Regarding a rare complication secondary to the peritoneal dialysis (PD) technique, our team is sending you this letter to disclose an unusual clinical presentation among the clinicians.
This case report describes a 48-year-old man with end-stage-renal disease secondary to primary focal and segmental glomerulosclerosis on to PD since October 2022. The patient presented with a previous history of arterial hypertension, diabetes mellitus and hyperuricemia. One month after starting PD, he was admitted in the nephrology ward with malaise, fever and dry cough, which started four days before. Blood analysis showed elevated levels of inflammatory markers and blood cultures were positive for Enterococcus faecalis. Transthoracic echocardiography revealed multiple aortic vegetations in the right coronary cuspid with valvular perforation and severe aortic regurgitation which led to a diagnosis of infective bacterial endocarditis. After three weeks of intravenous ampicillin administration, the patient's condition deteriorated with acute pulmonary edema. He was transferred to the cardiac surgery department and underwent aortic valve replacement. Automated PD was re-started three days postoperatively with mild hematic effluent drainage. A computerized tomography (CT) excluded active intra-abdominal bleeding. Two weeks later, he presented with recrudescence of fever and worsening of inflammatory markers despite antibiotic therapy with Piperacillin/Tazobactam. At this time, blood cultures revealed Klebsiella oxytoca bacteriemia, a new contrast-enhanced CT scan showed mediastinal collections and a diagnosis of post-surgical mediastinitis was presumed.
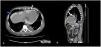
Regardless improvement of infectious parameters, the patient evolved with hypotension and peritoneal drainage with daily variable ultrafiltration volumes was observed. Echocardiography was performed revealing de novo pericardial effusion with signs of cardiac tamponade and, on day twenty-five after the surgery, the patient underwent evacuating pericardiocentesis. Evaluation of pericardial fluid for glucose revealed a concentration of 2000mg/dL, superimposed with that of peritoneal fluid, which led to a diagnosis of an iatrogenic peritoneal–pericardial leakage after pericardiocentesis. After two days of PD suspension the patient has restarted the technique, but extremely variable peritoneal ultrafiltration volumes persisted. Revaluating echocardiography revealed recrudescence of severe pericardial effusion. A CT peritoneography with iodinate contrast infusion through the peritoneum was performed and confirmed peritoneal–pericardial leakage (Fig. 1).
The pericardial effusion resolved with definitive PD suspension.
Peritoneal–pericardial leakage is a rare complication.1 Only 9 cases have been described in the literature.2 A history of cardiovascular interventions or multiple abdominal surgeries, which may be responsible for a breach; the presence of a congenital anomaly favoring tissue fragility with the occurrence of fistulas and hernias; history of malnutrition; long-term immunosuppression; previous peritonitis and excessive or too rapid increase in the volume of infusion represent the main risk factors for this condition.3 The clinical presentation varies from mild symptoms, such as cough and thoracalgia, to more severe clinical scenarios with dyspnea or hemodynamic instability in a cardiac tamponade setting.4
The presentation of our patient was exceptional, as he presented with variable ultra-filtration volumes with true ultrafiltration failure rarely observed. Biochemical analysis with glucose measurement of the pericardial fluid and radiologic investigations such as peritoneography with injection of iodinated contrast by CT or by magnetic resonance and peritoneal scintigraphy allow for a definitive diagnosis, as was the case of our patient.5
Therapeutic management varies from a conservative approach, in which a temporary suspension of the technique allows spontaneous resolution, to invasive procedures such as pericardiocentesis or thoracotomy for surgical repair.6 A definitive suspension of PD is sometimes required.1
Our case demonstrates the importance for a higher clinical suspicion compared to pleural–peritoneal leakage, especially if risk factors are present, as clinical presentation can be mild and ultrafiltration failure can be inapparent. The mediastinal collections and the hematic effluent could also represent early sings of diaphragmatic breaches that clinicians should be aware.
SupportNone.
Patient protectionsThe authors declare that they have obtained consent from the patient reported in this article for publication of the information about him that appears within this paper.
Financial disclosureThe authors declare that they have no relevant financial interests.