In chronic kidney disease (CKD) patients, the risk of kidney replacement therapy (KRT) is highly variable. In 2011, Tangri et al. developed the kidney failure risk equations (KFRE) to predict the 2 and 5-year probability of requiring kidney replacement therapy (KRT). The KFRE is an easily calculated 4-variable equation which has been extensively validated in multiple cohorts. The aim of this study was to validate this risk score in a Portuguese cohort.
MethodsWe conducted a retrospective analysis of CKD patients stage 3–5 referred for nephrology consult at Centro Hospitalar Universitário Lisboa Norte during the first 6 months of 2018. Age, gender, estimated glomerular filtration rate (eGFR) and albuminuria were assessed. The 4-variable kidney failure risk equation (KFRE) calibrated to a non-North American population was calculated. Requirement of KRT was assessed in a 2-year follow-up. We assessed the Cox logistic regression method of the KFRE to predict KRT requirement and the discriminatory ability was determined using the receiver operating characteristic (ROC) curve. A cut-off value was defined as that with the highest validity.
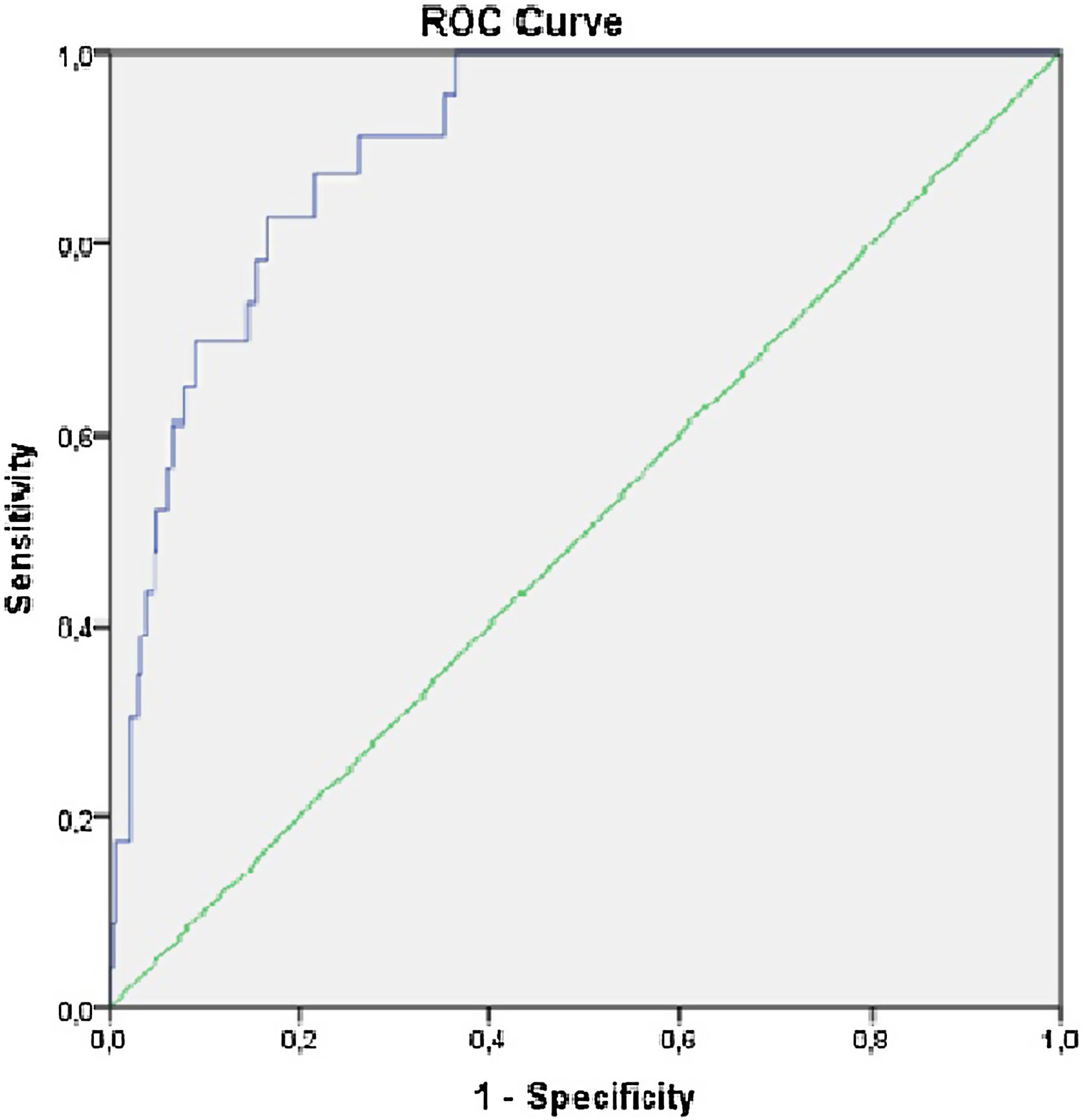
Results360 patients were included and 54.4% were male. Mean age was 74.9±12.2 years, serum creatinine was 1.97±0.84mg/dL, eGFR was 33.4±12.13ml/min/1.73m2 and albuminuria was 571.1±848.3mg/g. Mean calculated risk score was 6.2±11.2%. Twenty-three patients required KRT (6.4%) in the two-year follow-up. The hazard ratio was 1.1 [95% CI (1.06–1.12), p<0.001] for the 2-year risk of KRT. The KFRE predicted progression to KRT requirement with an auROC of 0.903, [95% CI (0.86–0.95), p<0.001], with a sensitivity 91.3% and specificity of 71.8%. The optimal KFRE cut-off was >4.5% for 2-year nephrologist referral, with an hazard ratio of HR 26.7 [95% CI (6.15–116.3), p<0.001] for 2-year risk of KRT requirement.
DiscussionWe have independently externally validated the 2-year KFRE and shown that it has excellent discrimination. The KFRE should be incorporated in clinical care of patients with CKD to improve patient-clinician dialogue and provide guidance on timing of referral for nephrology evaluation and planning for dialysis access.
