La esclerosis peritoneal encapsulante (EPE) es una complicación poco común, pero grave, de la diálisis peritoneal. La tasa de incidencia varía entre el 0,5 y el 4,4 %. Esta complicación está asociada a tasas significativas de morbilidad y mortalidad. El diagnóstico requiere la presencia de manifestaciones clínicas de obstrucción intestinal o función gastrointestinal alterada con signos patológicos y radiológicos de encapsulamiento intestinal. El mecanismo patogénico exacto de la EPE sigue siendo desconocido, aunque sí que se asocia firmemente con la duración de la diálisis peritoneal. Presentamos un caso clínico de EPE y analizamos las manifestaciones clínicas, diagnóstico, tratamiento, pronóstico y prevención.
The encapsulating peritoneal sclerosis is a rare but serious complication of peritoneal dialysis. The incident rate varies between 0.5 to 4.4%. This entity is associated with significant morbidity and mortality. The diagnosis requires the presence of clinical features of intestinal obstruction or disturbed gastrointestinal function with pathological and radiological evidence of bowel encapsulation. The exact pathogenic mechanism of EPS remains unknown, although it’s strongest associated with duration of peritoneal dialysis. We present a clinical case of EPS and discuss the clinical manifestations, diagnosis, treatment, prognosis and prevention.
INTRODUCTION
Changes to peritoneal membrane function over time result in the development of ultrafiltration failure in a proportion of peritoneal dialysis (PD) patients and pose a risk for the rarer condition of encapsulating peritoneal sclerosis.1
Encapsulating peritoneal sclerosis (EPS) is a rare complication of peritoneal dialysis (PD), but carries significant morbidity and high mortality of approximately 50%.2
Although EPS can be found in different settings, the condition is most frequently seen in patients treated with PD.2
The first cases of EPS in PD patients were reported by Gandhi et al. and Denis et al. in 1980.
The multiplicity of suspected etiologies and the its unknown pathogenesis are reflected in the variety of terms that have been used indiscriminately and interchangeably to describe this complication, such as peritoneal fibrosis/peritoneal sclerosis/sclerotic thickening of the peritoneal membrane/sclerotic obstructive peritonitis, calcifying peritonitis, abdominal cocoon and sclerosing peritonitis and sclerosing encapsulating peritonitis.3
