ADV7103 is a new prolonged-release treatment for distal renal tubular acidosis (dRTA), containing potassium citrate and potassium bicarbonate. Since acidosis may affect bone mineral contents, the effects of ADV7103 on bone mineral density (BMD) and growth in patients with dRTA over 24 months were evaluated.
Patients and methodsThirty patients (24 paediatric patients and 6 adults) were included in an open-label extension study after a phase II/III trial. BMD, measured by densitometry, was assessed at baseline and at 24 months. Growth was evaluated throughout the study. Plasma bicarbonate, parathyroid hormone, 25-hydroxy vitamin D, 1,25-dihydroxy vitamin D, bone alkaline phosphatase, calciuria and citraturia, were also determined. Safety and treatment compliance were evaluated as well.
ResultsAfter 24 months of treatment with ADV7103, mean spine BMD z-score values significantly increased as compared with baseline (p=0.024). In adults, spine and whole-body densitometry z-scores showed a significant correlation with plasma bicarbonate levels (rS=0.82 and rS=0.97, respectively, p<0.005). There was an increase>0.5 units in z-scores for height and weight in 18% and 36% of the paediatric patients, respectively. With treatment, plasma bicarbonate concentration and calciuria at the different visits were normal in 69–86% and 93–96% patients, respectively. Only nine treatment-related gastrointestinal AEs of mild/moderate severity, were reported in five patients.
ConclusionsTwo years of ADV7103 treatment improved growth and increased spine BMD. These results suggest that control of acidosis by ADV7103 treatment improves bone parameters.
El ADV7103 es un nuevo tratamiento de liberación prolongada para la acidosis tubular renal distal (ATRd), que contiene citrato potásico y bicarbonato potásico. Dado que la acidosis puede afectar al contenido mineral óseo, se ha evaluado el efecto de dicho medicamento a lo largo de 24 meses sobre la densidad mineral ósea (DMO) y el crecimiento en pacientes con ATRd.
Pacientes y métodosSe incluyeron treinta pacientes (24 pediátricos y seis adultos) en un estudio abierto de extensión tras un ensayo clínico de fase II/III. La DMO medida por densitometría se midió al inicio del estudio y los 24 meses. El crecimiento se evaluó a lo largo del estudio. Adicionalmente, se determinaron el bicarbonato plasmático, la parathormona, 25 hidroxivitamina D, 1,25 dihidroxivitamina D, fosfatasa alcalina ósea, calciuria y citraturia. La seguridad y el cumplimento terapéutico también fueron evaluados.
ResultadosTras 24 meses de tratamiento con ADV7103 la media del z-score de DMO de columna aumentó significativamente frente al valor basal (p = 0,024). En los adultos el z-score de la densitometría de columna y corporal total mostró una correlación significativa con los valores de bicarbonato plasmático (rS = 0,82 y rS = 0,97, respectivamente, p < 0,005). Se registró un incremento > 0,5 unidades de z-score para altura y peso en el 18 y 36%, respectivamente, de los pacientes pediátricos. Con el tratamiento, la concentración plasmática de bicarbonato y la calciuria fueron normales en las diferentes visitas en un 69-86% y un 93-96% de los pacientes, respectivamente. Solamente se notificaron nueve eventos adversos gastrointestinales relacionados con el tratamiento, todos de intensidad leve/moderada en cinco pacientes.
ConclusionesDos años de tratamiento con ADV7103 mejoraron el crecimiento y la DMO de columna. Estos resultados sugieren que el control de la acidosis con dicho tratamiento provoca una mejora de parámetros óseos.