En pacientes con enfermedad renal crónica (ERC), el riesgo de la terapia de reemplazo renal (TRR) es muy variable. En 2011, Tangri et al. desarrollaron las ecuaciones de riesgo de insuficiencia renal (KFRE) para predecir la probabilidad de 2 y 5años de requerir terapia de reemplazo renal (KRT). El KFRE es una ecuación de 4 variables de fácil cálculo que ha sido ampliamente validada en múltiples cohortes. El objetivo de este estudio fue validar esta puntuación de riesgo en una cohorte portuguesa.
MétodosSe realizó un análisis retrospectivo de pacientes con ERC estadio 3-5 remitidos para consulta de Nefrología en el Centro Hospitalario Universitário Lisboa Norte durante los primeros 6meses de 2018. Se evaluaron la edad, el sexo, el filtrado glomerular estimado (TFGe) y la albuminuria. Se calculó la ecuación de riesgo de insuficiencia renal (KFRE) de 4 variables calibrada para una población no norteamericana. La necesidad de KRT se evaluó en un seguimiento de 2años. Evaluamos el método de regresión logística de Cox del KFRE para predecir el requisito de KRT, y la capacidad discriminatoria se determinó utilizando la curva de característica operativa del receptor (ROC). Se definió como valor de corte el de mayor validez.
ResultadosSe incluyeron 360 pacientes, y el 54,4% eran varones. La edad media fue de 74,9±12,2 años, la creatinina sérica de 1,97±0,84mg/dl, la TFGe de 33,4±12,13ml/min/1,73m2 y la albuminuria de 571,1±848,3mg/g. La puntuación de riesgo media calculada fue de 6,2±11,2%. Veintitrés pacientes requirieron KRT (6,4%) en los 2años de seguimiento. El cociente de riesgos instantáneos fue de 1,1 (IC del 95%: 1,06-1,12; p<0,001) para el riesgo de 2años de KRT. El KFRE predijo la progresión al requerimiento de KRT con un auROC de 0,903 (p<0,001; IC del 95%: 0,86-0,95), con una sensibilidad del 91,3% y una especificidad del 71,8%. El punto de corte óptimo de KFRE fue >4,5% para la derivación al nefrólogo de 2años, con un índice de riesgo de HR 26,7 (IC del 95%: 6,15-116,3; p<0,001) para el riesgo de 2años de necesidad de KRT.
DiscusiónHemos validado externamente de forma independiente el KFRE de 2años y hemos demostrado que tiene una discriminación excelente. El KFRE debe incorporarse en la atención clínica de los pacientes con ERC para mejorar el diálogo entre el médico y el paciente y proporcionar orientación sobre el momento de la derivación para la evaluación nefrológica y la planificación del acceso a diálisis.
