Chronic kidney disease remains an important risk factor for morbidity and mortality among LT recipients, but its exact incidence and risk factors are still unclear.
Material and methodsWe carried out a retrospective cohort study of consecutive adults who underwent liver transplant (January 2009–December 2018) and were followed (at least 6 months) at our institution. CKD was defined following the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines. Long-term kidney function was classified into 4 groups: no CKD (eGFR, ≥60mL/min/1.73m2), mild CKD (eGFR, 30–59mL/min/1.73m2), severe CKD (eGFR, 15–29mL/min/1.73m2), and end-stage renal disease (ESRD).
ResultsWe enrolled 410 patients followed for 53.2±32.6 months. 39 had CKD at baseline, and 95 developed de novo CKD over the observation period. There were 184 (44.9%) anti-HCV positive, 47 (11.5%) HBsAg positive, and 33 (8.1%) HBV/HDV positive recipients. Recipient risk factors for baseline CKD were advanced age (P=0.044), raised levels of serum uric acid (P<0.0001), and insulin dependent DM (P=0.0034). Early post-transplant AKI was common (n=95); logistic regression analysis found that baseline serum creatinine was an independent predictor of early post-LT AKI (P=0.0154). According to our Cox proportional hazards model, recipient risk factors for de novo CKD included aging (P<0.0001), early post-transplant AKI (P=0.007), and baseline serum creatinine (P=0.0002). At the end of follow-up, there were 116 LT recipients with CKD – 109 (93.9%) and 7 (6.1%) had stage 3 and advanced CKD, respectively. Only two of them are undergoing long-term dialysis.
ConclusionThe incidence of CKD was high in our cohort of LT recipients, but only a slight decline in kidney function over time was recorded. Prevention of post-transplant AKI will improve kidney function in the long run. We need more studies to analyze the function of kidneys among LT recipients over extended follow-ups and their impact on mortality.
La enfermedad renal crónica (ERC) sigue siendo un importante factor de riesgo de morbimortalidad entre los receptores de un trasplante hepático (TH), su incidencia exacta y sus factores de riesgo aún no están claros.
Materiales y métodosLlevamos a cabo un estudio de cohortes retrospectivo de adultos incluidos de forma consecutiva que habían recibido un TH (de enero de 2009 a diciembre de 2018) e hicimos el seguimiento (mínimo 6 meses) en nuestra institución. La ERC se definió siguiendo las guías de práctica clínica Kidney Disease: Improving Global Outcomes (KDIGO) de 2012. La función renal a largo plazo se clasificó en 4 grupos: sin ERC (filtración glomerular estimada [FGe]>60ml/min/1,73m2), ERC leve (FGe: 30-59ml/min/1,73m2), ERC grave (FGe: 15-29ml/min/1,73m2) y enfermedad renal terminal (ERT).
ResultadosIncluimos a 410 pacientes a los que se hizo un seguimiento durante 53,2±32,6 meses: 39 tenían ERC al inicio y 95 desarrollaron ERC de novo durante el periodo de observación. Había 184 (44,9%) receptores con anticuerpos contra el VHC, 47 (11,5%) con positividad para el HBsAg y 33 (8,1%) portadores del virus de la hepatitis B (VHB) o el virus de la hepatitis D (VHD). Los factores de riesgo de los receptores para presentar ERC al inicio fueron la edad avanzada (p=0,044), unos niveles elevados de ácido úrico en suero (p<0,0001) y la presencia de diabetes mellitus (DM) insulinodependiente (p=0,0034). La aparición temprana de lesión renal aguda (LRA) postrasplante fue frecuente (n=95); un análisis de regresión logística reveló que la creatinina sérica al inicio era un factor predictivo independiente de LRA temprana después del TH (p=0,0154). Según nuestro modelo de riesgos proporcionales de Cox, los factores de riesgo de los receptores para presentar ERC de novo incluyeron la edad avanzada (p<0,0001), una LRA temprana postrasplante (p=0,007) y la creatinina sérica al inicio (p=0,0002). Al final del seguimiento, había 116 receptores de TH con ERC, 109 (93,9%) y 7 (6,1%) tenían ERC en estadio 3 y avanzada, respectivamente. Solo 2 de ellos estaban recibiendo diálisis a largo plazo.