In 2001 an Ad Hoc committee of the International Society for Peritoneal Dialysis on ultrafiltration management in PD changed the name of the syndrome to encapsulating peritoneal sclerosis (EPS), which is more descriptive of the morphologic changes, because findings of acute inflammation and peritonitis component is still absent in developed syndrome.3
Encapsulating peritoneal sclerosis is characterized by partial or diffuse bowel obstruction, accompanied by marked sclerotic thickening of the peritoneal membrane. The clinical features vary, but they frequently include abdominal pain, nausea, vomiting, weight loss, low-grade fever, hemorrhagic effluent, ultrafiltration failure, ascites and resistance to recombinant human erythropoietin.3 The development is insidious and probably starts with sterile visceral peritoneal inflammation with neovascularisation, followed by massive deposition of fibrous scar tissue that encases part or the entire bowel.2
Longitudinal and cross-sectional studies of peritoneal membrane function with duration of PD have identified that at least two processes seem to be occurring. The rate of small-solutes transport tends to increase with duration on PD reflects early change occurred in peritoneal membrane. The second change with time on treatment occurs in only proportion of patients is a reduction in the capacity of the membrane.1
Peritoneal sclerosis is generally recognized as a critical component of EPS. However, the onset of EPS does not result solely from the natural progression of peritoneal sclerosis. A “second hit” event, such bacterial peritonitis, abdominal bleeding, or abdominal surgery may be needed to trigger the onset of EPS in the face of advanced peritoneal sclerosis.4
With the peritoneal membrane, the epithelial to mesenchymal transition (EMT) is one of the earliest histologic changes in the peritoneum and is a critical step in the development of an abnormal membrane (Scheme 1). There may be more than on stimulus for EMT; candidates include transforming growth factor –β (TGF-β), angiotensin II, fibroblast growth factor, and connective tissue growth factor (CTGF).2
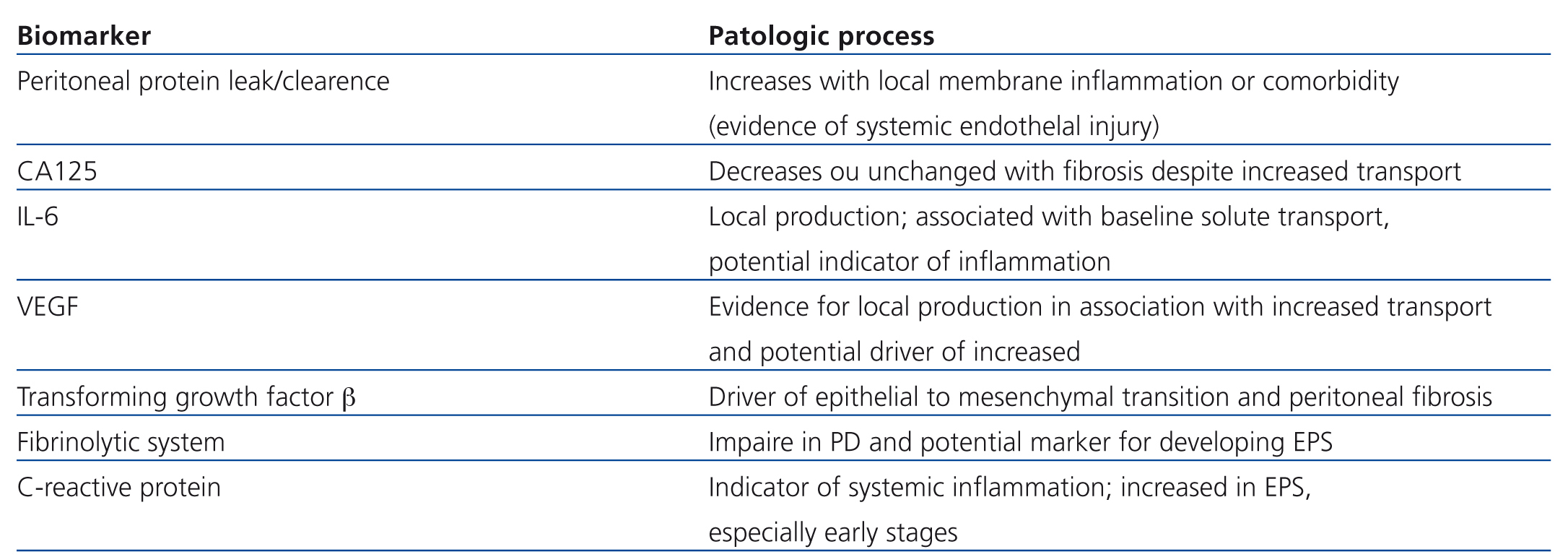
A number of potential mediators have been postulated in the progressive injury associated with membrane damage (Table 1).