Distal renal tubular acidosis (dRTA) is a rare disease characterized by an impaired net acid excretion at the distal tubule causing hyperchloremic metabolic acidosis. It is often associated with hypokalaemia, due to renal potassium wasting. Additionally, hypercalciuria occurs as excess of calcium is released from bone in an attempt to compensate metabolic acidosis.1,2 Low intracellular pH also affects calcium channel TRPV5 function, causing a reduction of calcium reabsorption in the distal tubule.3 Sustained metabolic acidosis impairs growth in children and causes a reduction in bone mass.4,5 Prolonged acidosis directly stimulates osteoclasts activity which increases bone resorption, and also reduces osteoblast activity resulting in decreased bone formation. In addition, acidosis stimulates parathyroid hormone (PTH) secretion, another factor involved in bone resorption.6–9 Finally, it has been shown that chronic acidosis exerts an antianabolic effect on bone growth by generating resistance to the effect of growth hormone and insulin-like growth factor-1 (IGF-1).10,11
In a retrospective study including 96 adult and paediatric patients with dRTA, bone pain or growth failure was observed in 19% of adults and in 52% of the children, respectively. Osteomalacia/rickets were reported in 9.6% of adult patients and 59% of the children.12 In a cohort of 89 patients with dRTA, 58% of the children presented failure to thrive.13 In patients with acquired dRTA due to Sjogren's syndrome, approximately a 33% presents osteomalacia and/or pseudo-fractures.14,15
Among patients with dRTA, lower blood pH values were observed in patients with bone abnormalities.16 Thus, correction of acidosis should improve growth defects, prevent bone mass loss and maintain a normal bone histology.4,17,18 Correction of acidosis with alkali therapy is achieved when plasma bicarbonate, urinary calcium and citrate excretion levels are restored to normal values. However, these objectives are not satisfactorily attained with current therapies.19,20
ADV7103 is formulated as prolonged-release alkalizing microgranules, containing potassium citrate and potassium bicarbonate in a proportion 1:2, which allows providing sustained alkali levels throughout day and night with only twice daily administration.21 It has been shown that administration of ADV7103 to patients with dRTA, increases plasma bicarbonate concentration more effectively than the standard of care treatment (SoC), as demonstrated by morning pre-dose serum bicarbonate concentrations (mean±SD) (mmol/L) of 21.2±3.1 and 23.0±1.6 after treatment with SoC and ADV7103, respectively.22 This effect was maintained in most patients during at least 24 months of treatment when they were treated with ADV7103.23 Other oral alkalizing products, such as oral bicarbonate salts are not sustained-release forms, require several administrations during the day and are not as well tolerated as ADV7103.23
Thus, our hypothesis was that treatment with ADV7103 would produce significant improvement in bone in dRTA patients. The objective of the present study was to evaluate the bone effects of 2-year treatment with ADV7103, assessing bone mineral density (BMD) variations from baseline, and measuring growth changes.
MethodsPatients and study designPatients with dRTA that completed a previous phase II/III study22 with ADV7103 were included in a 2-year extension study. It was a multicenter, single-arm, open-label trial (EudraCT 2013-003828-36, registered in September 2013), aiming to assess efficacy, safety, and tolerability, of treatment with ADV7103 in paediatric and adult patients.23 The study was approved by regional independent ethics committees (i.e. the Ethics Committee CPP Sud Est II in France, the Ethics Committee of the Bratislava Children's Hospital in Slovakia and the Ethics Committee of the Clinical Centre Nis in Serbia) and national regulatory health authorities (i.e. French National Agency for the Safety of Medicines and Health Products (ANSM), Slovakia State Institute for Drug Control (SUKL) and Medicines and Medical Devices Agency Serbia (ALIMS), and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
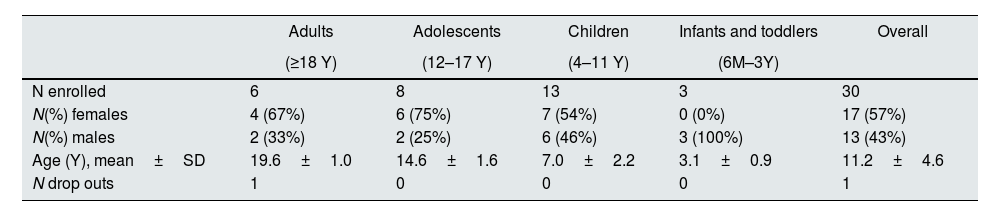
A total of thirty patients (24 paediatric and 6 adults) (Table 1), from 12 different centres in France, Serbia and Slovakia were included. They continued treatment with ADV7103 given twice daily at the optimal doses determined during the phase II/III study (the dose was further adapted if required).