ConclusiónLa incidencia de ERC fue alta en nuestra cohorte de receptores de TH, pero solo se registró una ligera disminución de la función renal a lo largo del tiempo. La prevención de la LRA postrasplante mejorará la función renal a largo plazo. Necesitamos más estudios para analizar la función de los riñones entre los receptores de TH durante seguimientos prolongados, así como su efecto sobre la mortalidad.
Chronic kidney disease (CKD) is a well-known complication in solid-organ transplant recipients with a frequency ranging between 10 and 90%.1,2 This large variability has been related to numerous factors including heterogeneity in the definition of post-transplant kidney disease, various follow-up lengths, methods of measurement of eGFR and differences in the types of transplantation studied.1,2 CKD after the transplantation of a non-renal organ leads to increased morbidity and mortality.1,2
LT recipients are an important group of non-renal solid-organ recipients: long-term survival of LT recipients is currently longer in comparison with the past due to better immunosuppressive therapies, better selection criteria and surgical procedures.3–14 The overall 1-year and 5-year patient survival is 90% and 75%, respectively. As survival time lengthened after liver transplant, CKD has emerged as a major long-term complication after LT.3–14 CKD is independently associated with poor survival among LT recipients.15,16
Some information in the medical literature regarding the occurrence of CKD after LT already exists. Many demographic, clinical and biochemical factors have been shown to play a role in the development of CKD after LT including arterial hypertension, immunosuppression, diabetes mellitus, metabolic syndrome, dyslipidemia, pre-operative GFR and hepatitis C.3–14 The impact of perioperative management factors, which are potentially modifiable, on the pathogenesis of progressive CKD is under active investigation.17
Since the implementation of MELD in 2002, the number of patients with impaired kidneys who develop CKD after LT has increased and will continue to increase, as the number of patients transplanted with MELD of 40 or greater is also increasing, at least in part because more patients will have renal dysfunction before LT.18,19 CKD has become currently one of the leading reasons for morbidity and death rate after LT. We have performed a retrospective study to assess frequency and risk factors for CKD in a large cohort of LT recipients followed up to 10 years at our institution. In addition, we have addressed incidence and pathogenesis of AKI in this population.
Material and methodsStudy subjects and designThis was a single-center retrospective cohort study. The study was conducted at the Ospedale Maggiore Policlinico, Fondazione ‘Ca’ Granda, IRCCS, Milano, Italy. Patients were identified using electronic healthcare data that included their medical, medication administration, and procedure records and laboratory results maintained in the study setting. All data were collected and analyzed to ensure data integrity and patient privacy. As listed in Supplementary File 1, this study was performed according to the guidelines from the STROBE (STrengthening the Reporting of OBservational Studies in Epidemiology) initiative20 and the Declaration of Helsinki (World Medical Association, Fortaleza, Brazil, October 2013).21,22
All adult patients who received LT at our institution from January 2009 to December 2018 were included. A total of 451 LT were performed during this period, and 410 were followed at our post-LT clinic and included in the study (follow-up ≥6 months). Of the 410 recipients regularly followed at post-LT clinic, 42 were censored (28 lost at follow-up, and 14 died). Patients who underwent combined kidney/liver transplant were excluded.
Preoperative parameters analyzed were: age, gender, ethnicity, etiology of liver disease, and history of chronic kidney disease (if present). Post-LT variables analyzed were: blood pressure and immunosuppressive medications, diabetes and type of diabetes treatment (oral agents or insulin), medications for dyslipidemia and hyperuricemia (uric acid in serum >7mg/dL). Laboratory data included: INR, serum albumin, total bilirubin, markers of viral hepatitis, and HIV status. Random urine test was made in LT patients with viral hepatitis. Baseline data on immunosuppressive therapy were collected at the time of discharge from the hospital where LT was made.
