Podocalyxin is an electronegative sialoglycoprotein that prevents the podocyte foot process from collapsing. The aim of this study was to detect an association between the glomerular immunohistochemical (IHC) expression of podocalyxin and the degree of podocyte effacement detected by electron microscopy, and to evaluate the role of podocalyxin IHC expression as a novel marker for disease activity in lupus nephritis (LN).
MethodsThirty-two renal biopsies of active lupus nephritis patients were studied. Clinical assessment by the systemic lupus activity measure (SLAM-R) score and laboratory data were included [serum creatinine, 24-h urinary protein, antinuclear antibodies (ANA), anti-double-strand DNA antibodies (anti-dsDNA), C3 and C4]. Light (L/M) and electron microscopic (E/M) examination was conducted. Podocyte loss was evaluated by immunohistochemistry with monoclonal anti-podocalyxin antibodies by means of a semiquantitative score that was graded from 0 to 4+ according to the percentage of glomerular involvement.
Results22 cases (68.8%) with LN class IV, 6 (18.8%) with class III and 4 (12.5%) with class V. The mean age was (25.41±10.13) years. There was a significant negative correlation between IHC podocalyxin score and LN class, and NIH activity parameters such as leukocyte infiltration, endocapillary proliferation, fibrinoid necrosis and cellular crescent and disease activity index but not chronicity index. There was a highly significant negative correlation between IHC podocalyxin and podocyte effacement by E/M (rs=−0.903, P=0.000), and E/M immune deposits (r=−0.53, P=0.001), and a significant association with degree of proteinuria, ANA and SLAM score (P<0.05).
ConclusionsPodocyte loss indicated by podocalyxin immunohistochemical expression reflects the degree of activity and severity of LN and the degree of podocyte effacement by E/M.
La podocalixina es una sialoglicoproteína electronegativa que evita el colapso del proceso podocitario. Nuestro objetivo fue detectar una asociación entre la expresión inmunohistoquímica (IHQ) glomerular de la podocalixina y el grado de borramiento podocitario detectado mediante microscopia electrónica, además de evaluar la función de la expresión IHQ de la podocalixina como un nuevo marcador de la actividad de la enfermedad en la nefritis lúpica (NL).
MétodosSe evaluaron 32 biopsias renales de pacientes con NL activa. Se incluyeron la evaluación clínica mediante la puntuación de la determinación de la actividad del lupus sistémico (systemic lupus activity measure, SLAM-R) y datos analíticos (creatinina sérica, proteína en la orina de 24h, anticuerpos antinucleares [AAN], anticuerpos anti-ADN de doble cadena [anti-ADNdc], C3 y C4). Evaluación mediante microscopio de luz (M/L) y microscopio electrónico (M/E). La evaluación de la pérdida podocitaria se realizó mediante inmunohistoquímica con anticuerpos antipodocalixina monoclonales, por medio de una puntuación semicuantitativa que se clasificó de 0 a 4+ en función del porcentaje de afectación glomerular.
ResultadosEncontramos 22 (68,8%) casos con clase iv de NL, 6 (18,8%) con clase iii y 4 (12,5%) con clase v. La media de edad fue de 25,41±10,13 años. Se observó una asociación negativa significativa entre la puntuación de la podocalixina en la IHQ con la clase de NL y los parámetros de actividad del NIH, como la infiltración leucocitaria, la proliferación endocapilar, la necrosis fibrinoide y los drepanocitos y el índice de actividad de la enfermedad, pero no el índice de cronicidad. Se observó una correlación negativa muy significativa entre la podocalixina en la IHQ y el borramiento podocitario mediante M/E (rs=−0,903; p=0,000), depósitos inmunes mediante M/E (r=−0,53; p=0,001) y una asociación significativa con el grado de proteinuria, AAN y puntuación en el índice SLAM (p<0,05).
ConclusionesLa pérdida podocitaria indicada mediante la expresión IQH de la podocalixina refleja el grado de actividad y la intensidad de la NL, así como el grado de borramiento podocitario mediante M/E.
