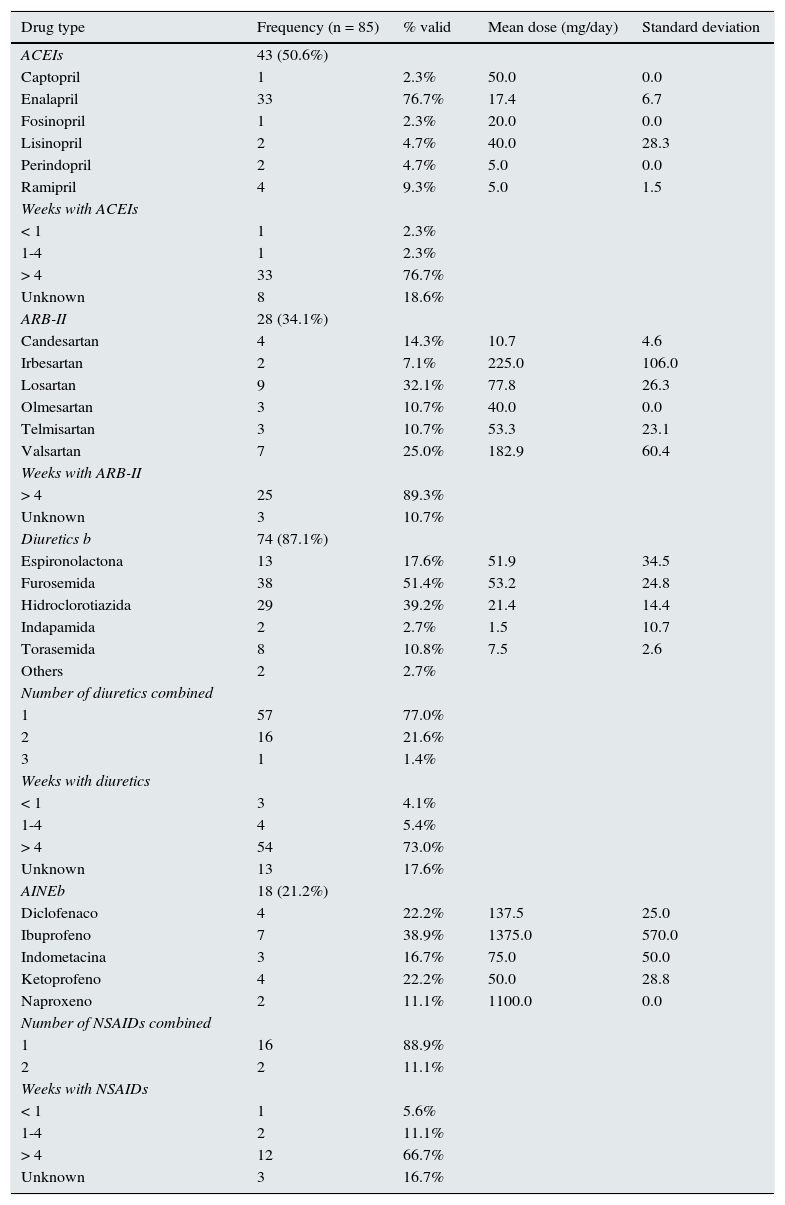
With respect to the drugs the patients were taking before admission, ACEI prescriptions were identified in half of cases; three-quarters of these patients had been taking them for more than four weeks. The most widely used ACEI was enalapril, which was consumed by three out of four ACEI users. One-third of patients were ARB-II users, most of whom had been on treatment for more than four weeks. The most widely used form was losartan, which was being taken by a third of ARB-II users. Diuretics were prescribed in almost nine out of ten cases to treat the following: HTN in 55.4% of cases, CHF in 33.8%, and edema in 6%. Almost three-quarters of patients had been on treatment for more than four weeks and almost one-quarter had been prescribed more than one type of diuretic. The most widely used diuretic was furosemide, followed by hydrochlorothiazide. NSAIDs were prescribed in just over one-fifth of cases; two-thirds of them had been on treatment for more than four weeks. The most widely used NSAID was ibuprofen (Table 2).
