The incidence of acute kidney injury (AKI) in coronavirus disease 2019 (COVID-19) patients ranges from 0.5% to 35% and has been associated with worse prognosis. The purpose of this study was to evaluate the incidence, severity, duration, risk factors and prognosis of AKI in hospitalized patients with COVID-19.
MethodsWe conducted a retrospective single-center analysis of 192 hospitalized COVID-19 patients from March to May of 2020. AKI was diagnosed using the Kidney Disease Improving Global Outcome (KDIGO) classification based on serum creatinine (SCr) criteria. Persistent and transient AKI were defined according to the Acute Disease Quality Initiative (ADQI) workgroup definitions.
ResultsIn this cohort of COVID-19 patients, 55.2% developed AKI (n=106). The majority of AKI patients had persistent AKI (n=64, 60.4%). Overall, in-hospital mortality was 18.2% (n=35) and was higher in AKI patients (28.3% vs. 5.9%, p<0.001, unadjusted OR 6.03 (2.22–16.37), p<0.001). In this multivariate analysis, older age (adjusted OR 1.07 (95% CI 1.02–1.11), p=0.004), lower Hb level (adjusted OR 0.78 (95% CI 0.60–0.98), p=0.035), duration of AKI (adjusted OR 7.34 for persistent AKI (95% CI 2.37–22.72), p=0.001) and severity of AKI (adjusted OR 2.65 per increase in KDIGO stage (95% CI 1.32–5.33), p=0.006) were independent predictors of mortality.
ConclusionAKI was frequent in hospitalized patients with COVID-19. Persistent AKI and higher severity of AKI were independent predictors of in-hospital mortality.
La incidencia de lesión renal aguda (LRA) en pacientes con enfermedad por coronavirus 2019 (COVID-19) oscila entre el 0,5 y el 35% y se ha asociado a peor pronóstico. El propósito de este estudio fue evaluar la incidencia, gravedad, duración, factores de riesgo y pronóstico de la LRA en pacientes hospitalizados con COVID-19.
MétodosRealizamos un análisis retrospectivo de un solo centro de 192 pacientes con COVID-19 hospitalizados de marzo a mayo de 2020. La LRA se diagnosticó utilizando la clasificación Kidney Disease Improving Global Outcome (KDIGO) basada en criterios de creatinina sérica (SCr). La LRA persistente y la transitoria se definieron de acuerdo con las definiciones del grupo de trabajo de la Iniciativa de Calidad de Enfermedades Agudas (ADQI).
ResultadosEn esta cohorte de pacientes con COVID-19, el 55,2% desarrolló LRA (n=106). La mayoría de los pacientes tenían LRA persistente (n=64; 60,4%). En general, la mortalidad hospitalaria fue del 18,2% (n=35) y fue mayor en los pacientes con LRA (28,3% frente a 5,9%; p<0,001), (OR no ajustada 6,03; IC 95%: 2,22-16,37; p<0,001). En este análisis multivariado, mayor edad (OR ajustada 1,07; IC 95%: 1,02-1,11; p=0,004), menor nivel de Hb (OR ajustada 0,78; IC 95%: 0,60-0,98; p=0,035), duración de la LRA (OR ajustada 7,34 para LRA persistente; IC 95%: 2,37-22,72; p=0,001) y la gravedad de LRA (OR ajustada 2,65 por aumento en el estadio KDIGO; IC 95%: 1,32-5,33; p=0,006) fueron predictores independientes de mortalidad.
ConclusiónLa LRA fue frecuente en pacientes hospitalizados con COVID-19. La LRA persistente y su mayor gravedad fueron predictores independientes de mortalidad hospitalaria.
Since late 2019, the coronavirus disease 2019 (COVID-19) outbreak has resulted in over 130 million cases worldwide as of April 2021.1,2 The World Health Organization (WHO) classified COVID-19 as a pandemic which has been associated with significant morbidity and caused over 2.8 million deaths.3
The majority of patients present with mild symptoms including fever, dyspnea, cough, headache and diarrhea or are even asymptomatic.4,5 More severe cases of pneumonia can lead to acute respiratory distress syndrome (ARDS), septic shock, multiple organ failure and death.6,5
Current literature reports that the incidence of acute kidney injury (AKI) in COVID-19 patients ranges widely from 0.5% to 45% and has been associated with worse prognosis. The disparities in incidence reports may result from different definitions to classify AKI, different populations studied, different admission criteria and different resources in countries studied.5,7–13
AKI is characterized by a rapid decrease in renal function defined as an increase in serum creatinine (SCr) and/or a decline in urine output (UO).14 AKI is a common in hospitalized patients, with an incidence which can reach 60% in critically ill patients and is associated with increased in-hospital mortality.15 AKI is a frequent complication in ARDS patients, namely in older patients and patients with significant comorbidities.16
In COVID-19 patients, kidney impairment appears to be multifactorial resulting from systemic inflammatory response to volume loss, sepsis, local disruption in renin angiotensin aldosterone system (RAAS) homeostasis, rhabdomyolysis, and it is also suggested that the virus might have direct cytopathic effects.13,17,18
The present study retrospectively analyzed data to study the incidence, severity, duration, risk factors and prognosis of AKI in hospitalized patients with COVID-19.
Materials and methodsThis study is a retrospective analysis of hospitalized patients admitted to a Dedicated Unit for COVID-19 patients (UICIVE) at the Department of Medicine of the Centro Hospitalar Universitário Lisboa Norte (CHULN), in Lisbon, Portugal, between March 2020 and May 2020. The Ethical Committee approved of this study, in agreement with institutional guidelines and informed consent was waived, given its retrospective and non-interventional nature.
PatientsWe selected as eligible all adult patients (≥18 years of age) who tested positive by polymerase chain reaction (PCR) testing of a nasopharyngeal sample for COVID-19 and were admitted at the UICIVE from March 1st to May 31st of 2020. For patients who had multiple qualifying hospital admissions, we included only the first hospitalization. Exclusion criteria comprised (a) chronic kidney disease (CKD) patients on renal replacement therapy, (b) patients who underwent renal replacement therapy one week prior to admission, (c) patients who had less than 2 determinations of SCr and (d) patients who were discharged or died less than two days after admission.