Lupus nephritis (LN) represents a major serious manifestation in systemic lupus erythematosus (SLE) as it is associated with significant morbidity and mortality.1 The pathogenesis of SLE includes a deregulation of humoral and cellular immune activity, reflected in a broad production of autoantibodies, cytokines, and tissue damage. Among the nephrogenic autoantibodies are anti-dsDNA, anti-nucleosomes, and autoantibodies to laminin and collagen IV, which induce renal lesions by the in situ formation of immune complexes that cause complement activation.2 Renal injury patterns in SLE including mesangial, endothelial, epithelial (podocytes), tubulointerstitial and vascular involvement, were associated with different pathogeneses, clinical presentations and outcomes. Although the 2004 International Society of Nephrology and Renal Pathology Society (ISN/RPS) LN classification has been widely used internationally,3 there still exist some intriguing controversies, such as podocyte damage in different LN classes. Podocytes are highly differentiated glomerular epithelial cells that line the outside of the glomerular capillary with foot processes linked to the glomerular basement membrane with their actin cytoskeleton. The foot processes form a characteristic inter-digitating pattern leaving filtration slits in-between. Integrity of this filtration barrier is important in order to prevent the loss of protein into the urine.4 There are different mechanisms of Podocyte injury In SLE including antibody-mediated, cell-mediated, and immune mediated podocyte injury.5 Many studies focused about lupus podocytopathy in mesangial LN, which indicated that the overt proteinuria occurring in LN might involve pathways related to podocyte injury other than classical immune complex deposition.6,7 Furthermore, The podocyte effacement was obvious in most LN patients and the degree of foot process fusion varied within the different pathological classes of LN,8 that may indicates that renal podocyte damage might also contribute to the pathogenesis, activity and progression of other types of LN, which may needs further studies. Podocyte injury is difficult to be evaluated by routine hematoxylin and eosin (H&E) method, that's why many studies assessed podocyte injury by the degree of podocyte loss in urine, and renal biopsy specimens by use of immunohistochemistry. Markers used to detect podocytes in renal tissue include CD10, podocalyxin, synaptopodin, glomerular epithelial protein 1, podocin, CD2AP, nephrin, and Wilms tumor antigen I (WT1).9,10 Podocyte effacement by the electron microscopy is due to rearrangement of the podocyte actin cytoskeleton leads to a change in podocyte shape and consequently function.7
The main podocyte surface antigen podocalyxin is a highly electronegative sialoglycoprotein normally located at the apical part of podocyte foot processes prevents the podocyte from collapsing because of its high negative charge and absence of this protein was reported to have detrimental effect on glomerular filtration function.11,12
D’Agati described activity and chronicity indices as a histologic grading system of LN,13 but didn’t include the degree of podocyte injury or effacement although podocytes loss seems to be a new marker for glomerular disease activity or adverse prognosis.
We aimed to detect an association between glomerular immunohistochemical (IHC) expression of podocalyxin and the degree of podocyte effacement detected by electron microscopy and to evaluate the role of IHC expression of podocalyxin as a marker for pathological and clinical activity in lupus nephritis.
Material and methodsThis is a cross sectional study was conducted on 32 randomly selected patients with renal biopsies of active LN recruited from Ain shams specialized hospital, Cairo, Egypt. Clinical and laboratory data were collected included: age, gender, blood pressure, serum creatinine, degree of proteinuria (nephrotic range proteinuria ≥3.5g/d, sub nephrotic range <3.5g/d and normal up to 150mg/d) by 24h urinary protein, urine analysis, serological tests for antinuclear antibodies (ANA), anti-double strand DNA antibodies (anti-dsDNA), C3 and C4. Systemic Lupus Activity Measure revised (SLAM-R) was measured for assessment the clinical disease activity of SLE (0–3=mild, 4–7=moderate, >7=severe).14 Light and electron microscopic evaluation for renal biopsy specimens were performed. The majority of cases were the first time biopsied for diagnosis and classification. Two cores of renal biopsies were received for each patient. Inclusion criteria: renal biopsy specimens with minimum of 10 glomeruli according to Weening et al.3 Class I, II and VI are excluded as our study is concerned with active lupus nephritis.