Glomerular filtration rate (GFR) was calculated using the CKD-EPI equation.23 CKD was defined as GFR <60mL/min and categorized according to the KDIGO 2012 guidelines.24 Diagnosis of early post-transplant AKI (within 2 weeks after LT) was made according to the KDIGO criteria25 – an increase in serum creatinine by ≥0.3mg/dL (≥26.5mmol/L) within 48h or an increase in serum creatinine to ≥1.5 times baseline within the previous 7 days. AKI was categorized in three stages according to the KDIGO criteria.25
ImmunosuppressionBasiliximab was adopted for induction therapy at the discretion of the transplant physician. A standardized maintenance immunosuppression protocol including calcineurin inhibitors, steroids, and mycophenolate mofetil was started within 24h of transplantation. The choice of calcineurin inhibitor (cyclosporine or tacrolimus) was made by the transplant team. Patients on tacrolimus-based regimen received tacrolimus in order to reach trough levels of 8–12ng/mL during the first 2 weeks after LT, 7–10ng/mL during the following 2 months, and 5–8ng/mL thereafter. In patients on cyclosporine-based regimen, cyclosporine was administered to an intended trough level of 200–300ng/mL during the first week after transplantation, 150–200ng/mL during the following 3 weeks, 100–150ng/mL during the following 2 months, and 75–100ng/mL thereafter. Intravenous methylprednisolone 500mg was administered during anhepatic phase and tapered gradually during the first week.
Statistical analysisWe carried out a descriptive analysis using mean±standard deviation and median values (with respective ranges) for continuous variables with normal distribution or not, respectively. Comparison between groups was made with t-test (continuous variables) or Chi-square test (categorical parameters). Mann–Whitney U test was adopted, when appropriate. In some cases, continuous variables without normal distribution underwent logarithmical transformation and managed with parametric tests. Logistic regression analysis and Cox proportional hazards model were adopted where appropriate. Statistical analysis was performed with the software Statistica (version 10) and StatView. All tests were two-tailed and a P value of less than 0.05 was considered statistically significant.
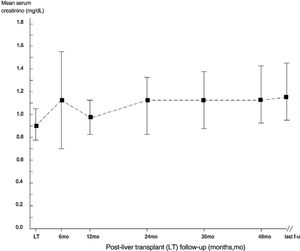
ResultsThe mean follow-up was 53.2±32.6 months. 410 patients were enrolled in the study, and the descriptive analysis is reported in Tables 1 and 2. The median serum creatinine was 0.90mg/dL (interquartile range, 0.71; 1.0) in the whole group at baseline. Some patients underwent re-transplant (n=24), one of them underwent three LT transplants. All recipients received livers from deceased donors. The course of serum creatinine over the follow-up in our cohort is reported in Fig. 1.
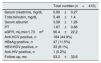
Characteristics of study patients at baseline (at LT).
| Total number (n=410) | |
|---|---|
| Age, yrs | 54.7±9.7 |
| Males, n | 296 (72.2%) |
| Caucasians, n | 388 (94.6%) |
| Chronic liver disease | |
| Alcohol, n | 44 (10.8%) |
| HCV, n | 71 (17.5%) |
| HBV, n | 41 (10.1%) |
| HCC, n | 159 (39.2%) |
| NASH, n | 19 (4.7%) |
| PBC, n | 21 (5.2%) |
| PSC, n | 12 (2.9%) |
| Others, n | 43 (10.5%) |
| Arterial hypertension, n | 94 (22.9%) |
| Non insulin dependent DM, n | 48 (11.7%) |
| Insulin dependent DM, n | 64 (15.6%) |
| Hyperuricemia, n | 37 (9%) |
| Dyslipidemia, n | 40 (9.7%) |
| Immunosuppression type | |
| Tacrolimus, n | 286 (69.7%) |
| Corticosteroids, n | 343 (83.6%) |
| Cyclosporine, n | 116 (28.3%) |
| Mycophenolate mofetil, n | 261 (63.6%) |
| Everolimus, n | 6 (1.46%) |
| Azathioprine, n | 2 (0.49%) |
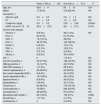
Characteristics of study patients at baseline (at LT).
| Total number (n=410) | |
|---|---|
| Serum creatinine, mg/dL | 0.90±0.27 |
| Total bilirubin, mg/dL | 5.49±1.4 |
| Serum albumin | 3.58±1.26 |
| PT | 1.67±0.86 |
| eGFR, mL/min/1.73m2 | 90.4±22.2 |
| Anti-HCV positive, n | 184 (44.9%) |
| HBsAg positive, n | 47 (11.5%) |
| HBV/HDV positive, n | 33 (8.1%) |
| Anti-HIV positive, n | 1 (0.2%) |
| Follow-up, mo | 53.2±32.6 |
Prior to LT, 39 (9.5%) had a eGFR <60mL/min/1.73m2 and had CKD, while 371 (90.5%) had eGFR >60mL/min/1.73m2. In the subset of patients with baseline CKD, 2 patients had CKD stage 3 and two with CKD stage 4. There were three patients with cryoglobulinemic GN, one had diabetic nephropathy, one had post-surgery single kidney, one renal tubular acidosis and 4 hepatorenal syndrome. Also, 14 patients had arterial hypertension, and 18 diabetes mellitus.
