Hypouricemia may be caused by disorders leading to decreased UA production, oxidation of UA to allantoin by drugs or increased renal tubular loss of filtered UA, renal hypouricemia (RHUC). RHUC may be resulted from familial or acquired disorders. Familial RHUC cases are classified according to the gene affected as type 1 (SLC22A12 gene) and type 2 (SLC2A9). Clinical importance of RHUC entity is mainly determined by emerging of acute kidney injury (AKI) after strenuous exercise and urolithiasis.
Case presentationHere, we report a case of RHUC with increased fractional excretion of uric acid value of more than 100%, serum uric acid level of nearly zero, and exercise-induced AKI episodes clinically and a new unpublished homozygous (biallelic) mutation of c.1419+2T>G (IVS11+2T>G) in the SLC2A9 gene genetically for the first time to our knowledge.
ConclusionClinicians should be aware of this rare entity defined as hereditary RHUC in order to provide long term renoprotection by advisements like simple precautions such as avoiding severe exercises.
Renal hypouricemia (RHUC) is defined as decreased serum uric acid (UA) level (less than 2mg/dL) due to severe renal uric acid loss with increased fractional uric acid excretion (FEUA).1 Majority of RHUC patients are asymptomatic and incidentally recognized.2 Clinical importance of RHUC is mainly determined by emerging of acute kidney injury (AKI) after strenuous exercise and urolithiasis.1 Long term renal outcome of these patients and factors affecting long term renal outcome are not known clearly.
Familial RHUC is a rare autosomal recessively transmitted genetic disorder mostly with variable mutations. So the diagnosis of familial RHUC is confirmed by detection of genetic mutations. In emergence of familial RHUC entity, mainly two types of genes with many mutations were considered. Type 1 familial RHUC is caused by the mutations in the urate anion exchanger 1 (URAT1) coding gene named as SLC22A12 gene and type 2 familial RHUC is caused by mutations in the glucose transporter 9 (GLUT9) coding gene named as SLC2A9 (located on 4p16.1). The mutations in these genes lead to defective renal proximal tubular uric acid transport through both decreased reabsorption and increased secretion.2
Here, we report a case of familial RHUC with exercise induced AKI clinically and a new unpublished homozygous (biallelic) mutation of c.1419+2T>G (IVS11+2T>G) in the SLC2A9 gene genetically for the first time to our knowledge.
Case presentationA 47-year-old male patient applied to our clinic with nausea, vomiting and history of two recurrent episodes of acute kidney injury (AKI) after exercise (12h of working at garden) during the years between 2014 and 2017. His family history revealed that parents were cousins of first degree. There was no history of medication (such as probenecid, sulfinpyrazon, enalapril, losartan, amlodipine, fenofibrate, atorvastatin, trimetoprim-sulfamathoxazole).