Light microscopyThe specimens were fixed in 10% neutral formalin, routinely processed and embedded in paraffin blocks, from which 3 micrometer (μm) thick serial sections were cut and stained with H&E, PAS “periodic acid Schiff” and Masson's trichrome. Sections examined were evaluated according to the ISN/RPS classification.3 Activity and chronicity indices were evaluated according to NIH scoring system.15,16
Electron microscopySpecimens were fixed in formaldehyde glutaraldehyde mixture pH 7.4 in cacodylate buffer, then embedded in Spurr's resin. Semithin sections, one micron thick were obtained by ultramicrotome using glass and/or diamond knife and stained with 1% toluidine blue and examined by light microscopy. Ultrathin sections (60–80nm thick), were cut from selected blocks then double stained with uranyl acetate and lead citrate. Examination was done by Philips-400 T transmission electron microscope at 80kV. The prints were examined for detection of changes in the mesangium, electron dense deposits (mesangial, subendothelial, intramembranous and subepithelial), epithelial cell changes (podocyte effacement) The electron dense deposits were graded as 1–3+ (minimum, moderate and massive) in accordance with their number and extension. Grade 1 was defined as one or two very small deposits, grade 2 as several small and large non-fused deposits and grade 3 as abundant amount of fused deposits. Most of them occupied a large part of the entire circumference of a capillary loop or most of the mesangial matrix.17 The amount of podocyte effacement (defined as the percentage of total glomerular capillary surface area over which the podocyte foot processes were effaced) was graded as 1+ with involvement of <25%, 2+ with involvement of 25–50%, and 3+ with involvement of >50% of glomerular capillary surface area.18
Immunofluorescence studyDirect immunofluorescence staining for IgA, IgG, IgM and C3 was done on frozen sections. The intensity of staining was graded as mild, moderated, and severe (1–3+).
Immunohistochemical studyPodocalyxin like antibody was used as podocyte/Apical marker. The primary antibody was rabbit polyclonal antibody, podocalyxin (NBP1-83348), concentrate antibody (commercial house: Novus Biologicals), Country of origin: USA. Podocalyxin immunohistochemical expression in renal glomeruli of normal kidney tissue, acquired from a case of renal cell carcinoma, was used as positive control for podocalyxin. Evaluation of immunohistochemically expression was done using modified method of Wang et al.19
A semi-quantitative score was graded from 0 to 4+ according to the percentage of total number of glomeruli involved in the renal biopsy specimen. The grade score was set as 0=0% when all glomeruli are negative for podoclyxin immunohistochemical expression, 1+=1–25% of glomeruli demonstrate mild to moderate segmental immunostaining, 2+=26–50% of glomeruli demonstrate moderate segmental and/or global immunostaining, 3+=51–75% of glomeruli demonstrate moderate to marked segmental and/or global immunostaining and 4+=76–100% of glomeruli demonstrate moderate to marked segmental and/or global immunostaining.19
Statistical analysisStatistical analysis was performed using SPSS version 20.0 for windows (IBM, USA). Categorical variables were presented as counts and percentages. Continuous variables were presented as mean±standard deviation or median (range) as indicated. Chi-square test for association and Spearman's correlation test were used as indicated. A two-sided P<0.05 was considered statistically significant.
ResultsThis study included thirty Two randomly selected patients with a clinical diagnosis of LN indicated for biopsy to determine the activity and chronicity indices of lupus nephritis, 6 (18.8%) of them were males and 26 (81.2%) were females, with The mean of age at presentation was (25.41±10.13) years. Basic demographics for the study population are shown in Table 1.