The characteristics of study patients with CKD versus non-CKD (at the time of LT) are shown in Table 3. The comparison between patients with or without eGFR <60mL/min/1.73m2 showed difference between the two groups with regard to arterial hypertension (P=0.026), insulin dependent DM (P=0.0039), and raised levels of serum uric acid (P<0.0001) (Table 3). No difference occurred in the frequency of anti-HCV positive, HBsAg positive and HBV/HDV positive patients between the two groups (Table 3). According to logistic regression analysis, age at LT (P=0.044), increased values of serum uric acid (P<0.0001), and insulin dependent DM (P=0.0034) were independently associated with CKD at baseline (Table 4).
Characteristics of study patients at baseline (LT): patients with CKD versus those without CKD.
| GFR<60mL/min/1.73m2 (n=39) | GFR>60mL/min/1.73m2 (n=371) | P | |
|---|---|---|---|
| Age, yrs | 58.1±7.96 | 54.3±9.8 | NS |
| Males | 30 (77%) | 266 (71.6%) | NS |
| Caucasian, n | 39 (100%) | 349 (89.2%) | NS |
| Albumin, g/dL | 3.48±0.69 | 3.6±1.3 | NS |
| PT | 1.56±0.63 | 1.68±0.88 | NS |
| Creatinine, mg/dL | 1.49±0.25 | 0.84±0.18 | 0.00001 |
| eGFR, mL/min/1.73m2 | 49.4±8.76 | 94.7±18.4 | 0.00001 |
| Total bilirubin, mg/dL | 3.77±6.5 | 5.67±7.9 | NS |
| Chronic liver disease | |||
| Alcohol, n | 5 (13.0%) | 39 (10.5%) | NS |
| HCV, n | 8 (21%) | 63 (17.1%) | |
| HBV, n | 4 (10.0%) | 37 (10%) | |
| HCC, n | 11 (28.9%) | 148 (40.1%) | |
| NASH, n | 5 (13.1%) | 14 (3.1%) | |
| PBC, n | 1 (2.0%) | 20 (5.4%) | |
| PSC, n | 0 | 12 (3.0%) | |
| Others, n | 4 (10.5%) | 35 (9.0%) | |
| HIV, n | 0 | 1 (0.02%) | NS |
| Anti-HCV positive, n | 14 (35.8%) | 170 (45.9%) | NS |
| HBsAg positive, n | 3 (7.7%) | 44 (11.8%) | NS |
| HBV/HDV positive, n | 4 (10.2%) | 29 (7.8%) | NS |
| Arterial hypertension, n | 15 (38.4%) | 79 (21.0%) | 0.026 |
| Non insulin dependent DM, n | 5 (12.8%) | 43 (11.5%) | NS |
| Insulin dependent DM, n | 13 (33%) | 51 (13.7%) | 0.0039 |
| Hyperuricemia, n | 12 (30.8%) | 25 (6.0%) | 0.0001 |
| Dyslipidemia, n | 3 (7.7%) | 37 (9.9%) | NS |
A group of patients developed CKD over the follow-up (n=95, 25.6%). The baseline characteristics of patients who developed de novo CKD after LT and those who did not are reported in Table 5. There was difference with regard to non insulin-dependent DM (P<0.01), raised uric acid levels (P<0.05), and serum creatinine (P=0.0002) (Table 5). Serum uric acid levels were greater in patients with de novo CKD compared with those without, 8.5±2.1 vs. 6.7±1.98mg/dL, P<0.04. Multivariate Cox regression model showed that three covariates were independently linked to incident CKD-age at LT (P<0.0001), early post-LT AKI (P=0.007), and serum creatinine at LT (P=0.0002) (Table 6).