– Drugs associated with drug-related acute kidney injury: types, dose and time of exposure (Palamós Hospital, January 2011–March 2012).
| Drug type | Frequency (n = 85) | % valid | Mean dose (mg/day) | Standard deviation |
|---|---|---|---|---|
| ACEIs | 43 (50.6%) | |||
| Captopril | 1 | 2.3% | 50.0 | 0.0 |
| Enalapril | 33 | 76.7% | 17.4 | 6.7 |
| Fosinopril | 1 | 2.3% | 20.0 | 0.0 |
| Lisinopril | 2 | 4.7% | 40.0 | 28.3 |
| Perindopril | 2 | 4.7% | 5.0 | 0.0 |
| Ramipril | 4 | 9.3% | 5.0 | 1.5 |
| Weeks with ACEIs | ||||
| < 1 | 1 | 2.3% | ||
| 1-4 | 1 | 2.3% | ||
| > 4 | 33 | 76.7% | ||
| Unknown | 8 | 18.6% | ||
| ARB-II | 28 (34.1%) | |||
| Candesartan | 4 | 14.3% | 10.7 | 4.6 |
| Irbesartan | 2 | 7.1% | 225.0 | 106.0 |
| Losartan | 9 | 32.1% | 77.8 | 26.3 |
| Olmesartan | 3 | 10.7% | 40.0 | 0.0 |
| Telmisartan | 3 | 10.7% | 53.3 | 23.1 |
| Valsartan | 7 | 25.0% | 182.9 | 60.4 |
| Weeks with ARB-II | ||||
| > 4 | 25 | 89.3% | ||
| Unknown | 3 | 10.7% | ||
| Diuretics b | 74 (87.1%) | |||
| Espironolactona | 13 | 17.6% | 51.9 | 34.5 |
| Furosemida | 38 | 51.4% | 53.2 | 24.8 |
| Hidroclorotiazida | 29 | 39.2% | 21.4 | 14.4 |
| Indapamida | 2 | 2.7% | 1.5 | 10.7 |
| Torasemida | 8 | 10.8% | 7.5 | 2.6 |
| Others | 2 | 2.7% | ||
| Number of diuretics combined | ||||
| 1 | 57 | 77.0% | ||
| 2 | 16 | 21.6% | ||
| 3 | 1 | 1.4% | ||
| Weeks with diuretics | ||||
| < 1 | 3 | 4.1% | ||
| 1-4 | 4 | 5.4% | ||
| > 4 | 54 | 73.0% | ||
| Unknown | 13 | 17.6% | ||
| AINEb | 18 (21.2%) | |||
| Diclofenaco | 4 | 22.2% | 137.5 | 25.0 |
| Ibuprofeno | 7 | 38.9% | 1375.0 | 570.0 |
| Indometacina | 3 | 16.7% | 75.0 | 50.0 |
| Ketoprofeno | 4 | 22.2% | 50.0 | 28.8 |
| Naproxeno | 2 | 11.1% | 1100.0 | 0.0 |
| Number of NSAIDs combined | ||||
| 1 | 16 | 88.9% | ||
| 2 | 2 | 11.1% | ||
| Weeks with NSAIDs | ||||
| < 1 | 1 | 5.6% | ||
| 1-4 | 2 | 11.1% | ||
| > 4 | 12 | 66.7% | ||
| Unknown | 3 | 16.7% |
Out of patients the admitted, 88.2% were on dual or triple therapy: 54% dual therapy, 39% combined ACEI/ARB-II + diuretics, and 34% Triple Whammy.