VariablesData was collected from individual electronic clinical records. The following variables were analyzed: patient demographic characteristics (age, gender, ethnicity, body weight and height); comorbidities [diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), cirrhosis, CKD and/or active malignancy]; current treatment with RAAS inhibitors; disease severity according to the Sequential Organ Failure Assessment (SOFA)19 score and Brescia-COVID Respiratory Severity Scale (BCRSS) at admission20; laboratory values at admission [serum hemoglobin, hematocrit, neutrophil, lymphocyte count and platelet count, serum albumin, serum ferritin, SCr, procalcitonin (PCT) and C-reactive protein (CRP), arterial blood gas and pH analysis, lactic acid dehydrogenase (LDH)]; exposure to nephrotoxins during the first week of admission [non-steroidal anti-inflammatory drugs (NSAIDS), radiocontrast, vancomycin, aminoglycosides]; need for intensive care unit (ICU) admission; need for mechanical ventilation; vasopressor use; treatment options used for COVID-19 (hydroxychloroquine, lopinavir/ritonavir, corticosteroids, tocilizumab).
Diagnosis of COVID-19 was based on the WHO interim guidelines as a positive PCR test.21
AKI, developing during hospital stay, was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) classification, using the serum creatinine (SCr) criteria, as follows: Stage 1: increase in SCr by 0.3mg/dL within 48h or a 1.5–1.9 times increase in SCr from baseline within 7 days; Stage 2: 2.9 times increase in SCr within 7 days; Stage 3: 3 times or more increase in SCr within 7 days or initiation of renal replacement therapy (RRT).22 Patients were stratified according to the highest AKI stage attained during their hospital stay. Persistent AKI was defined as continuance of AKI beyond 48h according to the consensus report of the ADQI 16 Workgroup.23 Transient AKI was defined as AKI of less than 48h duration.23
Pre-admission SCr (mean SCr within the previous three months) was considered as baseline value. The estimated glomerular filtration rate (eGFR) for patients with previous baseline SCr was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.24 When unavailable, baseline SCr was estimated from the MDRD equation, accepting the lower limit of a normal baseline GFR of 75mL/min/1.73m2, as previously proposed.22
Presence of CKD was estimated according to the baseline SCr as an estimated GFR of lower than 60mL/min/1.73m2.25
Diabetes mellitus was diagnosed according to the American Diabetes Association criteria.26 Hypertension diagnosed according to the 2018 European Society of Cardiology (ESC) and European Society of Hypertension Guidelines.27 COPD comprised emphysema and chronic bronchitis. CVD was considered whenever a history of cerebrovascular disease, chronic heart failure of any cause, cardiac ischemic disease and/or peripheral arterial disease was documented. Acidemia was defined as blood gas PH<7.35.28 N/L ratio at admission was calculated as: Neutrophil count/Lymphocyte count.
OutcomesThe analyzed outcomes were the development of AKI during the first week of admission and in-hospital mortality.
Statistical analysisThe Shapiro–Wilk test was used to assess the normal distribution of variables. Categorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean±standard deviation or median and range. Continuous variables were compared with the Student's t-test and categorical variables were compared with the Chi-square test.
All variables underwent univariate analysis to determine statistically significant factors which may have contributed to AKI development and in-hospital mortality. Subsequently, only variables with a significant statistical difference in the univariate analysis were included in the multivariate analysis using the logistic regression method.
Data were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as a p-value <0.05. Statistical analysis was performed with the statistical software package SPSS for windows (version 21.0).
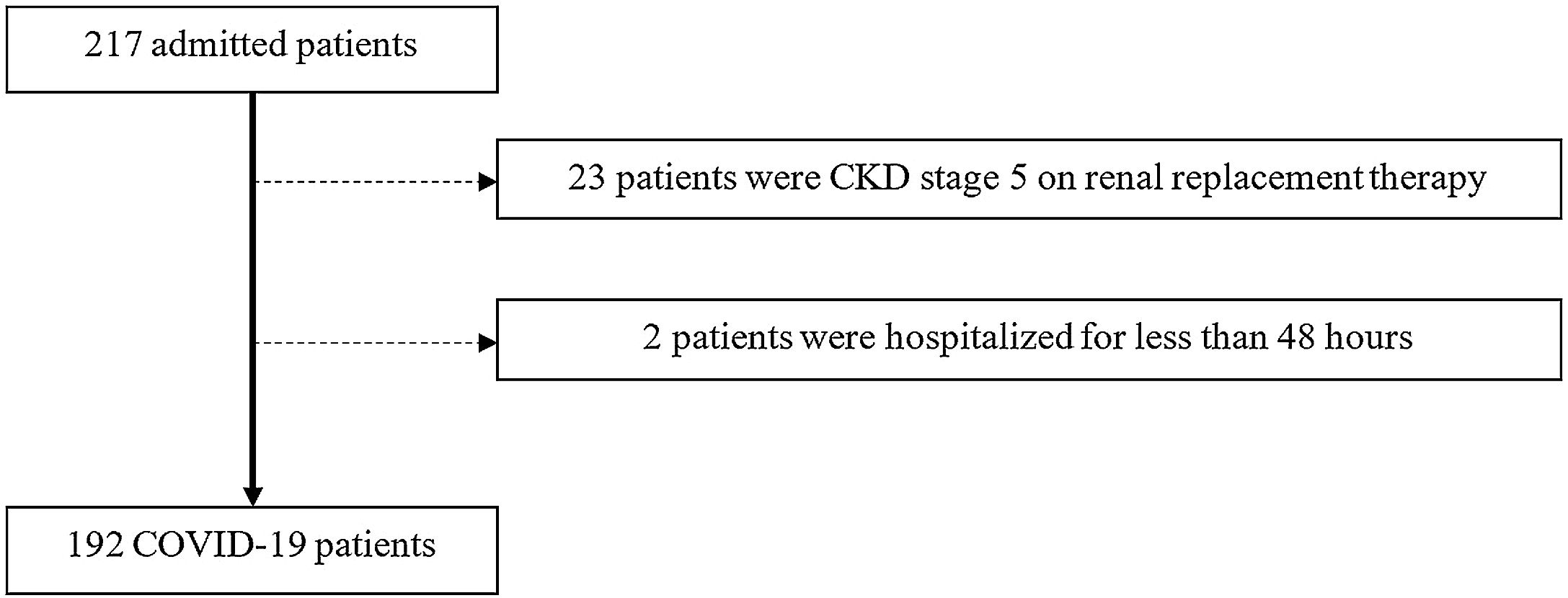
ResultsParticipantsFrom March 1st to May 31st, 217 patients were admitted to UICIVE with a diagnosis of COVID-19 on admission. We focused on 192 patients after excluding 25 patients as depicted in Fig. 1.