The potential to use these mediators as biomarkers is still under investigation, although with-in and between –patient variability is high and it may help be patterns rather than absolute values that will help the clinician in decision making.1
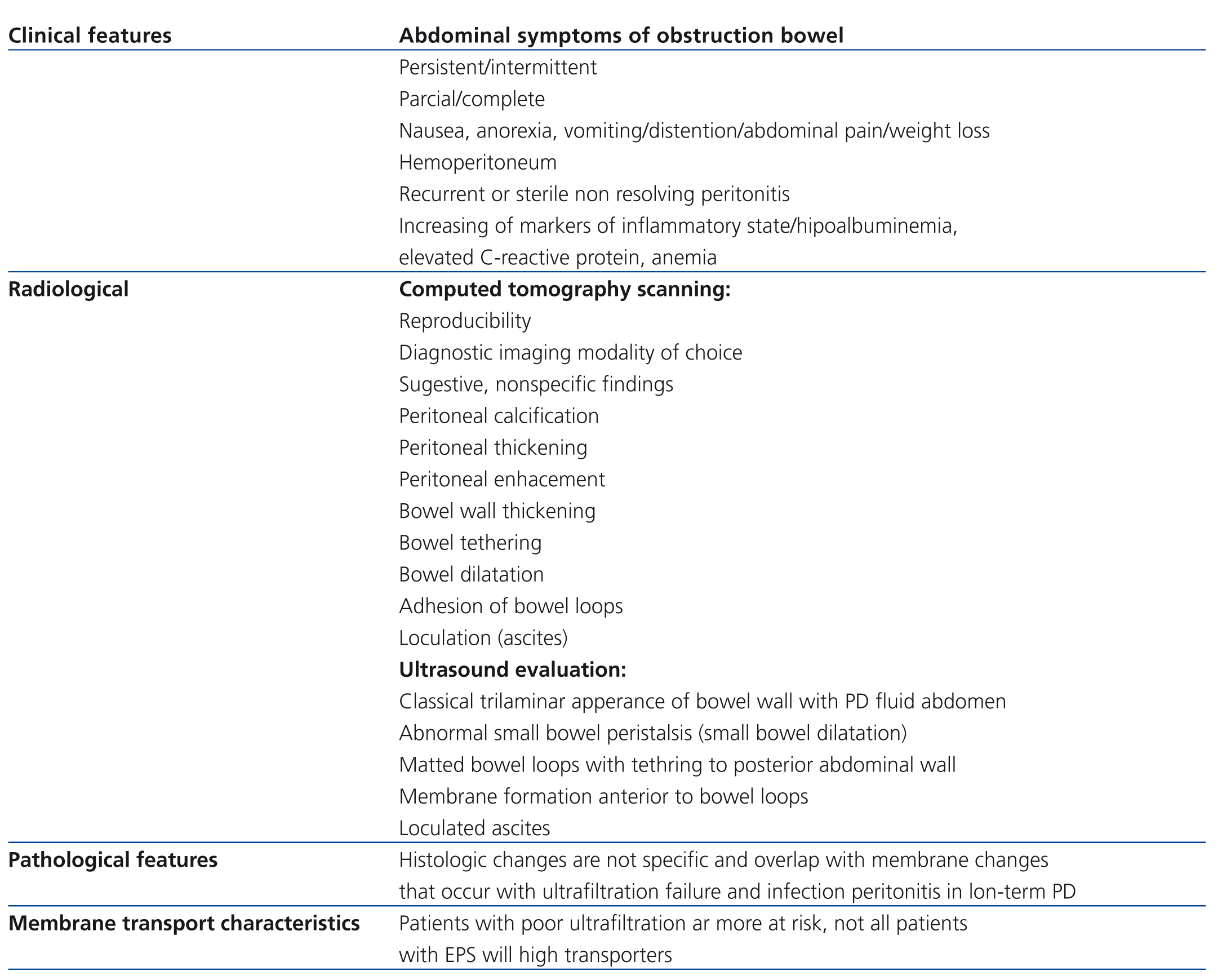
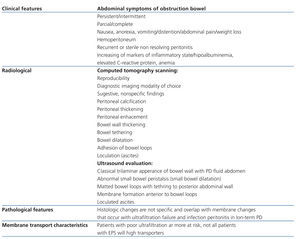
According with ISPD (International Society for Peritoneal Dialysis) Statement, the tentative diagnosis is made on the basis of a combination of bowel obstruction and features of encapsulation due fibrosis. Laparotomy is the only way to make a definitive diagnosis but usually not done as it can be associated considerable morbidity and mortality.5 The steps for the diagnosis of EPS are exposed on Table 2.
Medical and surgical management of EPS remains unsupported by randomized trials. The role of medical therapies and the timing of surgery are poorly defined.
The clinical course of EPS is very variable. Some patients progress rapidly to complete bowel encapsulation and obstruction in few weeks, however others can have a course relatively benign.