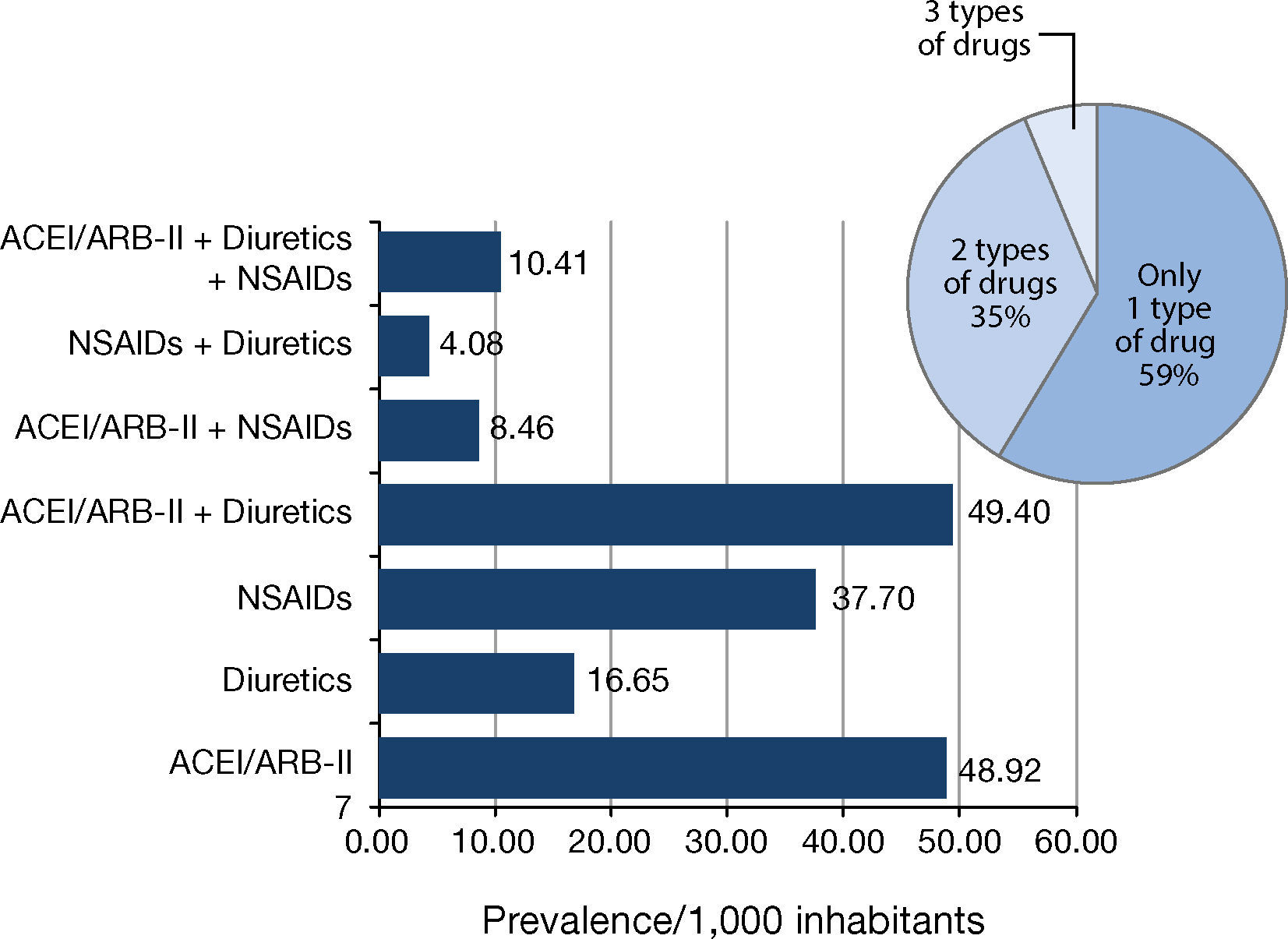
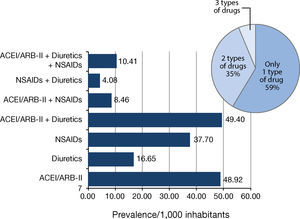
2. Second stage of the study: Use of ACEI/ARB-II, diuretics and NSAIDs in the population and associated AKIFrom a population of 87,160 people, a total of 15,307 were on the target drugs, which represented 175.62 users/1,000 inhabitants (Figure 1).
The population prevalence of use for each type of drug (alone and in various possible combinations among them) is shown in Figure 2. The most common type of use in the study population was monotherapy, which accounted for more than half of these users. The most common monotherapy was ACEI/ARB-II, and the least used were diuretics. More than one-third of these users were on dual therapy, the most frequent being ACEI/ARB-II + diuretics. Just over 1 person per 100 inhabitants had been prescribed the Triple Whammy.