A majority of Caucasian (n=174, 90.6%) males (n=100, 52.1%) were hospitalized with a mean age of 72.2±16.4 years. There was a large prevalence of hypertensive (n=131, 68.2%), CVD (n=68, 35.4%), diabetic (n=54, 28.1%) and CKD (n=38, 19.8%) patients. Baseline creatinine was estimated in 5.7% of patients (n=11). Forty-two percent of patients were medicated with RAAS inhibitors. Almost 20% of hospitalized patients (n=38) required admission to an intensive care unit (ICU) mostly due to respiratory failure, 15.1% of patients fulfilled ARDS criteria and 16.7% of patients required mechanical ventilation. Most patients had a SOFA score of at least 2 (57.8%), and 12.5% of patients had a SOFA score of at least 4. Almost 30% of patients had a BCRSS score of at least 2.
At admission, median SCr was 1.00 (0.37–19.10)mg/dL, mean hemoglobin was 13.0±2.1 and almost 40% of patients were anemic, mean NL ratio was 6.49±5.71, mean serum albumin was 3.37±0.59g/dL and more than 70% of patients had hypoalbuminemia, median serum ferritin was 707.0 (66.0–7884.0)μg/L, mean CRP was 9.71±8.72mg/dL, mean lactate level was 15.65±10.60mg/dL and 27% of patients were acidemic.
During the first week of admission, 20.8% of patients were exposed to nephrotoxins, namely NSAIDS, radiocontrast, vancomycin or aminoglycosides. Concerning treatment, a vast majority of patients were medicated with hydroxychloroquine (n=140, 72.9%) and lopinavir/ritonavir (n=128, 66.7%). Only 3 patients were treated with tocilizumab and 10.9% of patients required corticosteroids. Mean time to ICU admission was 3.2±1.8 days.
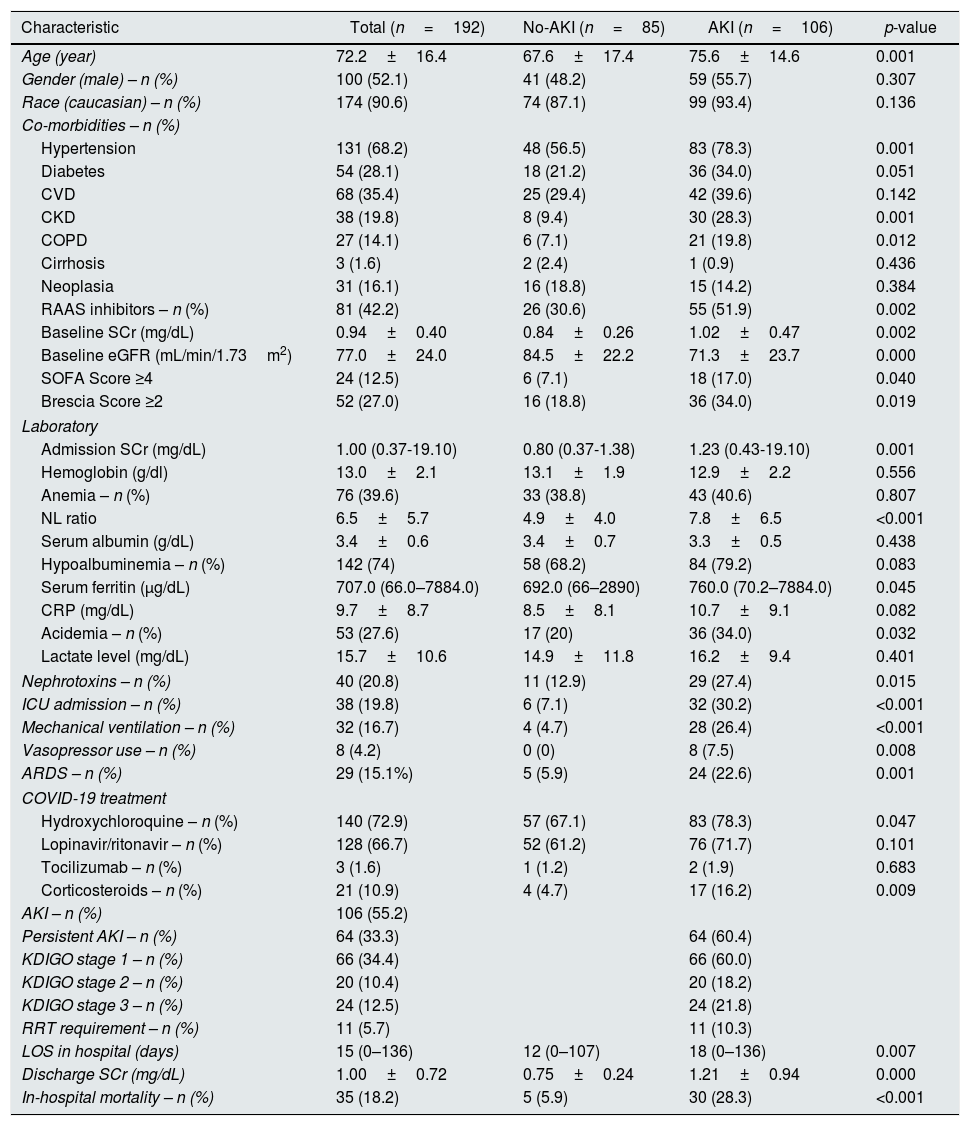
Median length of hospital stay was 15 (0–136) days. Baseline characteristics of this cohort are described in Table 1.