Basic demographics.
| Age (year) | 25.41±10.13 |
| Gender | 6 males (18.8%) |
| 26 females (81.2%) | |
| Blood pressure | |
| Systolic BP | 125±16.84 |
| Diastolic BP | 83.43±13.10 |
| Serum creatinine | 1.291±1.0418 |
| Proteinuria | |
| Normal range | 7 (21.9%) |
| Subnephrotic range | 12 (37.5%) |
| Nephrotic range | 13 (40.6%) |
| ANA | 27 (84.4%) |
| Anti-dsDNA | 29 (90.6%) |
| C3 | 53.63±22.023 |
| C4 | 10.38±5.399 |
| SLAM-R score | |
| Mild | 6 (18.75%) |
| Moderate | 10 (31.25%) |
| Severe | 16 (50%) |
| ISN/RPS lupus class | |
| III | 6 (18.8%) |
| IV | 22 (68.8%) |
| V | 4 (12.5%) |
Categorical data are presented as number (frequency); numerical data are presented as mean±standard deviation or median (range) as indicated.
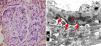
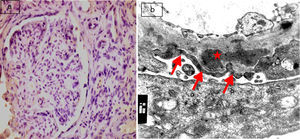
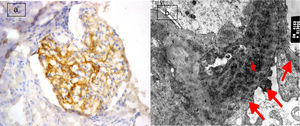
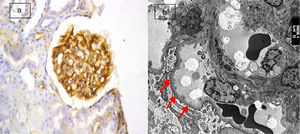
The most frequent class of LN in this study was found to be class IV (68.8%) with 22 cases, followed by class III (18.8%) with 6 cases. The least frequent class was class V (12.5%), consisting of 4 cases. Semiquantitative score of IHC podocalyxin was 0 in 4 (12.5%), +1 in 8 (25%), +2 in 7 (21.9%), +3 in 8 (25%) and +4 in 5 (15.6%) of the total cases. The degree of glomerular immunohistochemical expression of podocalyxin in different classes of LN was shown in Table 2 as number of cases with 0 expression of podocalyxin (Fig. 1a) was more in class V LN cases and number of cases with +4 IHC podocalyxin expression (Fig. 3a) was more in class III LN cases. Focal segmental immunostaining for podocalyxin 2+ was shown in (Fig. 2a). There was significant negative association between IHC podocalyxin score and LN class (r=−0.701, P=0.000). L/M activity and chronicity parameters were shown in Table 3. In our study; there was significant negative correlation between IHC podocalyxin score and activity parameters as leukocyte infiltration (r=−0.489, P=0.005) endocapillary proliferation (r=−0.473, P=0.006), fibrinoid necrosis (r=−0.351, P=0.049) and cellular crescent (r=−0.444, P=0.011) (P<0.05) but non-significant correlation with presence of wire loop or mononuclear cell infiltration (P>0.05).
Degree of glomerular immunohistochemical expression of podocalyxin in different classes of lupus nephritis.
| Semi-quantitative IHC podocalyxin scorea | Total | |||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | ||
| Lupus ISN class | ||||||
| Lupus class III | 0 | 0 | 0 | 3 | 3 | 6 |
| Lupus class IV | 1 | 7 | 7 | 5 | 2 | 22 |
| Lupus class V | 3 | 1 | 0 | 0 | 0 | 4 |
| Total | 4 | 8 | 7 | 8 | 5 | 32 |
Light microscopy activity and chronicity parameters of lupus nephritis.