DESCRIPTION
We report the clinical case of a forty-one years old woman suffering of chronic kidney disease in stage 5 secondary to chronic lithiasic pielonephritis. She started hemodialysis in April 2001. In May of 2002 she received a living donor kidney allograft from her mother and unfortunately she quickly lost it, in consequence of unsolved kinking of the renal artery. In December 2003 she restarted hemodialysis and by personal choice she was evaluated to peritoneal dialysis in consult.
In January 2004, she was submitted to implantation of the PD catheter and 4 weeks after she began this modality. The first peritoneal equilibration test revealed a high average transporter and she started automated PD (Nightly Tidal PD). During the first 3 years she used hypertonic PD solutions (3,86% glucose) to improve fluid balance and control blood pressure, because icodextrine PD solutions were not available. Despite of this, she was presenting a good peritoneal dialysis adequacy and ultrafiltration membrane capacity.
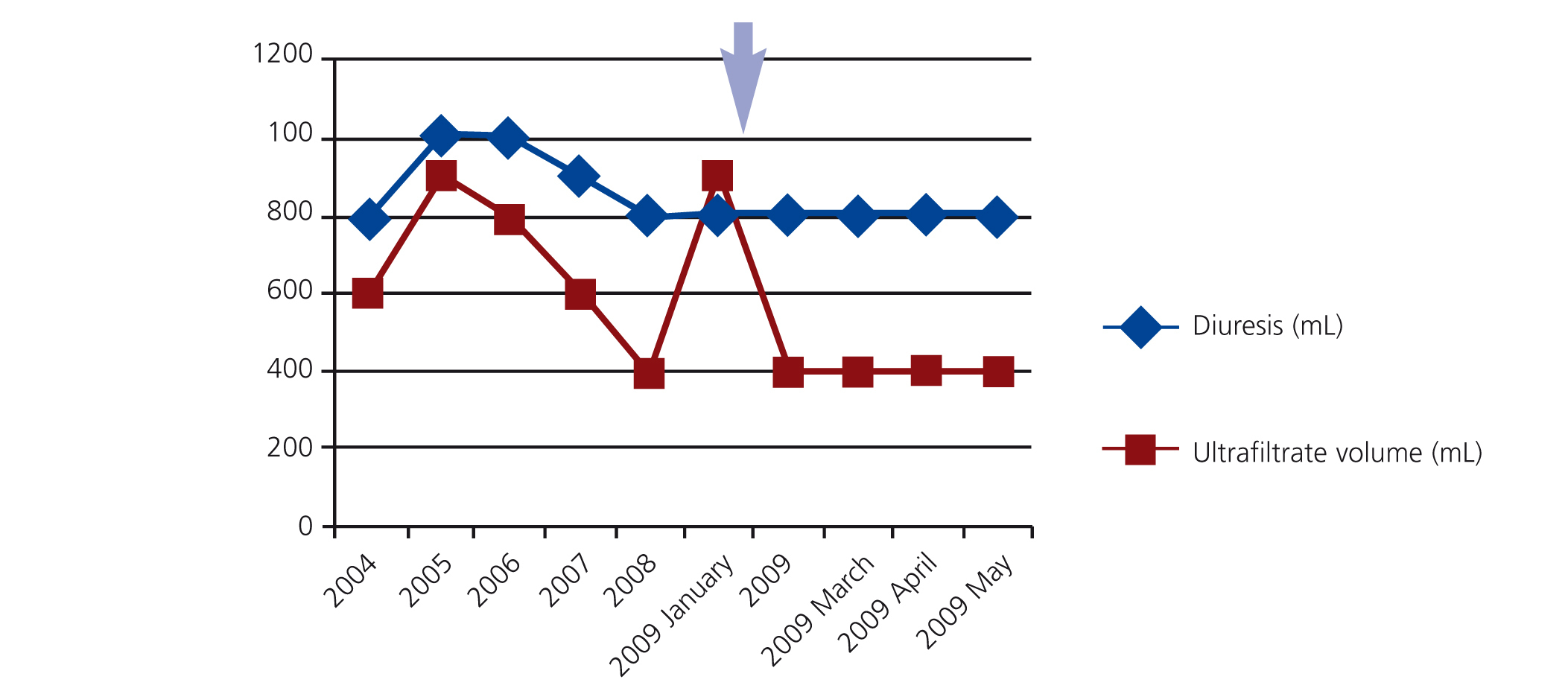
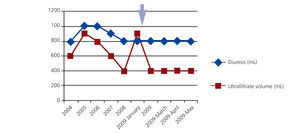
During the first years of PD 2 episodes of infection of the extern orifice exit were registered (unknown germen), and in the fourth year she had a haemoperitoneum (it was excluded infectious and gynecological causes) accompanied by ultrafiltration failure and her peritoneal membrane transporter status changed to high transporter. In this way, it was introduced 7,5% icodextrine PD solutions, that was associated with increased membrane ultrafiltration (Figure 1).