Patients’ baseline characteristics and according to AKI development.
| Characteristic | Total (n=192) | No-AKI (n=85) | AKI (n=106) | p-value |
|---|---|---|---|---|
| Age (year) | 72.2±16.4 | 67.6±17.4 | 75.6±14.6 | 0.001 |
| Gender (male) – n (%) | 100 (52.1) | 41 (48.2) | 59 (55.7) | 0.307 |
| Race (caucasian) – n (%) | 174 (90.6) | 74 (87.1) | 99 (93.4) | 0.136 |
| Co-morbidities – n (%) | ||||
| Hypertension | 131 (68.2) | 48 (56.5) | 83 (78.3) | 0.001 |
| Diabetes | 54 (28.1) | 18 (21.2) | 36 (34.0) | 0.051 |
| CVD | 68 (35.4) | 25 (29.4) | 42 (39.6) | 0.142 |
| CKD | 38 (19.8) | 8 (9.4) | 30 (28.3) | 0.001 |
| COPD | 27 (14.1) | 6 (7.1) | 21 (19.8) | 0.012 |
| Cirrhosis | 3 (1.6) | 2 (2.4) | 1 (0.9) | 0.436 |
| Neoplasia | 31 (16.1) | 16 (18.8) | 15 (14.2) | 0.384 |
| RAAS inhibitors – n (%) | 81 (42.2) | 26 (30.6) | 55 (51.9) | 0.002 |
| Baseline SCr (mg/dL) | 0.94±0.40 | 0.84±0.26 | 1.02±0.47 | 0.002 |
| Baseline eGFR (mL/min/1.73m2) | 77.0±24.0 | 84.5±22.2 | 71.3±23.7 | 0.000 |
| SOFA Score ≥4 | 24 (12.5) | 6 (7.1) | 18 (17.0) | 0.040 |
| Brescia Score ≥2 | 52 (27.0) | 16 (18.8) | 36 (34.0) | 0.019 |
| Laboratory | ||||
| Admission SCr (mg/dL) | 1.00 (0.37-19.10) | 0.80 (0.37-1.38) | 1.23 (0.43-19.10) | 0.001 |
| Hemoglobin (g/dl) | 13.0±2.1 | 13.1±1.9 | 12.9±2.2 | 0.556 |
| Anemia – n (%) | 76 (39.6) | 33 (38.8) | 43 (40.6) | 0.807 |
| NL ratio | 6.5±5.7 | 4.9±4.0 | 7.8±6.5 | <0.001 |
| Serum albumin (g/dL) | 3.4±0.6 | 3.4±0.7 | 3.3±0.5 | 0.438 |
| Hypoalbuminemia – n (%) | 142 (74) | 58 (68.2) | 84 (79.2) | 0.083 |
| Serum ferritin (μg/dL) | 707.0 (66.0–7884.0) | 692.0 (66–2890) | 760.0 (70.2–7884.0) | 0.045 |
| CRP (mg/dL) | 9.7±8.7 | 8.5±8.1 | 10.7±9.1 | 0.082 |
| Acidemia – n (%) | 53 (27.6) | 17 (20) | 36 (34.0) | 0.032 |
| Lactate level (mg/dL) | 15.7±10.6 | 14.9±11.8 | 16.2±9.4 | 0.401 |
| Nephrotoxins – n (%) | 40 (20.8) | 11 (12.9) | 29 (27.4) | 0.015 |
| ICU admission – n (%) | 38 (19.8) | 6 (7.1) | 32 (30.2) | <0.001 |
| Mechanical ventilation – n (%) | 32 (16.7) | 4 (4.7) | 28 (26.4) | <0.001 |
| Vasopressor use – n (%) | 8 (4.2) | 0 (0) | 8 (7.5) | 0.008 |
| ARDS – n (%) | 29 (15.1%) | 5 (5.9) | 24 (22.6) | 0.001 |
| COVID-19 treatment | ||||
| Hydroxychloroquine – n (%) | 140 (72.9) | 57 (67.1) | 83 (78.3) | 0.047 |
| Lopinavir/ritonavir – n (%) | 128 (66.7) | 52 (61.2) | 76 (71.7) | 0.101 |
| Tocilizumab – n (%) | 3 (1.6) | 1 (1.2) | 2 (1.9) | 0.683 |
| Corticosteroids – n (%) | 21 (10.9) | 4 (4.7) | 17 (16.2) | 0.009 |
| AKI – n (%) | 106 (55.2) | |||
| Persistent AKI – n (%) | 64 (33.3) | 64 (60.4) | ||
| KDIGO stage 1 – n (%) | 66 (34.4) | 66 (60.0) | ||
| KDIGO stage 2 – n (%) | 20 (10.4) | 20 (18.2) | ||
| KDIGO stage 3 – n (%) | 24 (12.5) | 24 (21.8) | ||
| RRT requirement – n (%) | 11 (5.7) | 11 (10.3) | ||
| LOS in hospital (days) | 15 (0–136) | 12 (0–107) | 18 (0–136) | 0.007 |
| Discharge SCr (mg/dL) | 1.00±0.72 | 0.75±0.24 | 1.21±0.94 | 0.000 |
| In-hospital mortality – n (%) | 35 (18.2) | 5 (5.9) | 30 (28.3) | <0.001 |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CRP, C reactive protein; CKD, chronic kidney disease; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; GFR, glomerular filtration rate; LOS, length of stay; NL, neutrophil and lymphocyte; RRT, renal replacement therapy; RAAS, renin angiotensin aldosterone system; SCR, serum creatinine; SOFA, sequential organ failure assessment.
In this cohort of COVID-19 patients, 55.2% developed AKI during the first week of admission (n=106). Of these, 64.2% of patients (n=68) presented AKI during the first 48h admission and the remaining developed AKI during the remaining period. Mean time to AKI development was 2.2±0.9 days. Patient characteristics according to AKI development are described in Table 1.
Patients with AKI were older (75.6±14.6 vs. 67.6±17.4, p=0.001), were more likely to have pre-existing hypertension (78.3% vs. 56.5%, p=0.001), CKD (28.3% vs. 9.4%, p=0.001) and COPD (19.8% vs. 7.1%, p=0.012), and to be medicated with RAAS inhibitors (51.9% vs. 30.6%, p=0.002). Mean baseline SCr was higher in AKI patients (1.02±0.47 vs. 0.84±0.26, p=0.002). Patients with BCRSS score higher than 2 developed more frequently AKI (34.5% vs. 18.8%, p=0.019).
At admission, patients with AKI had higher SCr (1.23 (0.43–19.10) vs. 0.80 (0.37–1.38), p=0.001; unadjusted OR 35.81 (95% CI 10.48–122.38). p<0.001), higher NL ratio (7.8±6.5 vs. 4.9±4.0, p<0.001) and were more likely acidemic (34.0% vs. 20%, p=0.032).
During the first week of hospitalization, patients more exposed to nephrotoxins were more likely to develop AKI (27.4% vs. 12.9%, p=0.015).
AKI patients required more ICU admission (30.2% vs. 7.1%, p<0.001), mechanical ventilation (26.4% vs. 4.7%, p<0.001) and vasopressor use (7.5% vs. 0%, p=0.008) and fulfilled more ARDS criteria (22.6% vs. 5.9%, p=0.001). More AKI patients were treated with hydroxychloroquine (78.3% vs. 67.1%, p=0.047) and corticosteroids (16.2% vs. 4.7%, p=0.009).