Chronic kidney disease (CKD) is a public health problem with an estimated prevalence of around 10% globally. Due to the population ageing and increase in cardiovascular comorbidities it is expected for the prevalence of CKD to increase.1 In Portugal, in 2018 a national survey has estimated CKD prevalence to be 20.1% in agreement with higher prevalence of CKD in Europe, with a majority of patients in stage 3.2–4
Patients with CKD are at increased risk of cardiovascular events and progression to end stage kidney disease (ESKD).5 CKD stages are defined by eGFR and albuminuria, which are traditionally used to estimate the risk of progression to ESKD. Predicting the risk of progression to ESKD is challenging as the declining pattern of kidney function is variable between different renal diseases and individually within the same disease. Therefore, knowledge of risk predictors for the progression to ESKD is crucial in determining the appropriate treatment plan.6,7
In 2011, Tangri et al. developed the kidney failure risk equations (KFRE), a four-variable model to predict the two-year probability of requiring KRT.8 This is an internationally validated risk prediction which accurately predicts the risk of progression to ESKD. The KFRE equation is calculated inputting routinely available variables such as age, gender, serum creatinine and albuminuria. KFRE was initially developed in Canadian population, but since then it has been extensively validated in multiple cohorts in non-North America setting.9–14 Other risk prediction scores have been published, but they have not undergone such robust validation.15,16
Since the accuracy of the prediction model might differ among different populations, the aim of this study was to validate the KFRE in a Portuguese cohort.
MethodsWe performed a single center retrospective analysis of adult patients with CKD referred to a nephrology consult at the Division of Nephrology and Renal Transplantation of Centro Hospitalar Universitário Lisboa Norte (CHULN) between January and June of 2018. This study was approved by the Ethical Committee in agreement with institutional guidelines and informed consent was waived due to the retrospective and non-interventional nature of the study.
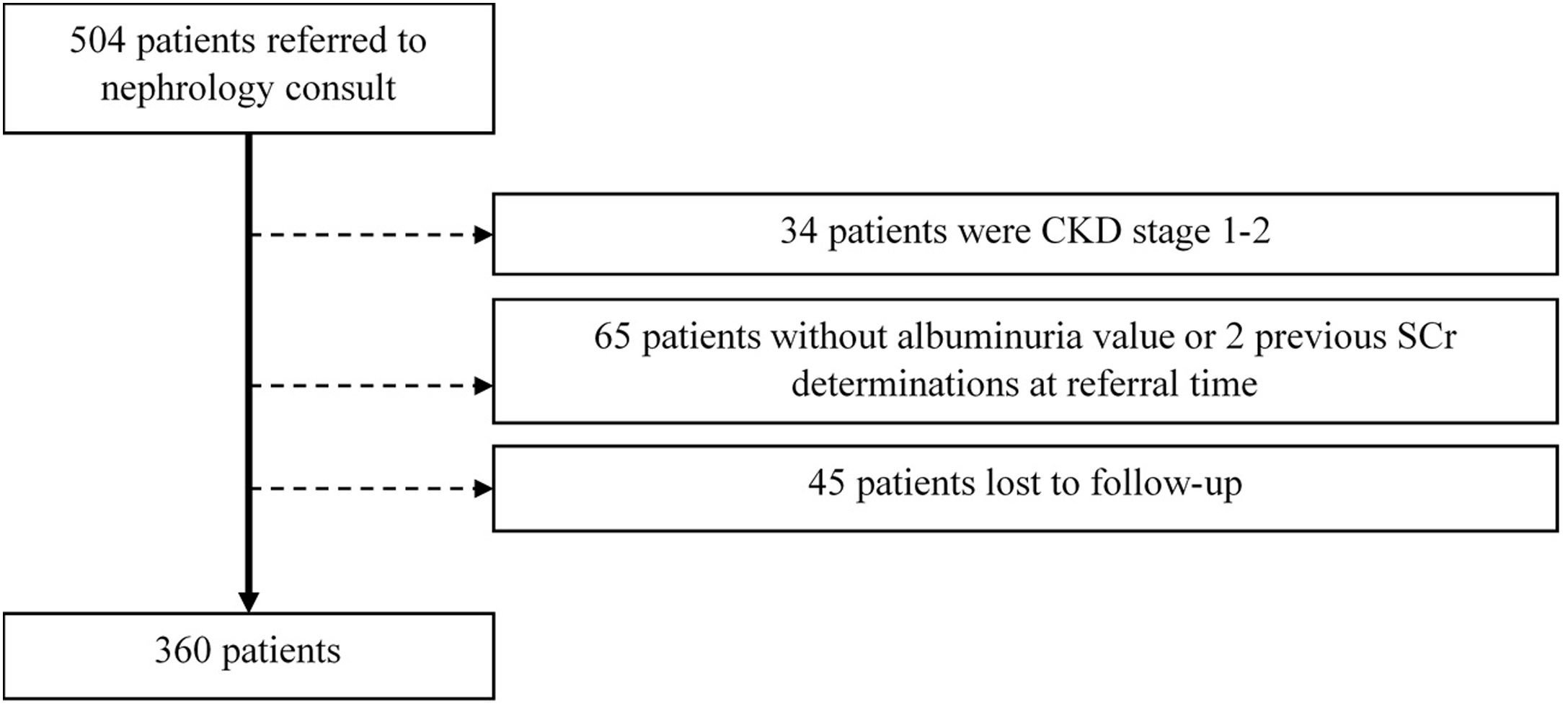
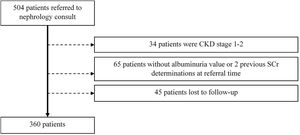