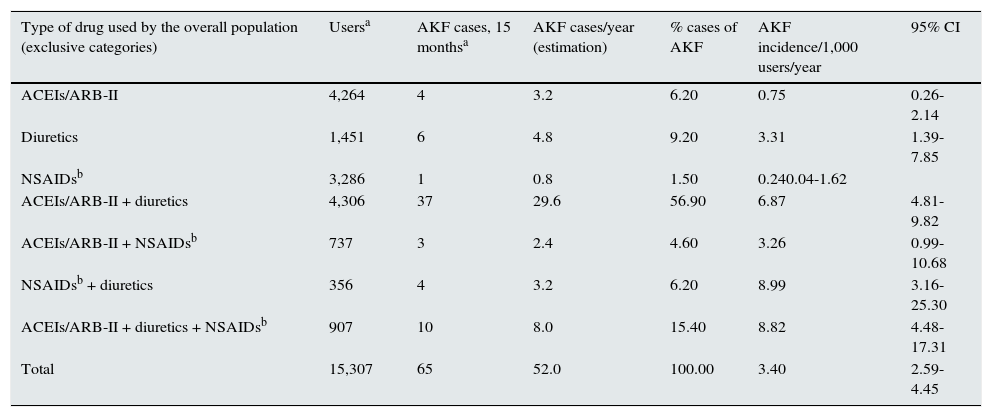
Table 3 shows the incidence of AKI associated with ACEI/ ARB-II, diuretics and NSAIDs in the population consuming these drugs. Overall incidence was 3.40 cases/year/1,000 users, which generally increased with the number of drugs combined. In monotherapy, the diuretics group had a relevant incidence, which increased when they were combined with ACEI/ARB-II or NSAIDs in a dual therapy. In contrast, the incidence of AKI associated with ACEI/ARB-II and NSAIDs, alone or combined among them, was lower. The incidence of associated AKI in Triple Whammy users was similar to that of users of dual therapies that included diuretics.
– Use of ACEI/ARB-II, diuretics and NSAIDs in the overall population and associated acute kidney injury (Baix Empordà, January 2011–March 2012).
| Type of drug used by the overall population (exclusive categories) | Usersa | AKF cases, 15 monthsa | AKF cases/year (estimation) | % cases of AKF | AKF incidence/1,000 users/year | 95% CI |
|---|---|---|---|---|---|---|
| ACEIs/ARB-II | 4,264 | 4 | 3.2 | 6.20 | 0.75 | 0.26-2.14 |
| Diuretics | 1,451 | 6 | 4.8 | 9.20 | 3.31 | 1.39-7.85 |
| NSAIDsb | 3,286 | 1 | 0.8 | 1.50 | 0.240.04-1.62 | |
| ACEIs/ARB-II + diuretics | 4,306 | 37 | 29.6 | 56.90 | 6.87 | 4.81-9.82 |
| ACEIs/ARB-II + NSAIDsb | 737 | 3 | 2.4 | 4.60 | 3.26 | 0.99-10.68 |
| NSAIDsb + diuretics | 356 | 4 | 3.2 | 6.20 | 8.99 | 3.16-25.30 |
| ACEIs/ARB-II + diuretics + NSAIDsb | 907 | 10 | 8.0 | 15.40 | 8.82 | 4.48-17.31 |
| Total | 15,307 | 65 | 52.0 | 100.00 | 3.40 | 2.59-4.45 |
In the comparison of the AKITW cases with paired non-AKI controls, no statistically significant differences were found for mean hospital stay between the two groups: 7.6 days (SD 6.4) for cases and 5.8 (SD 4.5) for controls (p = 0.07). The total number of hospital stays generated in one year due to AKI secondary to the target drugs was 473 days for 87,160 inhabitants. This represents an estimated 543 days of avoidable hospital stay/100,000 inhabitants/year.
Regarding costs, the mean cost was €3,016 (SD 2,468) for cases and €2,663 (SD 2,876) for controls, with no significant differences between them (p = 0.43). When we consider that the total one-year cost was €187,049 for 87,160 inhabitants, we can estimate an annual avoidable cost of €214,604/100,000 inhabitants/year.