The majority of AKI patients had persistent AKI (n=64, 60.4%). According to AKI severity, most patients were KDIGO stage 1 (n=66, 60.0%), followed by KDIGO stage 3 (n=24, 21.8%) and KDIGO stage 2 (n=20, 18.2%). Ten percent of AKI patients required renal replacement therapy (RRT), five patients required continuous RRT and the remaining intermittent RRT.
On a multivariate analysis, admission SCr (adjusted OR 48.01 (95% CI 10.46–220.45), p<0.001) and exposure to nephrotoxins (adjusted OR 3.60 (95% CI 1.30–9.94), p=0.014) were independent predictors of AKI.
Transient versus persistent AKIThere were no statistically significant differences between transient versus persistent AKI concerning demographic and clinical characteristics, nor laboratory values at admission.
Compared with transient AKI, patients with persistent AKI had a higher proportion of patients with more severe AKI (KDIGO stage 3 31.3% vs. 7.1%, KDIGO stage 2 21.9% vs. 14.3%, KDIGO stage 1 45.3% vs. 78.6%, p=0.002), required more often ICU admission (37.5% vs. 19.0, p=0.045), and RRT (14.1% vs. 2.4%, p=0.044). These patients had higher mortality rate than transient AKI patients (37.5% vs. 11.9%, p=0.004).
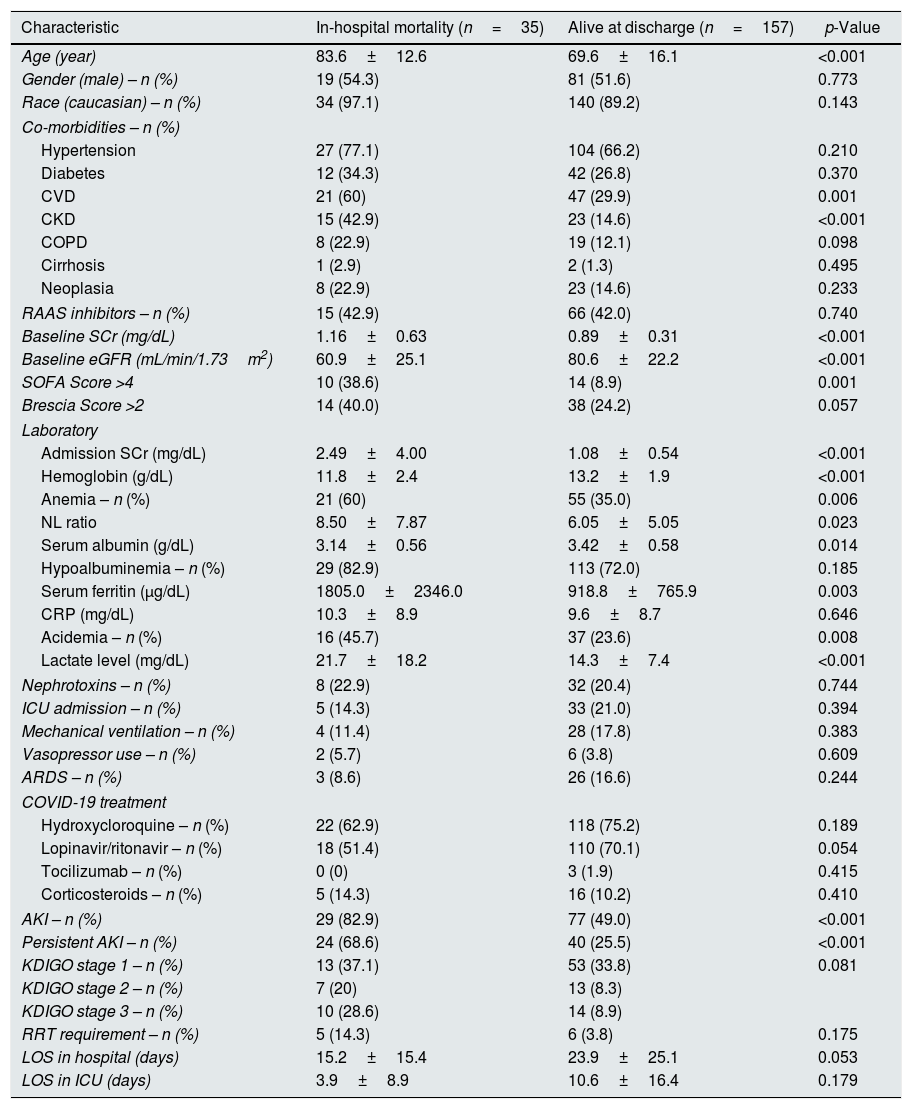
AKI and outcomesOverall, in-hospital mortality was 18.2% (n=35). Older age (83.6±12.6 vs. 69.6±16.1, p<0.001), pre-existing CVD (60% vs. 29.9%, p=0.001) and CKD (42.9% vs. 14.6%, p<0.001); lower Hb (11.8±2.4 vs. 13.2±1.9, p<0.001), higher SCr (2.49±4.00 vs. 1.08±0.54, p<0.001), lower albumin (3.14±0.56 vs. 3.42±0.58, p=0.014) and acidemia (45.7% vs. 23.6%, p=0.008) at admission were associated with mortality (Table 2).
Characteristics of patients according to in-hospital mortality.