Adult patients with CKD stages 3–5 (estimated GFR<60ml/min/1.73m2) at the time of initial nephrology referral were included. Exclusion criteria comprised (a) patients without two determinations of serum creatinine values more than 90 days apart, (b) patients without a quantifiable proteinuria value at referral time, (c) patients lost to follow-up. Kidney transplant patients were not included in this study.
Patient variables were collected from individual clinical records. The following variables were analyzed: patient demographic characteristics (age, gender); comorbidities (diabetes mellitus, hypertension, cardiovascular disease [ischemic cardiomyopathy, heart failure]); laboratory values at referral (serum creatinine, albumin-to-creatinine ratio (ACR)). CKD was defined and staged according to the KDIGO classification.17 Estimated GFR was calculated according to the CKD-EPI formula.18 Alternative measures of proteinuria (urine protein-creatinine ratio and 24-h urine total protein) were converted to ACR as is described in previous studies.14
The 4-variable KFRE (age, gender, baseline eGFR and log urine ACR) calibrated to a non-North American population was calculated, as proposed by Tangri et al.14
The outcomes measured were kidney replacement therapy (KRT) requirement and mortality. Follow-up was continued until 31st December 2020, and data extraction occurred between January and March 2021. Outcomes were ascertained by reviewing clinic records.
Statistical analysisCategorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean±standard deviation. Continuous variables were assessed for normality of distribution with Kolmogorov–Smirnov test and compared with the Student's t-test or Mann–Whitney test accordingly. Categorical variables were compared with the chi-square test. One-way ANOVA was used for comparisons between groups.
Cox regression analysis was performed to evaluate the correlation between KFRE and KRT requirement. The discriminatory ability for KFRE to predict KRT requirement in CKD patients was determined using the receiver operating characteristic (ROC) curve. Using the Youden's index a cut-off value was defined as that with the highest validity. Calibration was tested by the Hosmer–Lemeshow test.
Data were expressed as hazards ratios (HRs) with 95% confidence intervals (CIs). Statistical significance was defined as a p-value <0.05. Statistical analysis was performed with the statistical software package SPSS for windows (version 21.0).
ResultsWe focused on 360 patients after excluding 144 patients, as depicted in Fig. 1.