Patient demographic data by age group and overall.
| Adults | Adolescents | Children | Infants and toddlers | Overall | |
|---|---|---|---|---|---|
| (≥18 Y) | (12–17 Y) | (4–11 Y) | (6M–3Y) | ||
| N enrolled | 6 | 8 | 13 | 3 | 30 |
| N(%) females | 4 (67%) | 6 (75%) | 7 (54%) | 0 (0%) | 17 (57%) |
| N(%) males | 2 (33%) | 2 (25%) | 6 (46%) | 3 (100%) | 13 (43%) |
| Age (Y), mean±SD | 19.6±1.0 | 14.6±1.6 | 7.0±2.2 | 3.1±0.9 | 11.2±4.6 |
| N drop outs | 1 | 0 | 0 | 0 | 1 |
N=number of patients, M=months, Y=years.
Exclusion criteria were hyperkalaemia (plasma potassium>5.0mmol/L), kidney function impairment (eGFR <45mL/min/1.73m2), or any other condition that could be negatively affected by the study medication or that could affect the concomitant treatment with potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, or tacrolimus. Written informed consent was obtained from all adult patients, and from the parents or legal guardians of all children. Patients were evaluated at baseline (last visit of the phase II/III trial) and at 3, 6, 12, 18 and 24 months of treatment.
Bone mineral density evaluationsBone mineral density (BMD, g/cm2) was measured at baseline and after the 24-month treatment period in three skeletal regions (spine, hip and whole body) using dual-energy X-ray absorptiometry (DXA). Measurements for each patient were performed with the DXA tools available at their local hospital. Values of z-score≤−2.0 were considered below the normal range, according to the International Society for Clinical Densitometry (ISCD) recommendations.24
Anthropometric evaluationsHeight (cm), weight (kg) and BMI were measured at each study visit and z-scores were determined considering age and sex, according to WHO standards for paediatric patients<5 years25 and from 5 to 19 years old.26 Weight standards for paediatric patients 11–19 years old were obtained from the data provided by the French Auxology Group.27
Stunting and failure to thrive were considered, for height- and for weight-for-age z-scores≤−2.0. We determined the number of patients with an increase of height- or weight-for-age z-scores>0.5 units from baseline to the end of the 24-month treatment.
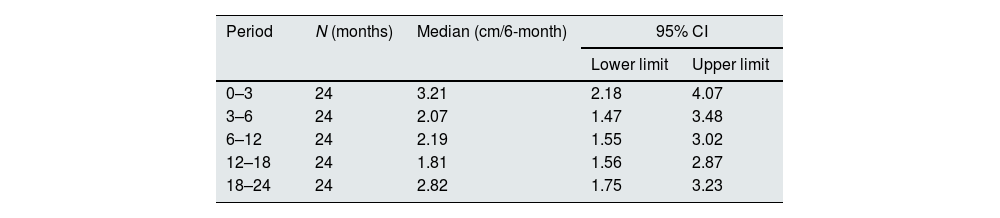
Growth velocity was calculated as the difference between height measurements performed during two consecutive visits and expressed as cm/6 month.28,29
Genetic target stature (GTS) was calculated according to the American College of Medical Genetics practice guideline.30 The difference between GTS and actual height was estimated in adult patients at baseline and after 24 months; short stature was considered if the difference was>10cm in men and >9cm in women.31
Blood and urine parametersDuring each study visit, blood samples were obtained, before the first morning dose of ADV7103. Serum bicarbonate, phosphate, calcium, and 25-hydroxy-vitamin D were determined by local laboratories. The serum concentration of bone alkaline phosphatases (bALP), 1,25-dihydroxy-vitamin D, and parathyroid hormone (PTH) were determined by immunoluminometry by a central laboratory. Normal values for bALP were adjusted to age and sex. Urine samples were also collected in the morning, to calculate the ratios calcium/creatinine (UCa/UCr), citrate/creatinine (UCi/UCr), calcium/citrate (UCa/UCi) and phosphate/creatinine (UP/UCr). These measurements were also performed by local laboratories.23 See supplemental document for all normal values.