| Characteristic | In-hospital mortality (n=35) | Alive at discharge (n=157) | p-Value |
|---|---|---|---|
| Age (year) | 83.6±12.6 | 69.6±16.1 | <0.001 |
| Gender (male) – n (%) | 19 (54.3) | 81 (51.6) | 0.773 |
| Race (caucasian) – n (%) | 34 (97.1) | 140 (89.2) | 0.143 |
| Co-morbidities – n (%) | |||
| Hypertension | 27 (77.1) | 104 (66.2) | 0.210 |
| Diabetes | 12 (34.3) | 42 (26.8) | 0.370 |
| CVD | 21 (60) | 47 (29.9) | 0.001 |
| CKD | 15 (42.9) | 23 (14.6) | <0.001 |
| COPD | 8 (22.9) | 19 (12.1) | 0.098 |
| Cirrhosis | 1 (2.9) | 2 (1.3) | 0.495 |
| Neoplasia | 8 (22.9) | 23 (14.6) | 0.233 |
| RAAS inhibitors – n (%) | 15 (42.9) | 66 (42.0) | 0.740 |
| Baseline SCr (mg/dL) | 1.16±0.63 | 0.89±0.31 | <0.001 |
| Baseline eGFR (mL/min/1.73m2) | 60.9±25.1 | 80.6±22.2 | <0.001 |
| SOFA Score >4 | 10 (38.6) | 14 (8.9) | 0.001 |
| Brescia Score >2 | 14 (40.0) | 38 (24.2) | 0.057 |
| Laboratory | |||
| Admission SCr (mg/dL) | 2.49±4.00 | 1.08±0.54 | <0.001 |
| Hemoglobin (g/dL) | 11.8±2.4 | 13.2±1.9 | <0.001 |
| Anemia – n (%) | 21 (60) | 55 (35.0) | 0.006 |
| NL ratio | 8.50±7.87 | 6.05±5.05 | 0.023 |
| Serum albumin (g/dL) | 3.14±0.56 | 3.42±0.58 | 0.014 |
| Hypoalbuminemia – n (%) | 29 (82.9) | 113 (72.0) | 0.185 |
| Serum ferritin (μg/dL) | 1805.0±2346.0 | 918.8±765.9 | 0.003 |
| CRP (mg/dL) | 10.3±8.9 | 9.6±8.7 | 0.646 |
| Acidemia – n (%) | 16 (45.7) | 37 (23.6) | 0.008 |
| Lactate level (mg/dL) | 21.7±18.2 | 14.3±7.4 | <0.001 |
| Nephrotoxins – n (%) | 8 (22.9) | 32 (20.4) | 0.744 |
| ICU admission – n (%) | 5 (14.3) | 33 (21.0) | 0.394 |
| Mechanical ventilation – n (%) | 4 (11.4) | 28 (17.8) | 0.383 |
| Vasopressor use – n (%) | 2 (5.7) | 6 (3.8) | 0.609 |
| ARDS – n (%) | 3 (8.6) | 26 (16.6) | 0.244 |
| COVID-19 treatment | |||
| Hydroxycloroquine – n (%) | 22 (62.9) | 118 (75.2) | 0.189 |
| Lopinavir/ritonavir – n (%) | 18 (51.4) | 110 (70.1) | 0.054 |
| Tocilizumab – n (%) | 0 (0) | 3 (1.9) | 0.415 |
| Corticosteroids – n (%) | 5 (14.3) | 16 (10.2) | 0.410 |
| AKI – n (%) | 29 (82.9) | 77 (49.0) | <0.001 |
| Persistent AKI – n (%) | 24 (68.6) | 40 (25.5) | <0.001 |
| KDIGO stage 1 – n (%) | 13 (37.1) | 53 (33.8) | 0.081 |
| KDIGO stage 2 – n (%) | 7 (20) | 13 (8.3) | |
| KDIGO stage 3 – n (%) | 10 (28.6) | 14 (8.9) | |
| RRT requirement – n (%) | 5 (14.3) | 6 (3.8) | 0.175 |
| LOS in hospital (days) | 15.2±15.4 | 23.9±25.1 | 0.053 |
| LOS in ICU (days) | 3.9±8.9 | 10.6±16.4 | 0.179 |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CRP, C reactive protein; CKD, chronic kidney disease; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; GFR, glomerular filtration rate; LOS, length of stay; NL, neutrophil and lymphocyte; RRT, renal replacement therapy; RAAS, renin angiotensin aldosterone system; SCR, serum creatinine; SOFA, sequential organ failure assessment.
Mortality was higher in AKI patients (28.3% vs. 5.9%, p<0.001), and AKI patients also had a more prolonged length of hospital stay (26.5±26.2 days vs. 17.1±19.6 days, p=0.007).
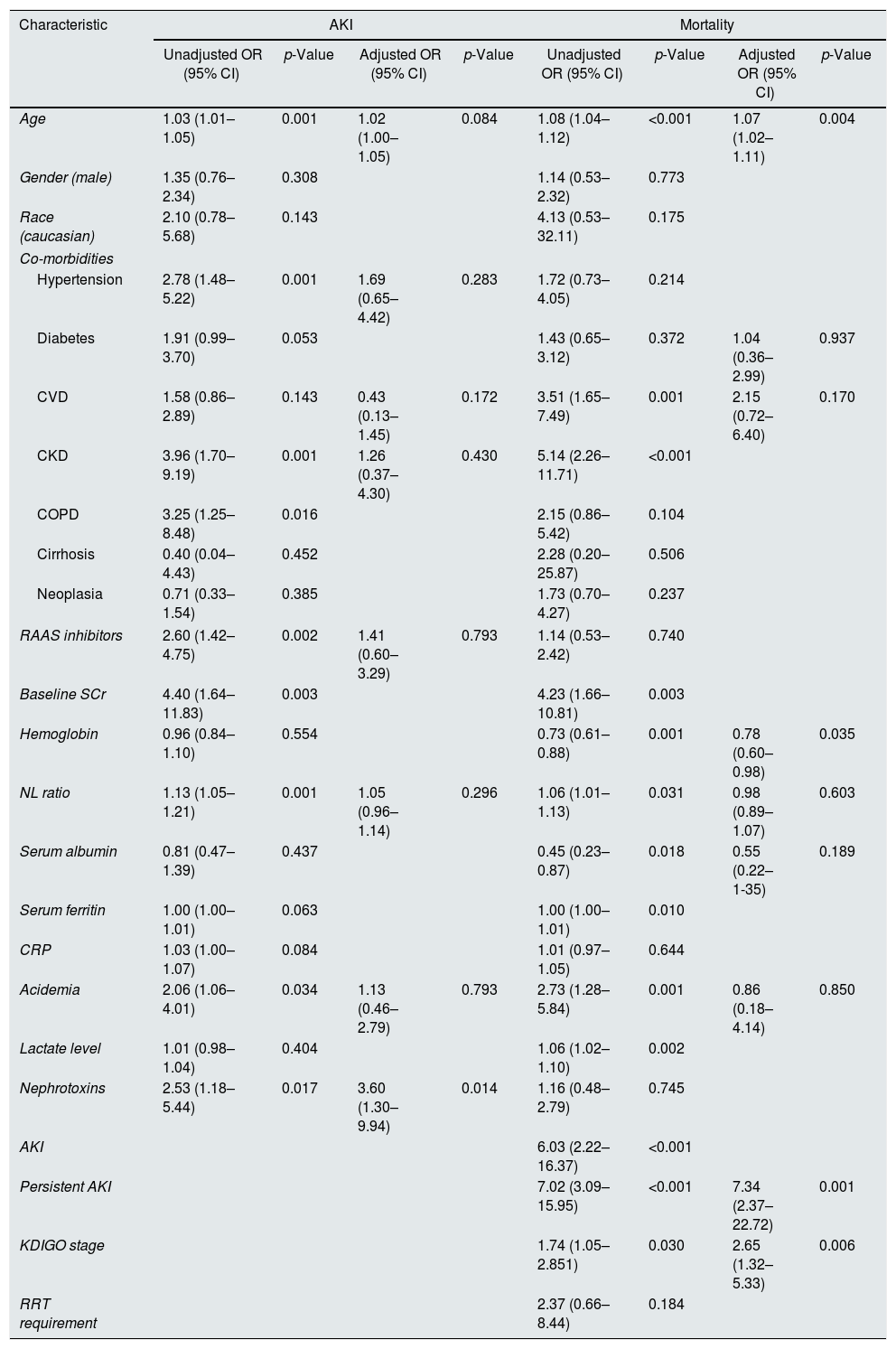
On a multivariate analysis, AKI was not an independent predictor of mortality (adjusted OR 3.00 (95% CI: 0.86–10.52), p=0.086). Thus, we performed two sensitivity analysis, one including only patients with persistent AKI and another assessing the impact of AKI severity in the multivariate model.