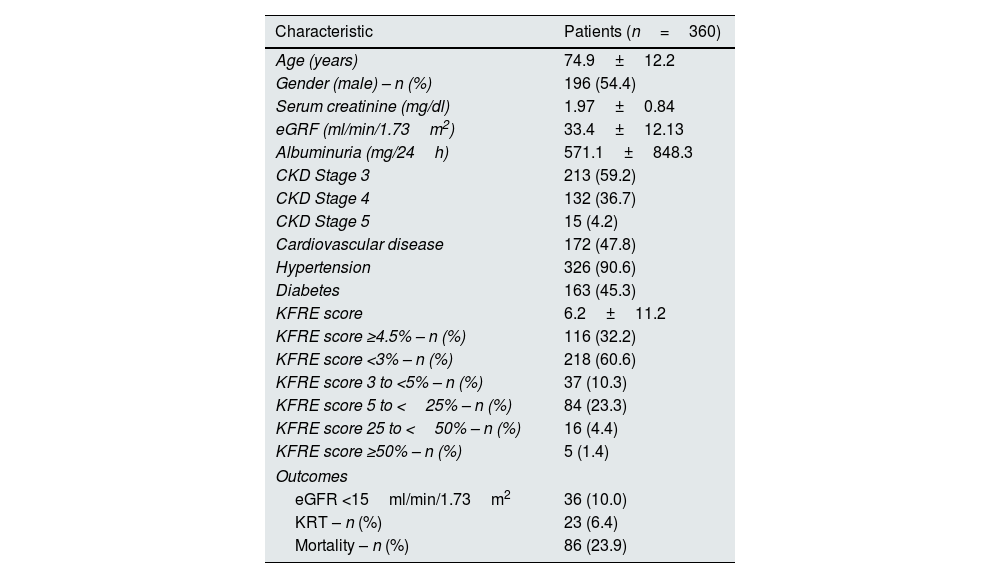
In this cohort of patients referred to nephrology consult, mean age was 74.9±12.2 years and the majority were male (54.4%). Mean eGFR was 33.4±12.13ml/min/1.73m2, mean proteinuria was 571.1±848.3mg/24h. Concerning comorbidities 90.6% of participants had hypertension, 47.8% had cardiovascular disease and 45.3% had diabetes. Baseline characteristics and outcomes are shown in Table 1.
Baseline characteristics and outcomes of the cohort.
| Characteristic | Patients (n=360) |
|---|---|
| Age (years) | 74.9±12.2 |
| Gender (male) – n (%) | 196 (54.4) |
| Serum creatinine (mg/dl) | 1.97±0.84 |
| eGRF (ml/min/1.73m2) | 33.4±12.13 |
| Albuminuria (mg/24h) | 571.1±848.3 |
| CKD Stage 3 | 213 (59.2) |
| CKD Stage 4 | 132 (36.7) |
| CKD Stage 5 | 15 (4.2) |
| Cardiovascular disease | 172 (47.8) |
| Hypertension | 326 (90.6) |
| Diabetes | 163 (45.3) |
| KFRE score | 6.2±11.2 |
| KFRE score ≥4.5% – n (%) | 116 (32.2) |
| KFRE score <3% – n (%) | 218 (60.6) |
| KFRE score 3 to <5% – n (%) | 37 (10.3) |
| KFRE score 5 to <25% – n (%) | 84 (23.3) |
| KFRE score 25 to <50% – n (%) | 16 (4.4) |
| KFRE score ≥50% – n (%) | 5 (1.4) |
| Outcomes | |
| eGFR <15ml/min/1.73m2 | 36 (10.0) |
| KRT – n (%) | 23 (6.4) |
| Mortality – n (%) | 86 (23.9) |
eGFR – estimated glomerular filtration rate; KFRE – kidney failure risk equation; KRT – kidney replacement therapy.
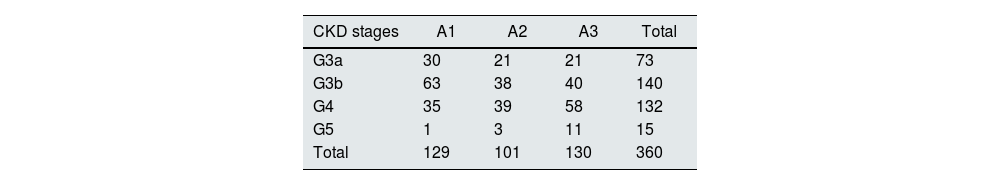
There were 213 (59.2%) patients classified as having CKD stage 3, 132 (36.7%) as CKD stage 4 and only 15 (4.2%) as CKD stage 5. Two hundred and forty six patients were at high risk of progression of CKD according to the KDIGO classification using GFR and albuminuria criteria, as depicted in Table 2.
Twenty-three patients required KRT (6.4%) and 86 (23.9%) died in the two-year follow-up. Need for KRT was significantly higher according to CKD stage [G3 1.4% (n=3) vs. G4 12.1% (n=16) vs. G5 26.7% (n=4), p<0.001] as was mortality [G3 16.4% (n=35) vs. G4 33.3% (n=44) vs. G5 46.7% (n=7), p<0.001]. There was no correlation between KRT requirement and mortality (23.4 vs. 30.4%, p=0.447).