In May 2009, she was admitted for a peritonitis diagnosis (abdominal pain and cloudy effluent with a WBC count of 6000/mm3 and 80% neutrophils).
She began an empiric antibiotic scheme with intermittently intraperitoneal vancomycin (2g every 5 days) and intraperitoneal ceftazidime (1g daily).
On day after, she developed sepsis and were prescribed intravenous antibiotics (meropenem 500mg/day and vancomycin 500mg/three times a week).
The peritoneal fluid culture revealed a methicillin - sensitive Staphylococcus aureus that led to addition of rifampin per os (600mg/day).
On the fifth day, the poor clinical evolution led to the diagnosis of refractory peritonitis and peritoneal catheter removal and she was transferred to hemodialysis.
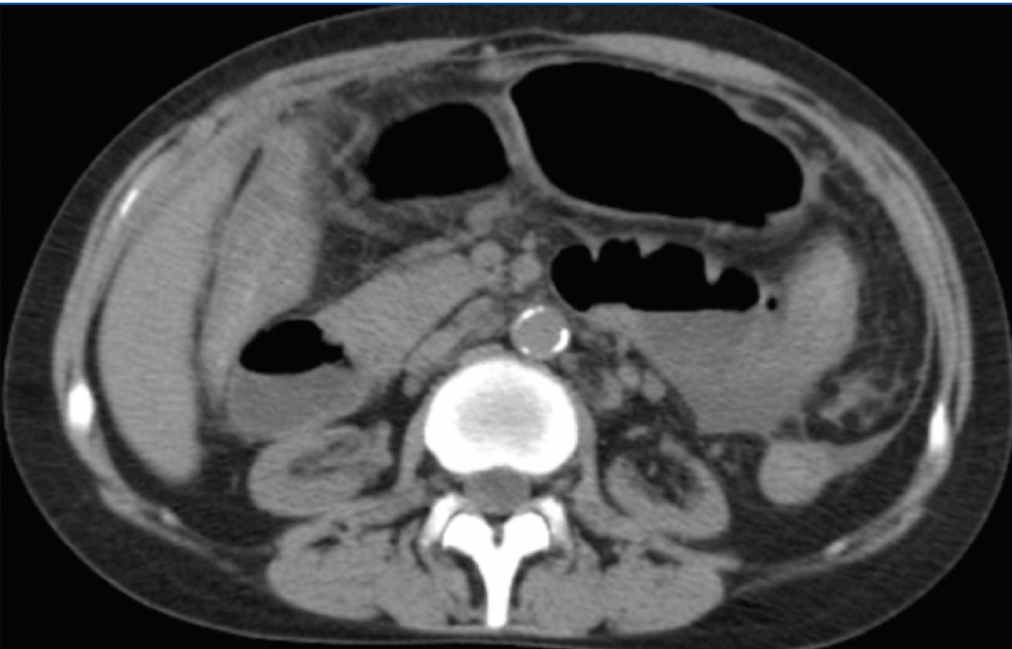
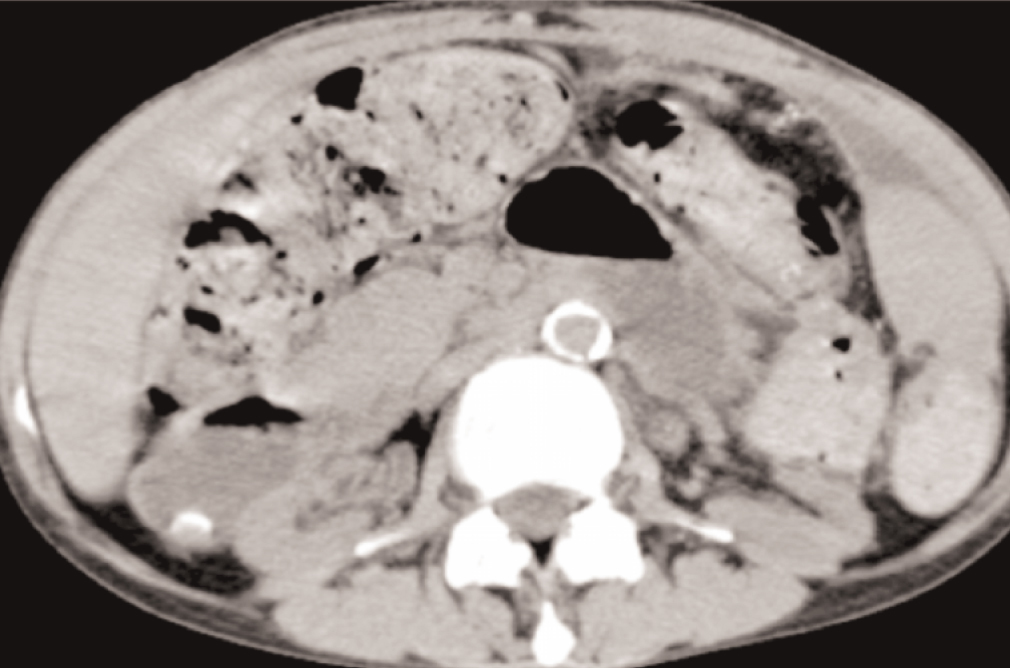
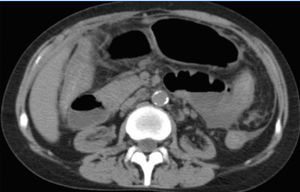
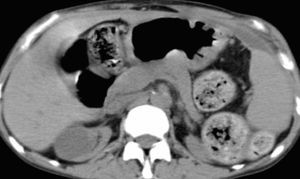
In spite of this, she maintained abdominal pain and distension, vomiting, diarrhea, severe protein malnutrition and emaciation. The radiological evaluation with computed tomography showed bowel wall thickening and enhancement, focal peritoneal calcification, bowel dilatation and loculated ascites (Figure 2 and Figure 3).
The laboratory findings reflected systemic inflammation (elevated reactive C protein) and electrolytes disturbances (particularly hypokalaemia).
In face of, the encapsulating peritonitis sclerosis diagnostic hypothesis was the more probable, nevertheless the absence of pathological confirmation.
In spite of the lack of consensus about pharmacological treatment we prescribed oral prednisone 20mg/day and tamoxifen (10mg/day) with institution of parenteral total nutrition.
After more than one month of hospitalization and symptom improvement she was discharged to home and extrahospitalar hemodialysis centre. On discharge date, her nutrition was composed by fluid diet and parenteral nutrition during hemodialysis session.
In December 2009, she presented with severe malnutrition (Mass Index Body of 13,6kg/m2), feeding intolerance and uncontrollable vomiting. She had discontinued the tamoxifen by gastrointestinal intolerance.