In this multivariate analysis, older age (adjusted OR 1.07 (95% CI 1.02–1.11), p=0.004), lower Hb level (adjusted OR 0.78 (95% CI 0.60–0.98), p=0.035), duration of AKI (adjusted OR 7.34 for persistent AKI (95% CI 2.37–22.72), p=0.001) and severity of AKI (adjusted OR 2.65 per increase in KDIGO stage (95% CI 1.32–5.33), p=0.006) were independent predictors of mortality (Table 3).
Univariate and multivariate analysis of factors predictive of AKI and mortality in COVID-19 patients.
| Characteristic | AKI | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Age | 1.03 (1.01–1.05) | 0.001 | 1.02 (1.00–1.05) | 0.084 | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.02–1.11) | 0.004 |
| Gender (male) | 1.35 (0.76–2.34) | 0.308 | 1.14 (0.53–2.32) | 0.773 | ||||
| Race (caucasian) | 2.10 (0.78–5.68) | 0.143 | 4.13 (0.53–32.11) | 0.175 | ||||
| Co-morbidities | ||||||||
| Hypertension | 2.78 (1.48–5.22) | 0.001 | 1.69 (0.65–4.42) | 0.283 | 1.72 (0.73–4.05) | 0.214 | ||
| Diabetes | 1.91 (0.99–3.70) | 0.053 | 1.43 (0.65–3.12) | 0.372 | 1.04 (0.36–2.99) | 0.937 | ||
| CVD | 1.58 (0.86–2.89) | 0.143 | 0.43 (0.13–1.45) | 0.172 | 3.51 (1.65–7.49) | 0.001 | 2.15 (0.72–6.40) | 0.170 |
| CKD | 3.96 (1.70–9.19) | 0.001 | 1.26 (0.37–4.30) | 0.430 | 5.14 (2.26–11.71) | <0.001 | ||
| COPD | 3.25 (1.25–8.48) | 0.016 | 2.15 (0.86–5.42) | 0.104 | ||||
| Cirrhosis | 0.40 (0.04–4.43) | 0.452 | 2.28 (0.20–25.87) | 0.506 | ||||
| Neoplasia | 0.71 (0.33–1.54) | 0.385 | 1.73 (0.70–4.27) | 0.237 | ||||
| RAAS inhibitors | 2.60 (1.42–4.75) | 0.002 | 1.41 (0.60–3.29) | 0.793 | 1.14 (0.53–2.42) | 0.740 | ||
| Baseline SCr | 4.40 (1.64–11.83) | 0.003 | 4.23 (1.66–10.81) | 0.003 | ||||
| Hemoglobin | 0.96 (0.84–1.10) | 0.554 | 0.73 (0.61–0.88) | 0.001 | 0.78 (0.60–0.98) | 0.035 | ||
| NL ratio | 1.13 (1.05–1.21) | 0.001 | 1.05 (0.96–1.14) | 0.296 | 1.06 (1.01–1.13) | 0.031 | 0.98 (0.89–1.07) | 0.603 |
| Serum albumin | 0.81 (0.47–1.39) | 0.437 | 0.45 (0.23–0.87) | 0.018 | 0.55 (0.22–1-35) | 0.189 | ||
| Serum ferritin | 1.00 (1.00–1.01) | 0.063 | 1.00 (1.00–1.01) | 0.010 | ||||
| CRP | 1.03 (1.00–1.07) | 0.084 | 1.01 (0.97–1.05) | 0.644 | ||||
| Acidemia | 2.06 (1.06–4.01) | 0.034 | 1.13 (0.46–2.79) | 0.793 | 2.73 (1.28–5.84) | 0.001 | 0.86 (0.18–4.14) | 0.850 |
| Lactate level | 1.01 (0.98–1.04) | 0.404 | 1.06 (1.02–1.10) | 0.002 | ||||
| Nephrotoxins | 2.53 (1.18–5.44) | 0.017 | 3.60 (1.30–9.94) | 0.014 | 1.16 (0.48–2.79) | 0.745 | ||
| AKI | 6.03 (2.22–16.37) | <0.001 | ||||||
| Persistent AKI | 7.02 (3.09–15.95) | <0.001 | 7.34 (2.37–22.72) | 0.001 | ||||
| KDIGO stage | 1.74 (1.05–2.851) | 0.030 | 2.65 (1.32–5.33) | 0.006 | ||||
| RRT requirement | 2.37 (0.66–8.44) | 0.184 | ||||||
AKI, acute kidney injury, ARDS, acute respiratory distress syndrome; CRP, C reactive protein; CKD, chronic kidney disease; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; GFR, glomerular filtration rate; NL, neutrophil and lymphocyte; RRT, renal replacement therapy; RAAS, renin angiotensin aldosterone system; SCR, serum creatinine; SOFA, sequential organ failure assessment.
In this retrospective cohort of hospitalized patients, we report a high incidence of AKI associated with COVID-19. More than 50% of infected patients developed AKI and the majority of these were persistent and had lower severity changes in renal function. Remarkably, only persistent AKI and higher severity AKI were associated with mortality in these patients.