Characteristics of study patients at baseline (at LT): patients who developed de novo CKD versus those who did not.
| De novo CKD (n=95) | Non-CKD (n=276) | P | |
|---|---|---|---|
| Age, yrs | 59.7±7.21 | 52.5±9.9 | 0.0001 |
| Males | 66 (69.4%) | 200 (72.4%) | NS |
| Caucasian, n | 89 (94%) | 260 (94%) | NS |
| Albumin, g/dL | 3.43±0.52 | 3.6±1.48 | 0.0001 |
| PT | 1.60±0.64 | 1.71±0.95 | NS |
| Creatinine, mg/dL | 0.91±0.16 | 0.81±0.18 | 0.0002 |
| eGFR, mL/min/1.73m2 | 85.3±17.7 | 98.0±17.5 | 0.00001 |
| Chronic liver disease | |||
| Alcohol, n | 7 (7.5%) | 32 (11.6%) | NS |
| HCV, n | 18 (19.3%) | 45 (16.3%) | |
| HBV, n | 9 (9.6%) | 28 (10.1%) | |
| HCC, n | 39 (41.9%) | 109 (39.6%) | |
| NASH, n | 8 (8.6%) | 6 (2.18%) | |
| PBC, n | 6 (6.4%) | 14 (5%) | |
| PSC, n | 2 (2.1%) | 10 (3.6%) | |
| Others, n | 4 (4.3%) | 31 (11.3%) | |
| HIV, n | 1 (0.01%) | 0 | NS |
| Anti-HCV positive, n | 48 (50.5%) | 122 (44.2%) | NS |
| HBsAg positive, n | 11 (11.5%) | 33 (11.9%) | NS |
| HBV/HDV positive, n | 5 (5.2%) | 24 (8.7%) | NS |
| Arterial hypertension, n | 24 (25.2%) | 55 (19.9%) | NS |
| Non-insulin dependent DM, n | 18 (18.9%) | 25 (9%) | 0.014 |
| Insulin dependent DM, n | 14 (14.7%) | 37 (13.4%) | NS |
| Hyperuricemia, n | 11 (11.6%) | 14 (5%) | 0.05 |
| Dyslipidemia, n | 10 (10.5%) | 27 (9.7%) | NS |
| Post-LT AKI | 34 (35.7%) | 46 (17.3%) | 0.0012 |
| Dialysis dependent AKI | 8 (8.4%) | 6 (2.2%) | 0.0058 |
Cox regression analysis (outcome: incident or de novo CKD post-LT).
| Covariate | B | Std. error | Wald | P | Exp(b) | 95% CI, Exp(b) |
|---|---|---|---|---|---|---|
| Age at LT | 0.09716 | 0.01642 | 34.9978 | <0.0001 | 1.1020 | 1.067; 1.1379 |
| Arterial hypertension | 0.0691 | 0.2380 | 0.08442 | 0.7714 | 1.0716 | 0.673; 1.704 |
| Post-transplant AKI | 0.6117 | 0.2274 | 7.2383 | 0.0071 | 1.8435 | 1.183; 2.872 |
| Hyperuricemia | 0.08367 | 0.3535 | 0.0560 | 0.8129 | 1.0873 | 0.5457; 2.1164 |
| Creatinine at LT | 2.2792 | 0.6083 | 14.0411 | 0.0002 | 9.7689 | 2.98; 31.98 |
Patients who developed early post-transplant AKI (n=95) were categorized in AKI stage 1 (n=35), AKI stage 2 (n=37) and 3 (n=23). There was difference between patients who developed de novo CKD and those who did not with regard to early post-transplant AKI (P<0.0001) (Table 5). No difference concerning the frequency of viral hepatitis was recorded between the two groups (Table 5). The characteristics of patients (at the time of LT) with early post-LT AKI and those with peri-operative normal kidneys are shown in Table 7. Dialysis dependent AKI was more common in patients who developed de novo CKD in comparison with those who did not (P<0.001) (Table 5). Logistic regression analysis reported that serum creatinine at baseline (P<0.0154) and MMF use (P<0.04) were associated with the occurrence of early post-transplant AKI.