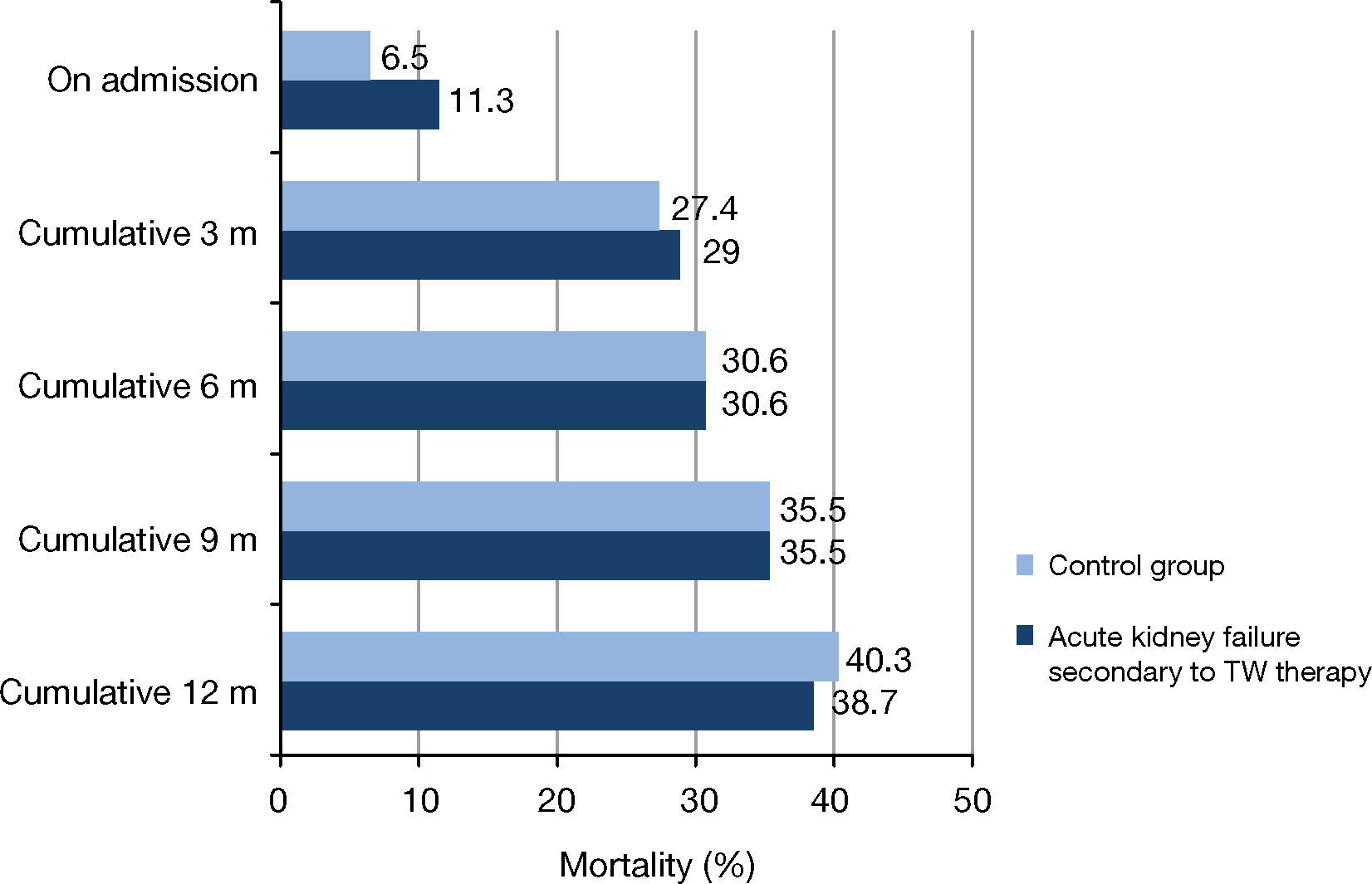
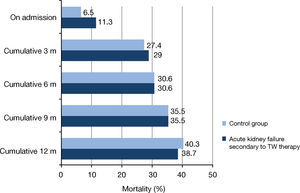
Mortality was 11.3% for cases and 6.5% for controls during hospitalization and 40.3% and 38.7%, respectively, during the 12-month follow-up. No statistically significant differences were found between the groups at those time points or during the follow-up at three, six and nine months (Figure 3).
– Mortality for AKI secondary to ACEI/ARB-II, diuretics and NSAIDs compared to admission of patients with no AKI (Palamós Hospital, 2011). (a) Control group: Hospitalizations of patients with no acute kidney injury paired by sex, age and comorbidity according to Clinical Risk Group (CRG) categories. All comparisons were non-significant (p > 0.05 based on Fischer's exact test). TW: Triple Whammy.