| L/M activity parameters | N (%) | L/M chronicity parameters | N (%) |
|---|---|---|---|
| Endocapillary proliferation | Glomerular sclerosis | ||
| Mild | 7 (21.9%) | Nil | 11 (34.4%) |
| Moderate | 14 (43.8%) | Mild | 16 (50.0%) |
| Severe | 11 (34.4%) | Moderate | 3 (9.4%) |
| Severe | 2 (6.3%) | ||
| Fibrinoid necrosis | Fibro cellular crescent | ||
| Nil | 7 (21.9%) | Nil | 17 (53.1%) |
| Mild | 17 (53.1%) | Mild | 13 (40.6%) |
| Moderate | 5 (15.6%) | Moderate | 2 (6.3%) |
| Severe | 3 (9.4%) | ||
| Cellular crescent | Tubular atrophy | ||
| Nil | 16 (50.0%) | Nil | 9 (28.1%) |
| Mild | 12 (37.5%) | Mild | 16 (50.0%) |
| Moderate | 1 (3.1%) | Moderate | 7 (21.9%) |
| Severe | 3 (9.4%) | ||
| Wire loop hyaline thrombi | Interstitial fibrosis | ||
| Nil | 1 (3.1%) | Nil | 8 (25.0%) |
| Mild | 19 (59.37%) | Mild | 16 (50.0%) |
| Moderate | 11 (34.4%) | Moderate | 8 (25.0%) |
| Severe | 1 (3.1%) | ||
| Leukocyte infiltration | Chronicity index (mean±SD) | 3.40±2.39 | |
| Nil | 1 (3.1%) | ||
| Mild | 13 (40.6%) | ||
| Moderate | 17 (53.1%) | ||
| Severe | 1 (3.1%) | ||
| Mononuclear cell infiltration | |||
| Nil | 4 (12.5%) | ||
| Mild | 15 (46.9%) | ||
| Moderate | 13 (40.6%) | ||
| Activity index (mean±SD) | 10.78±4.73 | ||
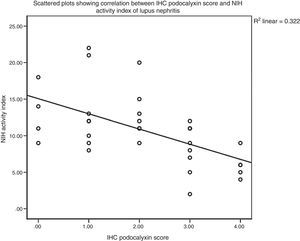
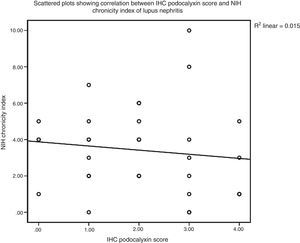
There was non-significant correlation between IHC podocalyxin score and L/M chronicity parameters of LN as glomerular sclerosis, presence of fibro cellular crescents, tubular atrophy and interstitial fibrosis (P>0.05). Finally there was a statistically significant negative correlation between IHC expression of podocalyxin and disease activity index in LN cases (rs=−0.609, P<0.0005) (Fig. 4). However, there was no statistical significant correlation with chronicity index (rs=−0.200, P=0.273) by Spearman's correlation test (Fig. 5). E/M podocyte effacement and immune deposits results were shown in Table 4. There was a Highly Significant negative correlation between IHC podocalyxin and podocyte effacement by EM (rs=−0.903, P=0.000) by Spearman's rho correlation test (Figs. 1–3b). Significant negative correlation between IHC podocalyxin score and E/M subepithelial immune deposits (r=−0.538, P=0.001) and intramembranous immune deposits (r=−0.541, P=0.001), no significant correlation with presence of mesangial or subendothelial immune deposits (P>0.05).
Electron microscopy studied parameters.
| E/M parameters | N (%) |
|---|---|
| Mesangial immune deposits | |
| Nil | 4 (12.5%) |
| Mild | 11 (34.4%) |
| Moderate | 13 (40.6%) |
| Severe | 4 (12.5%) |
| Sub endothelial immune deposits | |
| Nil | 1 (3.1%) |
| Mild | 5 (15.6%) |
| Moderate | 15 (46.9%) |
| Severe | 11 (34.4%) |
| Subepithelial immune deposits | |
| Nil | 22 (68.8%) |
| Mild | 6 (18.8%) |
| Moderate | 2 (6.3%) |
| Severe | 2 (6.3%) |
| Intramembranous immune deposits | |
| Nil | 28 (87.5%) |
| Mild | 2 (6.3%) |
| Moderate | 2 (6.3%) |
| Podocyte effacement | |
| No effacement | 2 (6.3%) |
| Mild | 11 (34.4%) |
| Moderate | 11 (34.4%) |
| Severe | 8 (25.0%) |
(a) Active lupus nephritis ISN/RPS class V (A), NIH activity index (12/24) NIH chronicity index (4/12) showing negative immunostaining for podocalyxin (original magnification ×400). (b) An electron micrograph for the same case showing marked diffuse fusion of podocyte foot processes (arrows) and subepithelial electron dense deposits (asterisks) (negative magnification ×22,000).