Characteristics of study patients at baseline (at LT): patients who developed early post-transplant AKI versus those who did not.
| Post-LT AKI (n=95) | Non-AKI (n=311) | P | |
|---|---|---|---|
| Age, yrs | 56.6±8 | 54±10 | 0.01 |
| Males | 77 (81%) | 216 (69.4%) | NS |
| Caucasian, n | |||
| Albumin, g/dL | 3.4±0.5 | 3.6±1.4 | NS |
| PT | 1.7±0.8 | 1.6±0.8 | NS |
| Creatinine, mg/dL | 0.9±0.2 | 0.87±0.26 | 0.0004 |
| eGFR, mL/min/1.73m2 | 85.8±24.2 | 92.1±21.3 | 0.016 |
| Chronic liver disease | |||
| Alcohol, n | 8 (8.4%) | 36 (11.5%) | NS |
| HCV, n | 20 (21%) | 51 (16.4%) | |
| HBV, n | 13 (13.7%) | 28 (9%) | |
| HCC, n | 36 (37.9%) | 122 (39.2%) | |
| NASH, n | 6 (6.3%) | 13 (4.1%) | |
| PBC, n | 2 (3.1%) | 19 (6.1%) | |
| PSC, n | 3 (3.1%) | 9 (2.9%) | |
| Others, n | 6 (6.3%) | 33 (10.6%) | |
| HIV, n | 0 | 1 (0.3%) | NS |
| Anti-HCV positive, n | 45 (47.3%) | 138 (44.4%) | NS |
| HBsAg positive, n | 14 (14.7%) | 32 (10.3%) | NS |
| HBV/HDV positive, n | 7 (7.4%) | 26 (8.4%) | NS |
| Arterial hypertension, n | 28 (29.5%) | 64 (20.6%) | NS |
| Non insulin dependent DM, n | 8 (8.4%) | 40 (12.9%) | NS |
| Insulin dependent DM, n | 18 (18.9%) | 45 (14.5%) | NS |
| Hyperuricemia, n | 8 (8.4%) | 94 (30.2%) | NS |
| Dyslipidemia, n | 8 (8.4%) | 32 (10.3%) | NS |
| Tacrolimus, n | 65 (68.4%) | 219 (70.4%) | NS |
| Corticosteroids, n | 76 (80%) | 264 (84.9%) | NS |
| Cyclosporine, n | 28 (29.5%) | 87 (27.9%) | NS |
| Mycophenolate mofetil, n | 69 (72.6%) | 190 (61%) | 0.028 |
| Everolimus, n | 1 (1.1%) | 5 (1.6%) | NS |
| Azathioprine, n | 0 | 2 (0.6%) | NS |
Thirty patients developed diabetes mellitus after LT (PTDM), in many of them non-insulin dependent diabetes occurred (Table 8). In the group of LT recipients having viral hepatitis (n=264), no patients with HBV-related and a few (n=12) with HCV-related cryoglobulinemic glomerular disease were recorded.
Descriptive analysis of the study group at the end of follow-up.
| Total number (n=410) | |
|---|---|
| Age, yrs | 59.4±11.3 |
| Serum creatinine, mg/dL | 1.15±0.17 |
| eGFR, mL/min/1.73m2 | 71.52±23.1 |
| Arterial hypertension, n | 265 (65.1%) |
| Non-insulin dependent DM, n | 68 (16.7%) |
| Insulin dependent DM, n | 74 (18.2%) |
| Post-transplant DM, n | 30 (10%) |
| Hyperuricemia, n | 74 (18.2%) |
| Dyslipidemia, n | 83 (20.4%) |
| Immunosuppression type | |
| Tacrolimus, n | 315 (76.8%) |
| Corticosteroids, n | 52 (12.7%) |
| Cyclosporine, n | 74 (18%) |
| Mycophenolate mofetil, n | 253 (61.7%) |
| Everolimus, n | 46 (11.2%) |
| Azathioprine, n | 8 (1.9%) |
At the end of follow-up, there were 116 LT recipients with CKD; 95 patients had de novo and 21 baseline CKD. 109 (93.9%) LT recipients had CKD stage 3, four (3.5%) and three (2.6%) had stage 4 and ESRD, respectively. Two LT recipients are undergoing regular dialysis. 368 patients were followed up at the end of the study period.