Recent studies have suggested the association of AKI and COVID-19, despite an initial report by Wang et al. which described there was no AKI in 116 patients in Wuhan.29 This study included a majority of mild pneumonia patients had no patients had previous CKD which may explain the absence of AKI. In fact, in our cohort previous CKD and baseline SCr were important risk predictors of AKI development.
One of the studies which reports the lower rate of AKI is a retrospective study of 1099 hospitalized patients and outpatients in China, in which AKI was only present in 6 patients (0.5%).5 In a retrospective cohort of 52 critically ill COVID-19 patients, Yang et al. reported a 29% incidence of AKI and a mortality of 61.5% which was associated with the severity of the pneumonia.10 In another study, the incidence of AKI ranged from 3.5% in moderate disease patients to 42.9% in critically ill patients.30 Furthermore, AKI patients had a higher mortality rate.30 Indeed, AKI was most often present in more severe cases of COVID-19.31
In our cohort of hospitalized COVID-19 patients, AKI was present in 55%. The more frequent use of hydroxychloroquine and corticosteroids in AKI patients in our cohort reflects the presence of moderate to severe disease in these patients, in the first months of the pandemic when this was common practice. This severity of COVID-19 in our cohort explains the large incidence of AKI. Indeed, patients with AKI had higher BCRSS scores. The fact that 30% of AKI patients required ICU admission within the first week of admission, also points out the contribution of AKI to disease severity.
The largest multicenter cohort included 5449 hospitalized COVID-19 patients in which 36.6% developed AKI. Hirsch et al. reported a majority of patients with lower severity of AKI, in this study 46.5% of patients were KDIGO stage 1, and also reported that most AKI cases developed early in the course of COVID-19.32 This is in accordance with our results as we reported a prevalence of 60% of KDIGO stage 1, and most patients presented with AKI at admission or within the first two days.
The etiology of AKI in patients with COVID-19 appears to be multifactorial, due to direct cytopathic effects and indirect lesion. The mechanisms include ischemic injury due to fluid loss and heart failure, cytokine release syndrome, exposure to nephrotoxins, rhabdomyolysis, microcirculatory thrombi, hypercoagulability, direct podocyte and epithelial lesion, RAAS activation and renal vasoconstriction.18,33,34 Consistent with this, in our cohort AKI was more frequent in patients with higher disease severity, higher inflammatory markers and also in patients exposed to nephrotoxins, namely contrast, nonsteroidal anti-inflammatory drugs, vancomycin and aminoglycosides.
Despite the considerable focus on the use of RAAS inhibitors and severity of COVID-19, as it is theorized that the intake of these drugs might enable virus entry and replication, which relies on bonding to the angiotensin converting enzyme 2 (ACE-2), in both Hirsch's study and in our cohort the use of RAAS inhibitors was not associated with AKI development or mortality.32,35,36,
AKI has been associated with an increased risk of in-hospital mortality in multiple settings.15,37 The mortality rate of COVID-19 is estimated to be around 6% worldwide, still 81% of COVID-19 cases are mild.1 The rate of mortality in hospitalized patients ranges from 10 to 30% and is much higher in critically ill patients.8,38,39
Cheng et al. demonstrated that renal dysfunction defined as either elevated baseline SCr, hematuria, proteinuria and AKI, was associated with mortality in a prospective cohort of 701 hospitalized patients with COVID-19.40 Despite reporting an incidence of AKI of only 5.1%, this study reported a higher risk of mortality according to AKI severity.40 Lim et al. studied 164 hospitalized patients with an AKI incidence of 18% and demonstrated that AKI KDIGO stage 3 was independently associated with mortality.41 AKI was also an independent risk factor for hospital mortality in a prospective study by Portolés et al.42 This is also consistent with the results of our cohort, as we reported an increased risk of mortality with the severity of AKI (adjusted OR 2.65 per increase in KDIGO stage (95% CI 1.32–5.33), p=0.006).
Our cohort is the first to study the impact of AKI duration in COVID-19 patients and its association with outcomes. Indeed, the association of rapid kidney function recovery and better short-term survival has been previously reported in other settings.23,43–45 The impact of AKI duration on prognosis led to the development of a standardized definition of transient and persistent AKI, based on recovery of kidney function within 48h, by the ADQI Workgroup.23 Transient AKI might reflect reversible renal impairment due to nephrotoxins or hemodynamic changes and persistent AKI is more likely a result of conditions less easily reversed and these patients may consequently require more RRT.46,47
Rubin et al. analyzed AKI in 77 critically ill patients with COVID-19 and demonstrated that persistent AKI was present in the majority of patients (93%).48 In their study, clinical and laboratory characteristics were similar between patients with persistent and transient AKI.48 These findings were also present in our cohort. Interestingly, a prospective study of 52 critically ill COVID-19 patients reported that 50% of patients with AKI stage 2 progressed to stage 3, and 28% required RRT.49
We demonstrated that persistent AKI was present in 60% of patients and was associated with a significant increase in mortality risk (adjusted OR 7.34 for persistent AKI (95% CI 2.37–22.72), p=0.001). In our study, transient AKI did not carry an increased risk for in-hospital mortality. This highlights the importance of assessing the severity and duration of AKI as both influences prognosis.
Our study has some important virtues. This is the first study demonstrating an association between duration of AKI and mortality in patients with COVID-19. We defined AKI according to the KDIGO classification using SCr criteria. Additionally, we applied the standardized definitions of transient and persistent AKI as defined by the ADQI workgroup to evaluate its impact on prognosis. Also, despite its retrospective design, the studied variables were routinely recorded in daily practice which allowed for the analysis of important covariates with impact on AKI development and outcome.
Nevertheless, this study has certain limitations. Firstly, the single-center and retrospective nature of our study limits generalizability. Secondly, the small size of our cohort may have compromised, at least in part, the results. Thirdly, 5.7% of patients did not have baseline SCr and baseline renal function had to be estimated with the MDRD equation, which might have led to overestimation of AKI. Finally, we could not determine the exact mechanisms contributing to AKI and mortality in these patients.
To conclude, we demonstrated that AKI was frequent in hospitalized patients with COVID-19 and that persistent and higher severity of AKI were predictors of in-hospital mortality. Although we could not find predictors of persistent AKI in this modest cohort size, further studies should focus on this matter to allow for the early recognition of high-risk patients.
Conflict of interestThe authors declare that they have no conflict of interest.