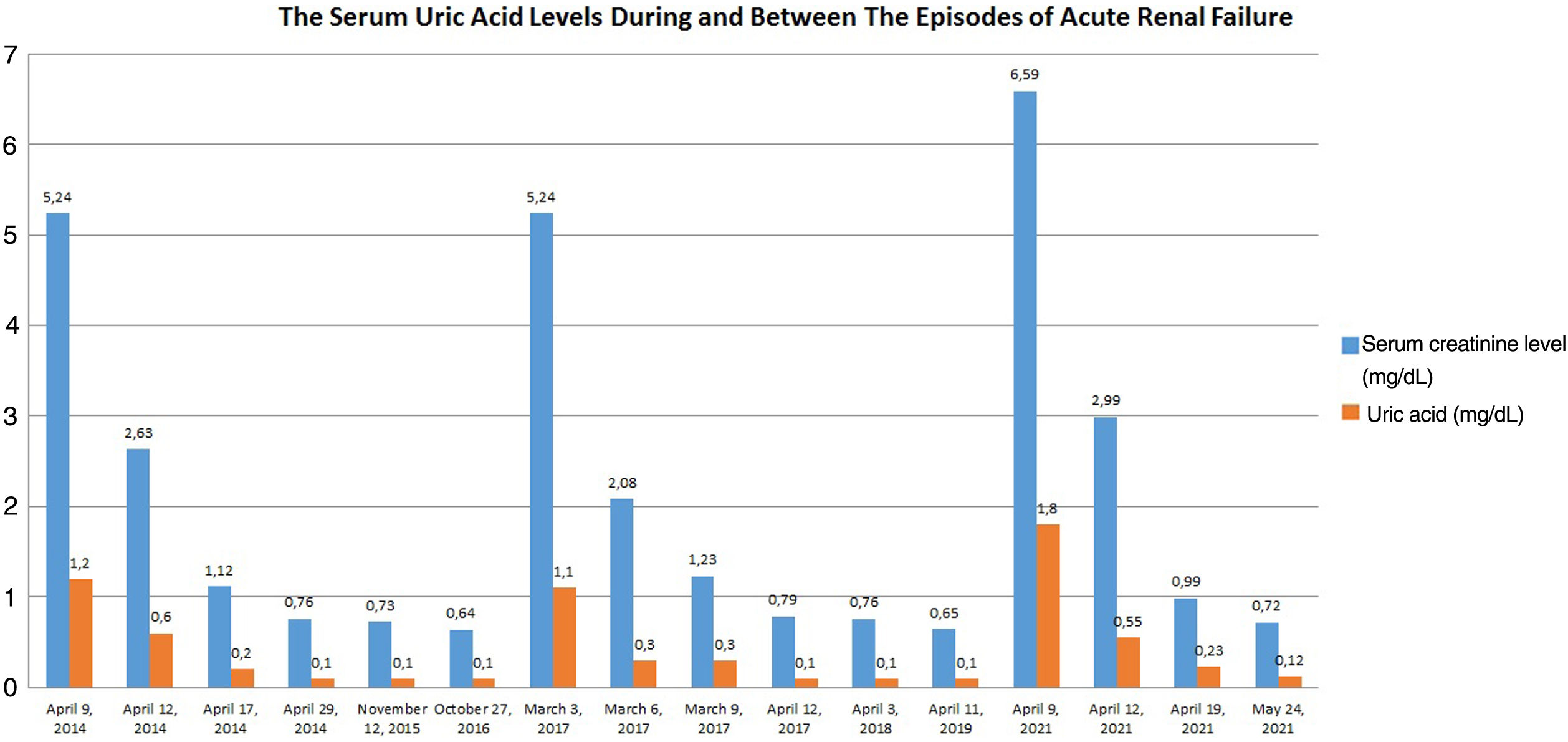
His blood pressure was 100/60mmHg. Body mass index was 31kg/m2. No other pathology was detected by physical examination. Biochemically his serum creatinine level was 6.59mg/dL and serum uric acid level was 1.8mg/dL. Ultrasonography revealed normal kidneys in size and echogenicity. Serum uric acid levels of the patient was noticed to be lower than normal (1.8mg/dL) although estimated glomerular filtration rate (eGFR) using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI creatinine) was severely decreased (9mL/min/1.73m2) due to acute kidney injury. Fractional uric acid (urate) excretion (FEUA) calculated via 24-hour urine collection was found to be inappropriately higher (Table 1). FEUA was calculated as 142% by the formula as follows: FEUA=100×(Urine urate×Plasma Cr)/(Plasma urate×Urine Cr).3
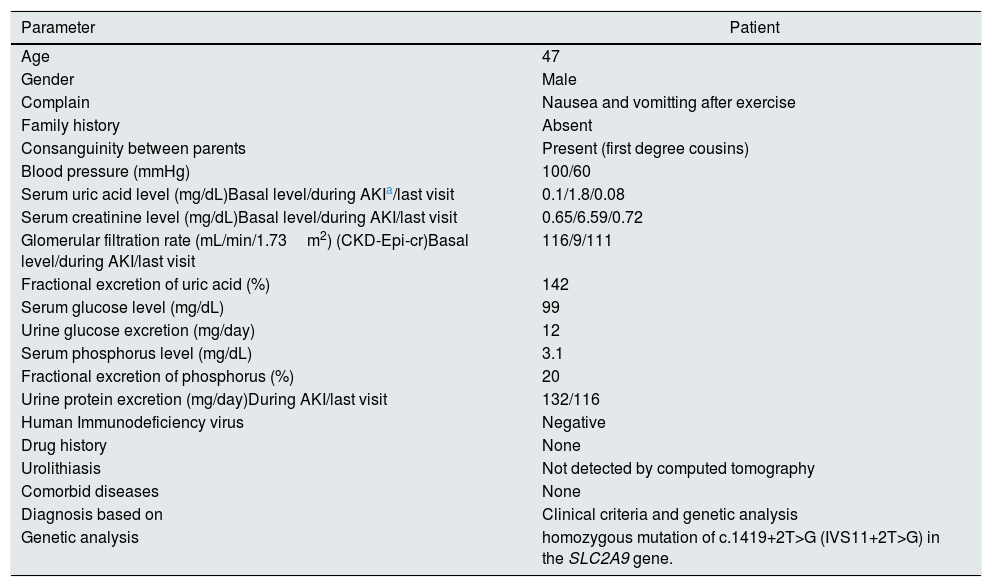
Clinical and laboratory findings of the patient.
| Parameter | Patient |
|---|---|
| Age | 47 |
| Gender | Male |
| Complain | Nausea and vomitting after exercise |
| Family history | Absent |
| Consanguinity between parents | Present (first degree cousins) |
| Blood pressure (mmHg) | 100/60 |
| Serum uric acid level (mg/dL)Basal level/during AKIa/last visit | 0.1/1.8/0.08 |
| Serum creatinine level (mg/dL)Basal level/during AKI/last visit | 0.65/6.59/0.72 |
| Glomerular filtration rate (mL/min/1.73m2) (CKD-Epi-cr)Basal level/during AKI/last visit | 116/9/111 |