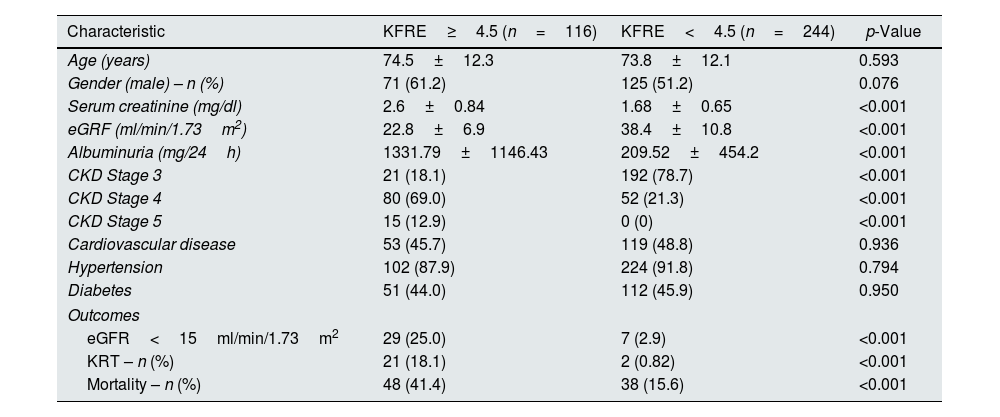
The mean calculated risk score was 6.2±11.2%. There was no statistical difference between CKD stages and KFRE score (G3 5.1±1.0% vs. G4 6.4±1.4% vs. G5 5.0±1.2%, p=0.611). Baseline characteristics and outcomes according to KFRE score outlined in Table 3.
Baseline characteristics and outcomes according to KFRE score.
| Characteristic | KFRE≥4.5 (n=116) | KFRE<4.5 (n=244) | p-Value |
|---|---|---|---|
| Age (years) | 74.5±12.3 | 73.8±12.1 | 0.593 |
| Gender (male) – n (%) | 71 (61.2) | 125 (51.2) | 0.076 |
| Serum creatinine (mg/dl) | 2.6±0.84 | 1.68±0.65 | <0.001 |
| eGRF (ml/min/1.73m2) | 22.8±6.9 | 38.4±10.8 | <0.001 |
| Albuminuria (mg/24h) | 1331.79±1146.43 | 209.52±454.2 | <0.001 |
| CKD Stage 3 | 21 (18.1) | 192 (78.7) | <0.001 |
| CKD Stage 4 | 80 (69.0) | 52 (21.3) | <0.001 |
| CKD Stage 5 | 15 (12.9) | 0 (0) | <0.001 |
| Cardiovascular disease | 53 (45.7) | 119 (48.8) | 0.936 |
| Hypertension | 102 (87.9) | 224 (91.8) | 0.794 |
| Diabetes | 51 (44.0) | 112 (45.9) | 0.950 |
| Outcomes | |||
| eGFR<15ml/min/1.73m2 | 29 (25.0) | 7 (2.9) | <0.001 |
| KRT – n (%) | 21 (18.1) | 2 (0.82) | <0.001 |
| Mortality – n (%) | 48 (41.4) | 38 (15.6) | <0.001 |
The KFRE accurately predicted the two-year risk of progression to KRT, with an hazard ratio of 1.1 [95% CI (1.06–1.12), p<0.001]. The Hosmer–Lemeshow test indicated good fit of this model (p=0.081).
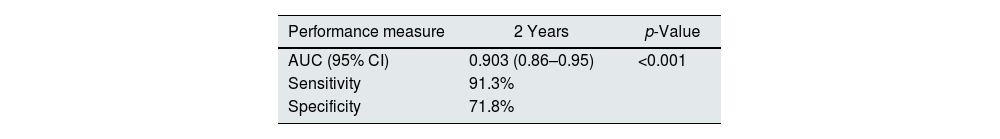
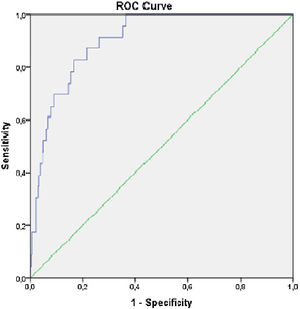
The KFRE predicted progression to requirement of KRT with an auROC of 0.903, [95% CI (0.86–0.95), p<0.001] (Fig. 2), with a sensitivity 91.3% and specificity of 71.8% (Table 4). The optimal KFRE cut-off was >4.5%, with an hazard ratio of 26.7 [95% CI (6.15–116.3), p<0.001] for 2-year risk of KRT.