DiscussionIn this retrospective cohort study of stable LT recipients followed at our institution, we found that a large (n=95, 25.6%) number of LT recipients developed CKD over the observation period. At the end of the follow-up, there were 116 LT recipients with CKD. An overwhelming majority of liver recipients did not have advanced CKD – two LT recipients undergo regular dialysis at the last follow-up.
Post-transplant CKD is a major public health problem among all non-renal solid organ transplant recipients. Previous studies reported on the occurrence of CKD after LT, with an incidence ranging between 20% and 80%.3–14 In addition to the evidence reported above, this wide range of results is related to other factors such as patient selection, or differences in managing patients, among others.3–14 We addressed the incidence of CKD among LT recipients in the MELD era; the current MELD system gives consistent weight to serum creatinine and this translated into greater incidence of post-transplant CKD.26,27 We adopted the CKD EPI equation to estimate GFR. According to a meta-analysis of serum creatinine based equations that estimated GFR among solid organ transplant recipients (around 40% LT recipients), CKD EPI equation was better at higher GFR compared to others.28
No role of viral hepatitis in the development of post-transplant CKD in our cohort was reported, unlike what others reported.3,4 Various factors could explain this – we made diagnosis of viral hepatitis by serologic assays instead of nucleic acid testing (NAT), HBV- or HCV-related cryoglobulinemic glomerular disease was uncommon, antiviral treatment with DAAs or other agents (data not shown) could be have improved renal outcomes.
Although the number of LT recipients who underwent long-term dialysis at the last follow-up was extremely small, the high frequency of CKD highlights the burden of cardiovascular risk in this population. It is well known that reductions in estimated GFR predict the development of fatal and non-fatal cardiovascular events, regardless of traditional CV risk factors (blood pressure, smoke, cholesterol, age, gender, among others), in the general population and in high-risk cohorts.29,30
A consistent group of patients developed de novo CKD post-LT in our study, and the frequency of AKI in the early postoperative period was independently associated with de novo CKD. The link between AKI and CKD after LT remains controversial31 – in vitro studies highlighted the occurrence of permanent kidney damage following AKI.32 It has been suggested that patients who experience AKI, even those who showed complete recovery from AKI, remain at risk for CKD.33,34 Careful monitoring of kidney function is needed in these patients post-LT. According to our regression logistic analysis, we found that the most important predictors for early post-transplant AKI were baseline serum creatinine and therapy with MMF. MMF was adopted more frequently by LT recipients with early post transplant AKI compared to those without. Patients with early post-transplant AKI received immunosuppression without CNI (or reduced dose CNI) to preserve kidneys and consequently adopted immunosuppressive therapy with MMF.
Our study involved the assessment of the etiology of chronic kidney disease after LT. Unlike other studies, we did not find a role of dyslipidemia, or extended criteria grafts.35 Metabolic syndrome at baseline or blood pressure and CNI dosing 1 year post-transplant were not evaluated.36 Lack of knowledge of CKD among LT recipients is a barrier to patient engagement and self-management of chronic disease risk factors and has been associated with progression of CKD post-transplant.37,38
Despite the large cohort of transplant recipients, our single-center study had some limitations. First is the retrospective design, which hampered the analysis of treatment changes over time by the physicians, including detailed changes in the immunosuppression. Second, we were not able to collect data on pre-operative proteinuria. Limited evidence exists on the impact of proteinuria on patient/kidney survival following LT39–41; unfortunately, dipstick urine analysis is made in selected circumstances in LT candidates at our institution. Third, the design of our study did not allow to understand fully the etiology of early post-transplant AKI as we did not include in our model intra-operative factors (i.e., surgical techniques, intra-operative bleeding, hemodynamic instability, or volume of transfused blood products) or donor factors. Finally, our analysis may have been biased toward the selection of healthier patients with better long-term survival, which may have decreased the impact of covariates such as pre-transplant kidney dysfunction.42
In conclusion, we found that a good number of long-term liver transplant survivors developed CKD after transplant. Viral hepatitis had no role in the pathogenesis of CKD. New onset CKD was associated with early post-transplant AKI, according to our multivariate analysis. The timely management of post-transplant AKI may potentially improve patient survival and decrease post-transplant death risk.
FundingThis research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interestNone declared.