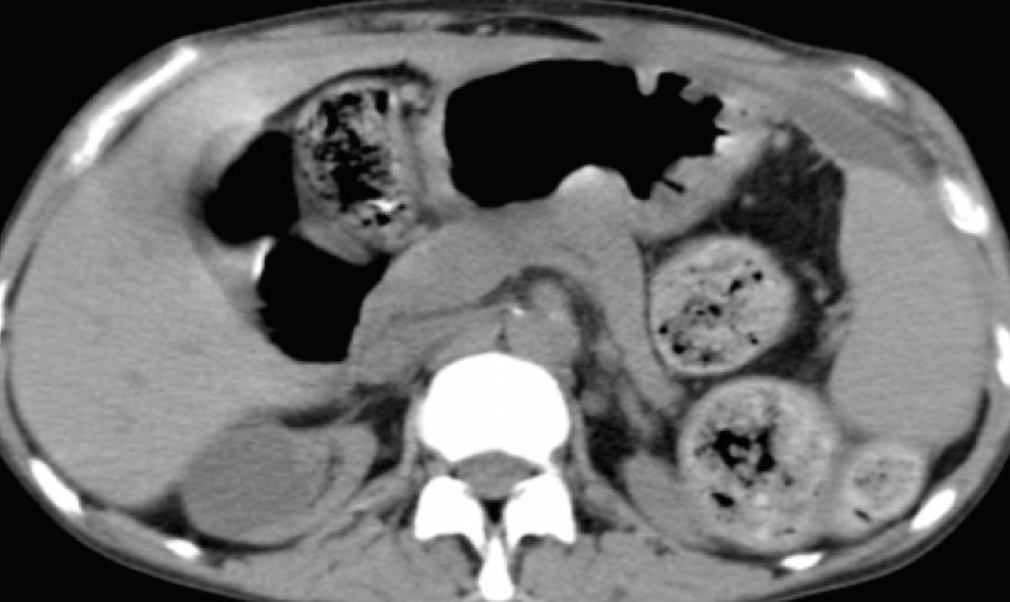
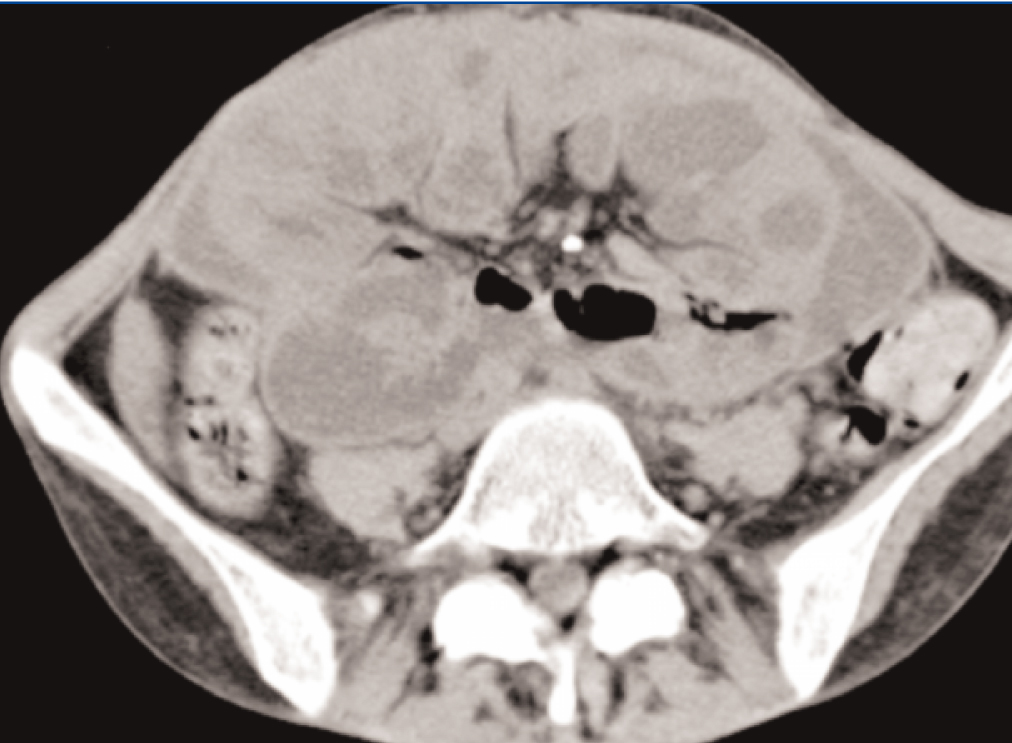
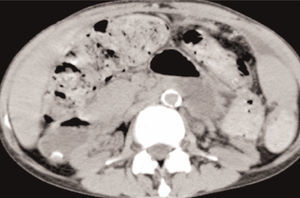
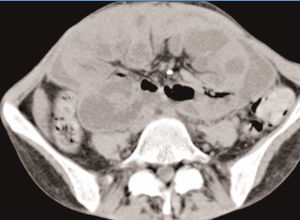
She was readmitted to the Hospital, submitted once again, to an Abdominal Computed Tomography, which showed similar findings to previous ones, with larger areas of loculated ascites (Figure 4 and Figure 5).
