Native arteriovenous fistulas (nAVF) are the vascular access of choice in patients with stage 5 chronic kidney disease on dialysis (CKD 5D). However, some studies report maturation failure rates ranging from 20% to 50%.1 According to the latest data from the Registre de Malalts Renals de Catalunya (RMRC) [Catalan Registry of Renal Patients],2 the rate of use of nAVF has reduced (55.8%) and only 36.1% of incidents in 2020 involved this type of vascular access.
From a pathophysiological point of view, low shear stress has been associated with increased stress in the walls of the efferent vein, turbulent flow and the generation of accelerated venous neointimal hyperplasia as the main cause of juxta-anastomotic stenosis. These mechanisms are related to the release of a host of inflammatory factors that induce platelet aggregation, migration of myofibroblasts from the media to the intima layer and, ultimately, thrombosis and loss of vascular access.3
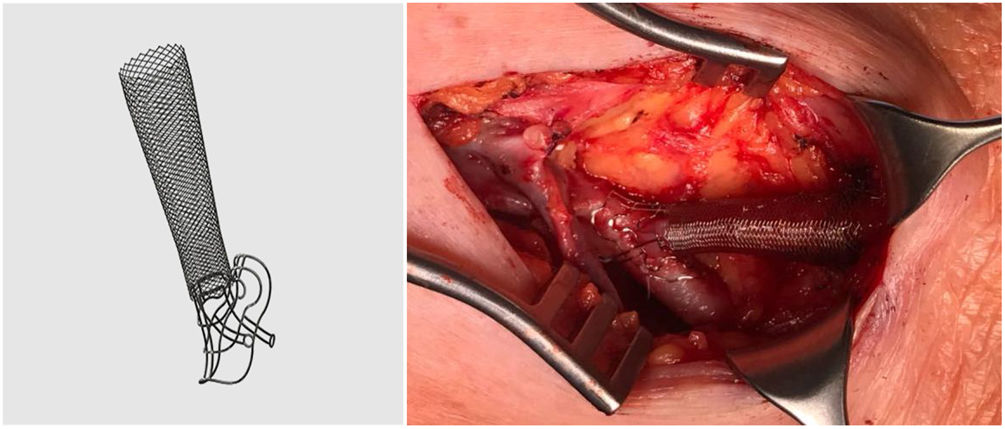
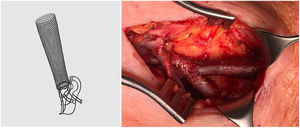
The VasQ™ external device (Laminate Ltd., Tel Aviv, Israel) was developed with these problems in mind. It consists of two parts, a neck and arm, made of nitinol, and is implanted by the vascular surgeon at the surgical anastomosis and outside the first portion of the efferent vein (Fig. 1). This device enables the angle at the surgical anastomosis to be increased to 40−50°, resulting in a more uniform or laminar vascular flow and less stress at the juxta-anastomotic area of the vein (conical arrangement), as well as having a possible protective effect in terms of cardiac haemodynamic factors (cardiac output).4,5Theoretically, this new device should reduce the development of stenosis in the first portion of the vein, improving patency rates in nAVF.
In the medical literature, there are few studies and all with small sample sizes.6–8 To our knowledge, we are the first national centre in Spain to use this new vascular device and evaluate its results.
Our main objective was to evaluate our experience of implanting the VasQ™ device in the maturation of nAVF in a subgroup of patients (2018–2019). Sociodemographic, ultrasound and revascularisation procedure data were analysed. For the statistical analysis, descriptive techniques were used: Chi2 test for proportions and t-test for continuous variables. Kaplan-Meier survival curves were used to calculate primary patency (PP) and secondary patency (SP), and Cox regression analysis for subgroup comparison, using the SPSS® v. 21 statistical package. Significance was considered if p ≤ 0.05. For the study of clinical and ultrasound maturation, the standards of the current Spanish vascular access guidelines were used.9
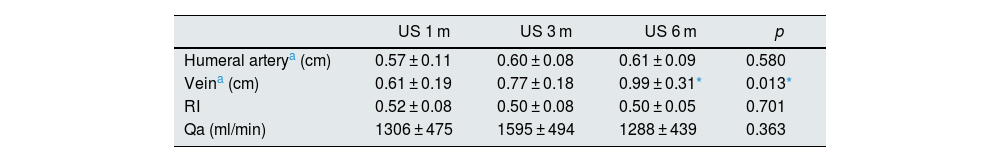
A total of 21 patients with 5D CKD were included (six female and 15 male), with a mean age of 69.8 ± 13.2 years (26–85 years), nine with radiocephalic and 12 with humerocephalic nAVF over which the VasQ™ device was implanted. Adequate clinical and ultrasound maturation rates were achieved in 95.2% of patients. There were no surgical complications of note or significant differences in the location of the nAVF (distal vs proximal). Preoperative mapping showed a mean feeding artery of 0.31 ± 0.16 cm and vein of 0.31 ± 0.15 cm. Table 1 shows the different ultrasound parameters during the first six months of the follow-up period. As can be seen, there was a significant increase in the diameter of the efferent vein between months one and six (0.61 ± 0.19 vs 0.99 ± 0.31 cm; p = 0.002). The PP rates obtained at 1, 3, 6 and 12 months were 95.2%, 90.5%, 71.4% and 52.4%, respectively, and the SP rates at the same times points were 95.2%, 90.5%, 85.7% and 83%, respectively. A total of 20 balloon-catheter percutaneous transluminal angioplasties (PTA) were performed during the follow-up period, although 14 of these procedures were carried out on just three of the patients. We found no significant differences in patency when comparing these results with a similar small retrospective cohort with isometric exercises (30 patients). The six-month PP and SP rates reported by other VasQ™ device studies were similar to those obtained by our group (PP 79–87.5% and SP 79–100%).6–8 The main limitation of these investigations lies in the inclusion of few patients (<35), as well as the lack of randomisation with a control group based on conventional surgery, which makes interpretation of the results difficult.
Changing ultrasound parameters after VasQ™ device (Laminate Ltd., Tel Aviv, Israel) implantation.
| US 1 m | US 3 m | US 6 m | p | |
|---|---|---|---|---|
| Humeral arterya (cm) | 0.57 ± 0.11 | 0.60 ± 0.08 | 0.61 ± 0.09 | 0.580 |
| Veina (cm) | 0.61 ± 0.19 | 0.77 ± 0.18 | 0.99 ± 0.31* | 0.013* |
| RI | 0.52 ± 0.08 | 0.50 ± 0.08 | 0.50 ± 0.05 | 0.701 |
| Qa (ml/min) | 1306 ± 475 | 1595 ± 494 | 1288 ± 439 | 0.363 |
RI: estimated resistance index in the humeral artery; Qa: estimated vascular flow in the humeral artery.
Based on our preliminary data, we conclude that the VasQ™ device is useful and safe, providing adequate maturation and good patency rates in nAVF. However, we are designing a prospective, randomised study in patients with radiocephalic (distal) nAVF in order to assess whether or not this new device represents a cost-effective advance in the field of vascular access.