One hundred and sixteen (32.2%) patients had KFRE≥4.5% the majority (61.2%) being males with mean age of 74.5±12.3 years.
These patients had significantly lower baseline eGFR (22.8±6.9 vs. 38.4±10.8ml/min/1.73m2, p<0.001), higher albuminuria (1331.79±1146.43 vs. 209.52±454.2mg/24h, p<0.001) and higher serum creatinine (2.6±0.84 vs. 1.68±0.65mg/dl, p<0.001).
Additionally, this group required more KRT (18.1 vs. 0.82%, p<0.001) and mortality was also higher in these patients (41.4 vs. 15.6%, p<0.001).
Mortality was higher in patients older than 70 years (89.5 vs. 55.6%, p<0.001), patients with hypertension (95.4 vs. 87.7%, p=0.038), cardiovascular disease (65.5 vs. 38.5%, p<0.001) and patients with KFRE≥4.5% (52.6 vs. 18.0%, p<0.001). On a multivariate analysis including hypertension, cardiovascular disease and KFRE≥4.5%, cardiovascular disease [OR 2.97, 95% CI (1.76–5.00), p<0.001] and KFRE≥4.5% [OR 4.48, 95% CI (2.67–7.49), p<0.001] were significant predictors of mortality.
DiscussionIn this cohort of 360 patients referred to nephrology consult 6.4% required KRT and 23.9% died in the two-year follow-up. The KFRE accurately predicted the two-year risk of progression to KRT, with a good performance [AUC 0.903 (0.86–0.95, p<0.001)] and a 91.3% sensitivity and 71.8% specificity. This is in line with the performance of KFRE in other populations, meaning that the use of this risk score in the Portuguese population is as adequate.14 Additionally, we identified that KFRE≥4.5% was a significant risk predictor of mortality on a two-year follow-up.
The original KFRE development cohort included 3449 Canadian patients with CKD stages 3–5 of whom 11% (386) progressed to ESKD in the 2-year follow-up. Tangri et al. developed several predictive models for the risk of progression of CKD. The model including only age and gender performed poorly but the addition of baseline eGFR and uACR improved the predictive model significantly. The improvement in discrimination between these models highlights the importance of eGFR and albuminuria for predicting progression of CKD. Indeed, the 4-variable model including age, gender, baseline eGFR and uACR had a good discrimination (C-statistic of 0.910; 95% CI, 0.894–0.926; p<0.001), which maintained good discrimination in the validation cohort (C-statistic 0.83).8 This is an easily calculated score which incorporates demographic and laboratory data which is routinely obtained in CKD patients.
Further studies identified differences in the estimated risk between regions which required usage of calibration factor to account for the increased baseline risk in North America populations.14
Wang et al. used the recalibrated KFRE in a cohort of 17,271 participants from Southeast Asia with ESKD incidence of 2.8% and achieved good discrimination (auROC 0.96, 95% CI 0.95–0.97). The recalibration model accounted for baseline risk differences between populations and at a 2-year follow-up, a threshold risk of >9% presented a sensitivity of 93% and specificity of 86% for ESKD.19 The recalibrated KFRE was also studied in a cohort of 35,539 patients referred from primary care in the United Kingdom, of whom only 1.21% progressed to ESKD at a 5-year follow-up. The use of KFRE in this population also revealed good discrimination of the prediction model (C-statistics 0.926), and a KFRE threshold of ≥5% they achieved a sensitivity of 6.8% and specificity of 99.7%. The low sensitivity might be explained by the low incidence of ESKD in the cohort.20 In a cohort of 595 Dutch CKD patients with mean eGFR 33.3ml/min/1.73m2 and an incidence of ESKD of 19%, Peters et al. demonstrated that the 4-variable KFRE performed similarly with good discrimination (auROC 0.88, 95% CI 0.85–0.91). Defining a threshold of lower risk of ESKD with KFRE≤20% achieve a sensitivity of 89% and specificity of 69%.21 This was an important study as it validated the KFRE in an European cohort.
In our study, the recalibrated KFRE performed similarly with an auROC of 0.903, a sensitivity 91.3% and specificity of 71.8%, which means that this score accurately predicts the risk of CKD progression in the Portuguese population.