(a) Active lupus nephritis ISN/RPS class IV (A), NIH activity index (14/24) NIH chronicity index (4/12) showing focal segmental immunostaining for podocalyxin 2+ (left half of the glomerulus) with lost immunostaining in the right half of the glomerulus involved by leukocyte infiltration and endocapillary proliferation (original magnification ×400). (b) An electron micrograph for the same case showing marked diffuse fusion of podocyte foot processes (arrows) and subendothelial electron dense deposits (asterisks) (negative magnification ×22,000).
(a) Active lupus nephritis ISN/RPS class III (A), NIH activity index (4/24) NIH chronicity index (3/12) showing strong diffuse global immunostaining for podocalyxin 4+ (original magnification ×400). (b) An electron micrograph for the same case showing minimal fusion of podocyte foot processes (arrows) (negative magnification ×2800).
There was a statistically significant association between score for immune-histochemical expression of podocalyxin and degree of proteinuria as a clinical marker for lupus activity (χ2=17.611, P=0.024) by chi square test, with a strength of association as observed by Cramer's V=0.525, P=0.024 (P<0.05). No significant correlation between podocalyxin expression and serum creatinine (P>0.05). Significant statistical negative correlation between SLAM-R score of clinical disease activity and IHC podocalyxin score (rs=−0.709, P<0.000) by Spearman's correlation test.
Significant association between IHC podocalyxin and positive serum ANA (χ2=9.759, P=0.045) but no significant association with positive dsDNA, C3 or C4 (P>0.05) as laboratory markers of SLE by chi square test. There was negative significant correlation between IHC podocalyxin expression score and diastolic BP (r=−0.432, P=0.014), and non-significant with systolic BP (r=−0.342, P=0.055), age or gender (P>0.05).
DiscussionIn this study, there was a significant negative association between IHC podocalyxin score and LN class. We observed that all cases of class III LN showed high immunoexpression for podocalyxin (3+ and 4+) denoting minimal podocyte loss in this class, while in class IV, 15 cases showed negative to low expression (0–2+) indicating severe podocyte loss. This could be attributed to the percentage of glomeruli affected, as glomerular lesions including podocyte detachment in focal proliferative LN (class III) affect less than 50% while in diffuse proliferative LN (class IV); more than 50% of glomeruli are affected.
Podocytes might also provide some potential antigen targets for circulating antibodies that directly injured the podocytes or formed immune complex deposits and subsequent complement activation, especially the formation of the membrane attack complex (MAC), might inflict cytotoxic injury on the podocytes in lupus membranous nephropathy (class V),20 which showed zero to low expression of IHC podocalyxin score in this study.
We reported in this study a significant negative correlation between IHC podocalyxin score and activity parameters as leukocyte infiltration, endocapillary proliferation, fibrinoid necrosis, cellular crescents and total NIH activity index score.