Two weeks after admission, she was submitted to surgical management with enterolysis conducted by an experient Surgeon. After this procedure, she began solid diet with progressive tolerance without abdominal pain.
She was discharged and her convalescence was short and without intercurrences.
In December 2011, she received an allograft from a deceased donor kidney allograft, which took place without major complications.
DISCUSSION
This is a successful clinical case of EPS, which is a poor prognostic entity.
In this case, we found factors that seem implicated in the development of EPS; that were: First, the duration of PD (5 years); second, the chronic use of “bioincompatibles” PD solutions.
The treatment of this entity implies supportive therapy to control the ileus (aggressive nutritional support), medical therapy (anti-fibrotic and anti-inflammatory drugs) and surgical interventional (partial or complete enterolysis without enterectomy).
The support care is the first step because the bowel obstruction causes intense malnutrition that implies early nutritional support with either total parenteral (TPN) or enteral nutrition5,6 and often time requires long-term nasogastric tube placement. In severely malnourished patients there is a role for TPN for 7 to 10 days before planned surgery.7
There have been no controlled trials of corticosteroid therapy in EPS patients. The timing of steroid use may at least partly explain variations in observed efficacy because corticosteroids would not be expected to treat established fibrosis.
Tamoxifen has been used as an antifibrotic in retroperitoneal fibrosis. It is a selective oestrogen receptor modulator (SERM), predominantly used in the treatment the breast cancer. Another characteristic of tamoxifen is itself activity on the profibrotic cytocine TGF-β and has been show to be effective in fibrotic diseases.8
The efficacy of tamoxifen in EPS has been described only in case series. Korte et al. make the largest study about efficacy of tamoxifen in EPS, from The Netherlands, between 1996 and 2007. Tamoxifen had been given to 24 patients and not used in 39 patients. The duration of tamoxifen use was for 4 weeks or more. Mortality in the tamoxifen group was 45,8% as compared with 74,4% in the untreated group (p 0.03), and multivariate Cox regression analysis confirmed a trend to better survival in the treated group.9
The surgical management should follow a trial of medical therapy. The time of surgery is thus as important and as yet unresolved factor. Mortality rate after surgery in the published literature has suffered variations; this reflects an increasing surgical expertise. The first experience suggested high mortality when enterectomy and bowel anastomosis was required, but recent reported improved outcome using enterolysis.10
CONCLUSION
Talking about prevention of EPS implies to know that the duration on PD is the only consistent risk factor. There are no prospective data demonstrating any benefit of preemptively switching long-term PD patients to HD. The incidence of EPS increases particularly after 5 or more years of the treatment, however the majority of long-term PD patients will not develop EPS.5 When considering switching patients from long-term PD to HD preemptively, it may appropriate to select those patients with potentially adverse features such ultrafiltration failure or low ultrafiltration capacity and requirement for high glucose containing concentration dialysate and recurrent episodes of peritonitis. The usually prescription and utilization of “biocompatible” PD fluids requires future randomized trials to support these practices.5
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 1. Potential biomarkers for monitoring membrane injury
Table 2. Diagnosis of encapsulating peritoneal sclerosis
Figure 2. Bowel dilatation, diffuse peritoneal enhancement
Figure 3. Focal peritoneal calcification
Figure 4. Diffuse peritoneal enhancement
Figure 5. Loculated ascites
Figure 1. Evolution over time of residual kidney function and ultrafiltration peritoneal membrane capacity