The KFRE is a simple and easily calculated risk score which relies on routinely collected laboratory data, and could be integrated into electronic medical records and information systems. The widespread use of this risk score in clinical practice can lead to the improvement in the management of CKD patients.6 Firstly this could be used in determining nephrology referral as patients at high risk benefit from strategies to delay CKD progression. As such, the existence of a tangible score can improve the risk communication and better educate patients of their disease and prognosis, as it has been shown that up to 40% of CKD patients have a misperception of the risk of progression of their disease.22 Thus, based on the risk of CKD progression, the timing of modality education may also be determined and timely vascular access creation addressed. In low-risk patients, modality education and planning might create unnecessary anxiety resulting in outdated or irrelevant treatment plans as these low risk patients also tend to have higher risk of mortality than ESKD. As for dialysis access creation, the routine use of the KFRE might minimize starting dialysis with a catheter while avoiding access creation in patient who might die before ESKD. Tangri et al. suggests vascular access planning in patients with a eGFR<20ml/min/1.73m2 and a two year KFRE≥40% although this has not been prospectively evaluated. Therefore, not only can the KFRE be used to improve patient management and communication, it might also enhance the allocation of appropriate resources and deliver cost-effective care to CKD patients.
Interestingly, Kwek et al. studied 1.128 CKD patients from Singapore in whom the KFRE had a good predictive ability and categorized patients into low (<5%), medium (5–14.99%) and high risk (≥15%), which might aid in management of patients with CKD stage 3 and 4. They proposed that patients with low risk could be managed in primary care and high risk patients should be referred to a nephrologist.23 The triage of nephrologist referral based on the KFRE was also studied recently. In their study, a risk lower than 3% at five years was used as threshold and these CKD patients were returned to primary care.24 In our cohort a cut-off at 4.5% had the best performance, as these patients had significantly more KRT events (HR 26.7 [95% CI 6.15–116.3, p<0.001]) on a 2-year follow-up. This clearly establishes a threshold for nephrologists to be aware of when caring for CKD patients as these should be referred for KRT planning and vascular access creation.
In our cohort there was a 24% mortality on the two-year follow-up. This might explain the low percentage of patients which required KRT over this time period (6.4%) as a higher KFRE was also associated with a higher mortality risk. Indeed, mortality was higher in older patients, with more advanced CKD and more cardiovascular disease. As we did not assess causes of mortality, we can assume cardiovascular death was it is one of the most frequent causes of mortality in CKD patients.5 Nevertheless, we might presume this significant mortality rate may be a consequence of the COVID-19 pandemic in a fragile population. We highlight the importance of the KFRE to identify patients at-risk.
This study has certain limitations which must be addressed. Firstly, the relatively small size of our cohort, the single-center and the retrospective nature of our study limit its generalizability. Secondly, we did not assess for the CKD causes. Thirdly, we did not determine if KRT requirement was promoted by an acute event. We assume a possible risk of selection bias due to referral of patients who empirically appeared to have greater risk of kidney disease. Fourthly, we did not assess if patients were selected for palliative care. And finally, we did not assess the causes of mortality in these patients.
Our study has some important noteworthy virtues. This is the first study to validate the risk score of CKD progression in a Portuguese population. Secondly, we included a recent cohort of patients, meaning that the KFRE is still reliable and up-to-date. Despite the retrospective design, the studied variables were routinely recorded in daily practice which allowed for the analysis of important covariates with impact on CKD progression.
In conclusion, we have independently validated the 2-year KFRE and shown that it has excellent discrimination in a Portuguese cohort. The KFRE should be incorporated in clinical care of patients with CKD to improve patient-clinician dialogue and provide guidance on timing of referral for nephrology evaluation and planning for dialysis access.
Ethics approval and consent to participateThe study was approved by the Ethical Committee at the Centro Hospitalar Universitário Lisboa Norte, EPE, in agreement with institutional guidelines. Informed consent was waived by the Ethical Committee due to the retrospective and non-interventional nature of the study.
Consent for publicationThe authors give their consent for publication.
Availability of data and materialNot applicable.
Authors’ contributionsThe authors participated as follows: BMS drafted the article, JC participated in the collection of data. CO and JG made substantial contributions to the study concept and design, analysis and interpretation of data, and were involved in revising it critically for important intellectual content.
FundingThere was no funding for this study.
Conflict of interestsThere is no conflict of interest.
The authors have no acknowledgements.