Endocapillary hypercellularity with or without leukocyte infiltration and with substantial luminal reduction is an active glomerular lesion specified by the 2004 ISN/RPS lupus nephritis classification.21 The significant association of podocyte loss detected by IHC podocalyxin score with endocapillary proliferation in active proliferative LN classes, could be explained by the immune-complex deposition in the capillary wall aggravating inflammation response with consequent podocyte injury.5 Cell mediated immunity might play also an important role in podocyte lesions and also several autoantibodies were found to directly cross-react with podocyte proteins, like a-actinin-4, and lead to the podocyte cytoskeleton reorganization that presented with podocyte fusion morphologically in proliferative LN.22
The significant association of IHC podocalyxin score with leukocyte infiltration could be attributed to the role of infiltrating leukocytes in inducing apoptosis of podocytes followed by podocyte loss.23 The presence of cellular crescents association with low IHC podocalyxin score may indicate that podocyte loss may be inducible factor in crescent formation in active LN. In Le Hir et al.20 study; they observed that podocytes detached from the glomerular basement membrane (GBM) migrate and infiltrate the parietal basement membrane initiating proliferation of parietal epithelial cells, resulting in a crescent. Using genetic tags in mouse models, it has been reported that up to 50% of the cells within crescents may originate from podocytes.24,25 Bariety and colleagues reported also the presence of podocytes in crescents identified by immunostaining for podocyte antigens in human glomerulonephritis including type IV lupus glomerulonephritis.26 Thorner and colleagues24 identified Nestin-positive cells, as podocyte marker, in glomerular crescents in human renal biopsies in a variety of renal pathologies including LN classes III and IV. Although the presence of podocytes in crescents remains unclear, the significant negative association of IHC podocalyxin score and cellular crescent observed in this study may indicates that podocyte injury is an important parameter of severity and activity in lupus nephritis.
Other study concluded that counting and scoring shed Bowman's space podocytes showed a statistically significant association to the pattern of proliferation, necrosis and cellular crescents as parameters of activity index but not of chronicity index.27 Although podocyte loss leading to initiation of inflammatory response with protein mediated cytotoxicity causes endothelial damage ends into sclerosis and fibrosis of the glomerulus and even end stage renal disease; we did not observed statistically significant correlation between IHC podocalyxin score and chronicity index.
In the present study, there was a highly significant negative correlation between IHC podocalyxin score and the degree of podocyte effacement by EM. We suggest that decreased immunohistochemical expression of podocalyxin is due to podocyte morphogenesis and the maintenance of structural integrity through the negative charge leads to ultrastructural changes of foot processes, which disturb their function, eventually leading to fusion of foot processes. As Wang et al. study8 reported that degree of podocyte effacement in proliferative LN assessed by foot process width by electron microscopy was positively correlated with the level of proteinuria and renal pathological indices, including cellular crescents, interstitial inflammatory and total chronicity index, and effacement of podocyte may be considered an important pathological parameter in active lupus nephritis.
We also found a statistically significant correlation between negative podocalyxin immunoexpression and electron dense deposits by electron microscopy indicating that podocyte loss is related to amount of immune complex deposition. We assume that mesangial and subendothelial immune complexes may induce podocyte injury indirectly through complement activation and release of inflammatory mediators aggravating the inflammatory response with consequent apoptosis could result in podocyte loss.
In the current study, IHC podocalyxin score was significantly associated with degree of proteinuria. Proteinuria is considered a very important clinical biomarker in the assessment of renal injury and LN activity, also considered as prognostic factor for renal outcomes in lupus nephritis, high proteinuria is associated with multi-systemic vascular damage leading to worsening GFR.28 We observed a significant negative correlation between the IHC podocalyxin score with SLE disease activity (SLAM-R) score as degree of effacement of podocytes may be considered a measurement not only for activity and severity of lupus nephritis but for global SLE activity, that's may need further studies.
As hypertension is an important factor influencing the prognosis of patients with LN.29 we reported a significant negative correlation between IHC podocalyxin score and diastolic blood pressure but not systolic BP, as podocyte injury with hypertension may be explained by mechanical stress and oxidative stress that are key mediators of podocyte damage in hypertension. We observed also the association between IHC podocalyxin and presence of ANA. Interventional studies with drug therapy that preserve expression of podocalyxin as target therapy in active lupus nephritis may be needed.
ConclusionsImmunohistochemical expression of podocalyxin significantly correlated to degree of podocyte effacement by EM and may be a novel marker for assessment of disease activity and severity of lupus nephritis.
Conflict of interestThe authors declare no conflict of interest.