| Fractional excretion of uric acid (%) | 142 |
| Serum glucose level (mg/dL) | 99 |
| Urine glucose excretion (mg/day) | 12 |
| Serum phosphorus level (mg/dL) | 3.1 |
| Fractional excretion of phosphorus (%) | 20 |
| Urine protein excretion (mg/day)During AKI/last visit | 132/116 |
| Human Immunodeficiency virus | Negative |
| Drug history | None |
| Urolithiasis | Not detected by computed tomography |
| Comorbid diseases | None |
| Diagnosis based on | Clinical criteria and genetic analysis |
| Genetic analysis | homozygous mutation of c.1419+2T>G (IVS11+2T>G) in the SLC2A9 gene. |
Clinical diagnostic criteria of RHUC was defined as serum uric acid levels less than 2mg/dL and in the mean time increased FEUA>11%.3 Confirmatory genetic analysis had been performed and identified a novel homozygous (biallelic) mutation of c.1419+2T>G (IVS11+2T>G) in the SLC2A9 gene. Computed tomography did not detect renal or ureteral nephrolithiasis. Serum phosphorus levels were normal (3.1mg/dL). Glycosuria was not detected in complete urine analysis and the glucose level in a 24-h urine sample was found to be 12mg/day (normal, <0.5g/day).4
After bed rest and fluid resuscitation had been given, serum creatinine level of the patient decreased to 0.72mg/dL and serum uric acid level was undetectable (Fig. 1). Restriction of heavy exercise was repeatedly advised and the patient was discharged.