Our results suggest that the consumers of these drugs have an elevated incidence of AKI. Greater incidence was observed for diuretics, as monotherapy or in dual or triple therapy; the highest incidence was for the dual therapy of diuretics + NSAIDs. Triple Whammy (ACEI/ARB-II + diuretics + NSAIDs) users had an AKI incidence similar to those taking dual therapies that included diuretics. The main reason for the prescription of diuretics in our patients was high blood pressure. Out of all the patients admitted due to AKITW, 87% used diuretics, 84.6% used ACEI/ARB-II and 38.5% used NSAIDs. In the population who had been prescribed one of these drugs, 7.2% were on dual or triple therapy.
Similarly to Loboz et al.2, we observed that 54% of our admitted patients were on dual therapy, of which almost 40% were prescribed the ACEI/ARB-II + diuretics combination. Loboz et al.2 noticed that an increase in the dose of diuretics was related to a decrease in GFR and, for the remaining drugs, the risk was present even at low doses. Likewise, in our case, patients were consuming therapeutic or even low doses of diuretics and other drugs. This suggests that administering small doses of these drugs in combination fails to reduce the risk of AKI. Lou LM et al.6 and Huerta et al.15 also observed an increase in AKI incidence with the combination of ACEI and diuretics.
The association of ACEI/ARB-II + NSAIDs presented an elevated incidence, although with a very broad confidence interval, probably due to the limited number of cases.
Huerta et al.15 did not find an increased risk for the combination of NSAIDs with renin-angiotensin system inhibitors.
Our data differ from the data found by Lapi et al.16, who found no risk for AKI when using dual therapy with diuretics or ACEI/ARB-II + NSAIDs. They only found a risk for AKI with the Triple Whammy, which was particularly noticeable in the first 30 days of treatment. In our study, we have seen that the hospitalized patients had been receiving treatment for more than four weeks, regardless of the drug group, and the triggering factor for AKI had been a concurrent disease causing hypovolemia.
The profile of a patient at risk of suffering AKI with these drugs is an elderly patient with multiple conditions and on multiple concomitant medicantions. This risk exists even with drugs at low therapeutic doses. The presence of hypoalbuminemia < 3 g/dL is alarming in 51% of hospitalization episodes due to AKITW, of which 19% were severe cases (< 2.5 g/ dL). This fact reflects the degree of malnutrition in a large part of the elderly population despite living at home. In 67% of cases, there was a triggering factor for AKI, such as fever, vomiting or diarrhea, in previous days. What probably makes the glomerular filtration drop abruptly is a concurrent disease causing volume depletion or, simply, an increase in baseline metabolism in a patient with intraglomerular pressure decreased by vasodilated efferent arterioles secondary to treatment with ACEI or ARB-II, combined with a circulating volume reduced by diuretics. Elderly patients are known to maintain their GF thanks to a complex system of intraglomerular resistance. Due to the vessel rigidity present in these patients, the kidneys find it hard to adapt to changes in intraglomerular pressure and, therefore, are very sensitive to volume changes. This seems to suggest that the cause of the AKI was not the treatment with these drugs, but the onset of a concurrent disease. Of the admitted patients, 74% were on chronic diuretic treatment (more than four weeks) and 21.6% were on two diuretics combined.
These were patients with decreased baseline GF, in many cases with a history of CKD or CHF, who were treated with diuretics, primarily for hypertension. They generally had mild or moderate AKI, with small creatinine increases that were resolved with hydration in most cases. Nevertheless, 10% of patients had CKD as a sequela and three patients died of hyperkalemia during admission. In four of five cases, the patients’ medical records did not specify a cause of pain justifying the chronic use of NSAIDs, and in 59% of cases where the cause was specified, it was chronic joint pain. These data reflect that NSAID therapies are started at times of acute pain and are not properly withdrawn afterwards.
The 2012 KDIGO guidelines17 already recommended starting treatment with ACEI and ARB-II at low doses in patients with GFR < 45 mL/min and discontinue it temporarily in cases with concurrent disease. As for NSAIDs, the recommendation is to avoid their use in patients treated with renin-angiotensin system inhibitors, and prolonged therapies are not recommended if GFR is < 60 mL/min.