DiscussionHypouricemia may be caused by hereditary (xanthine oxidase deficiency) or acquired (inhibitor of xanthine oxidase) disorders leading to decreased UA production, oxidation of UA to allantoin by drugs (rasburicase) or increased renal tubular loss of filtered UA.5 RHUC was first reported in Japan in 1975 and mostly seen among Japanese with a prevalence of 0.3%.6 Although most of these patients are Asians, cases also have been reported from many countries including Spain.7 A few RHUC cases were published in national journals of Turkey, but large scale epidemiological survey searching RHUC prevalence is absent.8
RHUC may be caused by familial or acquired disorders [such as Fanconi syndrome (cystinosis, multiple myeloma), volume expansion, acquired immunodeficiency syndrome, intracranial diseases, pregnancy, uricosuric drugs (probenecid, trimetoprim-sulfamathoxazole, etc.), amanita phalloides poisoning].5 Clinically, absence of renal glycosuria, and normal serum phosphorus levels without renal phosphorus loss made us ruled out RHUC due to Fanconi syndrome. Patients with low serum uric acids due to increased renal excretion should be screened for uricosuric drug history such as probenecid, trimethoprim-sulfamethoxasole, high-dose salicylate, fenofibrate, losartan. Intake of these drugs was absent in our patient's history. So, these acquired disorders could not have been identified in our patient.
Two types of genetic defects in tubular UA transporters were described for familial RHUC cases. Mutations either on chromosome 11q13 coding URAT1, or chromosome 4p15.3-p16 coding GLUT9 were described.5,7 Homozygous mutations for URAT1 mostly lead to serum uric acid levels of less than 1mg/dL, FEUA of 40–90% in affected patients and more often nephrolithiasis episodes than general population. On the contrary less common exercise-induced AKI cases were seen in cases with the genetic mutations in the gene coding URAT1.5 While patients with homozygous mutations in the gene coding GLUT9 present with serum uric acid levels close to zero with FEUA of 100% and high incidence of exercise-induced AKI and renal calculi.5 Serum uric acid levels of our patient were 0.1–0.3mg/dL and FEUA level was 142%. He had recurrent episodes of AKI with exercise without renal calculi. Based on these results, our patient's findings were found to be consistent with the phenotypic features of mutations in the gene coding GLUT9. The presence of increased FEUA value of more than 100%, serum UA level of nearly zero, and exercise-induced AKI episodes makes us think that this novel mutation (c.1419+2T>G (IVS11+2T>G) in GLUT9 coding gene (SLC2A9) of our case is pathogenic. Although many mutations regarding GLUT9 coding gene (SLC2A9) had been reported, most of them were found as benign, at the present time, only 12 pathogenic mutations were identified as far as we know.9
Even though definition of RHUC comprises serum uric acid levels <2mg/dL with FEUA>10%, it might be difficult to notice RHUC cases based on this criteria when their renal functions deteriorated with low GFR due to repetitive exercise induced AKI episodes.10 Long term renal and patient survival of these patients are not known clearly. Clinicians should be aware of this rare entity defined as hereditary RHUC in order to provide long term renoprotection by advisements like simple precautions such as avoiding severe exercises.
In conclusion, prevalences of renal hypouricemia among each countries and implications of genetical mutations on clinical course, long term renal and patient prognosis should be searched thoroughly in order to diagnose and treat appropriately affected individuals before irreversible renal injuries occur.
Authors’ contributionsAll authors have read and agreed to the final manuscript. Kubra Kaynar participated in conception, design, interpretation of data of the patient as well as drafting the article and revising it; Beyhan Güvercin contributed in design, interpretation of data of the patient, drafting the article. Mustafa Şahin: reviewed the literature and participated in writing the manuscript. Nilay Turan collected the patient's data, reviewed the literature, Ferhat Açıkyürek collected the patient's data.
Informed consentWritten informed consent for publication of this case report was taken from the patient.
Compliance with ethical standardsInformed consent was obtained from the patient.
Conflicts of interestAll of the authors declare no conflict of interests. No fund was taken.