The STOPP/START18 criteria determine that prescribing diuretics for isolated ankle edema is inappropriate in patients older than 65 years because there is no evidence of their efficacy. They are also not recommended as first-line monotherapy in hypertension. NSAIDs should be avoided in moderate to severe hypertension and CHF, and their use for more than three months to relieve mild joint pain should be avoided. In cases of CKD, they are not recommended either.
Even though NSAIDs are not formally contraindicated in the elderly, they are a high-risk population for adverse effects, particularly AKI, and their use should be the result of a thorough risk-benefit assessment.
Only 58% of physicians noticed evident signs of dehydration on admission. Therefore, it is advisable that, in case of a concurrent condition, treatment with at least one of the target drugs be discontinued. This should be applied primarily to diuretics, even if the physician does not notice signs of dehydration. When initiating these combinations, renal function and creatinine values are to be monitored.19
The Fournier, et al.20 group from Toulouse has recently performed a new analysis of the review they conducted on their pharmacovigilance database of adverse pharmacological effects in 2012. Their aim was to study whether NSAID half-life had an impact on the possibility of developing AKI. An association was found, not with the drugs’ half-life, but with the number of associated drugs, which coincided with the findings in previous publications and with our results. In our case, due to the small size of our sample, it was impossible to analyze incidence differences based on the drugs’ half-lives.
A cost of €214,604/100,000 inhabitants/year for hospitalization episodes derived from a combined prescription of these drugs is a significant economic problem and, evidently, the three deaths that occurred during admission due to hyperkalemia could have been avoided with correct prescription.
Our study is not limited to the utilization of large databases. It is focused on drug-related AKI in conjunction with the auditing of medical records by a nephrologist to ensure that the AKITW included in this study are only due to the treatment with these drugs, either alone or combined, without the contribution of any other factor except for the prerenal situations discussed above. This avoids confusion due to the high mortality associated with AKI for other causes. Most of the studies conducted so far are based on the utilization of large databases focused on overall AKI treatment. We analyzed the clinical patient characteristics to create a profile of at-risk patients, and we quantified the personal (mortality) and economic costs of AKI caused by incorrect drug combinations.
The limitations of our study are the small number of cases and not having included treatment with ASA at low doses (100 and 300 mg) as an NSAID but instead as antiplatelet drug, as indicated in the pharmaceutical coding, since a large part of the patients admitted (19) were receiving prophylactic treatment at these doses. Paracetamol at doses greater than 4 g/d could also have an NSAID effect. Both Paracetamol and the remaining NSAIDs are over-the-counter drugs in our pharmacies (our data only include NSAIDs prescribed by their physicians), so the use of NSAIDs could be underestimated. We have also not analyzed the impact of impaired renal function cases that do not require admission. Therefore, the true incidence of AKITW may be higher and may also be generating elevated expenditures in the outpatient setting.
ConclusionDiuretics in monotherapy, dual and triple combination therapy (Triple Whammy) have an elevated incidence of AKI. Dual therapies including diuretics show the same incidence of AKI as the Triple Whammy. The profile of the at-risk patient is an elderly patient with an underlying renal or cardiac disease. The problem does not seem to be the drug itself but the onset of a concurrent disease causing hypovolemia. Monitoring renal function and potassium levels is recommended when these therapies are initiated and in situations of concurrent disease, following the recommendations from scientific societies (KDIGO and STOPP-START criteria).
In cases of concurrent disease, the temporary discontinuation of the Triple Whammy drugs, particularly diuretics, is recommended. The use of NSAIDs should be minimized and treatment with diuretics should be monitored following hospital discharge. Patient treatment should be adjusted to the situation at hand, with special attention given to people over the age of 80 who have concurrent conditions. AKI derived from a combination of Triple Whammy drugs has demonstrated high health care-related expenditures and avoidable deaths. Health care authorities should be aware of this serious issue and limit the over-the-counter availability of NSAIDs at pharmacies.
Conflicts of interestThere are no conflicts of interest; this study was conducted for non-profit purposes.
FundingNo funding has been received.
The authors would like to thank the Sociedad Española de Nefrología (SEN) [Spanish Society of Nephrology] for the grant to help publish this paper.
Likewise, we would like to thank Lluis Palé Mena and Maritza López Pereira for their cooperation with the fieldwork required to carry out this study.
We also thank the assistance provided by the Serveis de Salut Integrats Baix Empordà research fund for the publication of this paper.