Adverse eventsThe occurrence of adverse events (AEs) was recorded throughout the study, including abnormal values of laboratory data, electrocardiogram (ECG) findings (12-lead resting ECG standard including heart rate, PR, QRS, and QT intervals), vital signs (blood pressure, heart rate, and respiratory rate), and physical examinations (articular system, bone system, general appearance, and muscular system); the severity of the adverse events was recorded.
Adherence to treatmentThe adherence to the treatment was assessed in all patients based on the returned empty boxes. This was done at each study visit up to the completion of the 24-month period. Four categories of adherence were considered: excellent (>90%), good (75-90%), average (50-74%), and poor (<50%). In the absence of returned boxes, the investigators classed the patient in one of the four categories on the basis of interviews and laboratory results.
Other exploratory evaluationsClinical signs, such as diffuse bone and joint pain, myalgia, muscle weakness, abnormal posture, and abnormal height were registered throughout the follow up.
Statistical analysisDescriptive statistics were performed to obtain the mean and standard deviation (SD) or median (range), as well as changes from baseline and 95% confidence intervals, at the different time points in all patients and in groups separated by age.
Comparison of mean values of the parameters evaluated at the different time points and between age groups was performed by non-parametric methods (Kruskal–Wallis and Mann–Whitney U tests). Comparison of the densitometric parameters was performed using Student's t-test. The groups have been considered as random samples to avoid the missing data effect.
Pearson's correlation coefficient or the Spearman's Rho coefficient were used to assess the potential correlations between age, growth velocity and other parameters, including densitometry, including data from the evaluations at baseline and at the 24-month visit.
All the tests were two-sided, considering the differences as statistically significant when p≤0.05. Statistical analyses were performed using IBM SPSS Statistics, version 27.0.0.0.
ResultsInformation relative to the 30 dRTA patients included in the study is shown in Table 1. A female adult patient discontinued the study after 12 months for personal reasons; thus 29 completed the 24-month study. At the end of the study a total of 79% of patients presented adherence rates≥75% after two years of treatment. The mean dose±SD at this time was 2.3±1.3mEq/kg/day in adults, while it was 2.6±1.7mEq/kg/day in adolescents, 3.4±1.3mEq/kg/day in children, and 4.8±2.0mEq/kg/day in toddlers, with two daily administrations, in the morning and in the evening.
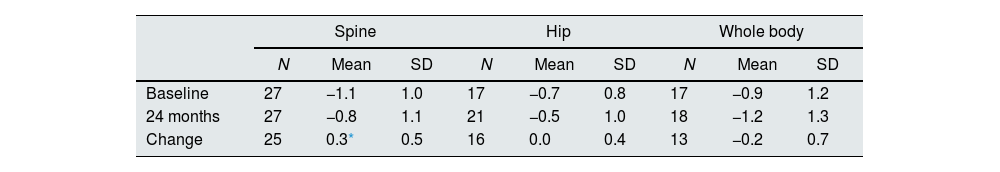
Bone mineral densityChanges in BMD z-scores after 24 months of treatment are shown in Table 2. The overall mean spine z-score significantly increased from -1.1at baseline to −0.8 at 24 months (Student's t-test; p=0.024), while no significant change was observed in hip and whole-body z-scores.
Evolution of overall spine, hip and whole-body densitometry z-scores.
| Spine | Hip | Whole body | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Baseline | 27 | −1.1 | 1.0 | 17 | −0.7 | 0.8 | 17 | −0.9 | 1.2 |
| 24 months | 27 | −0.8 | 1.1 | 21 | −0.5 | 1.0 | 18 | −1.2 | 1.3 |
| Change | 25 | 0.3* | 0.5 | 16 | 0.0 | 0.4 | 13 | −0.2 | 0.7 |
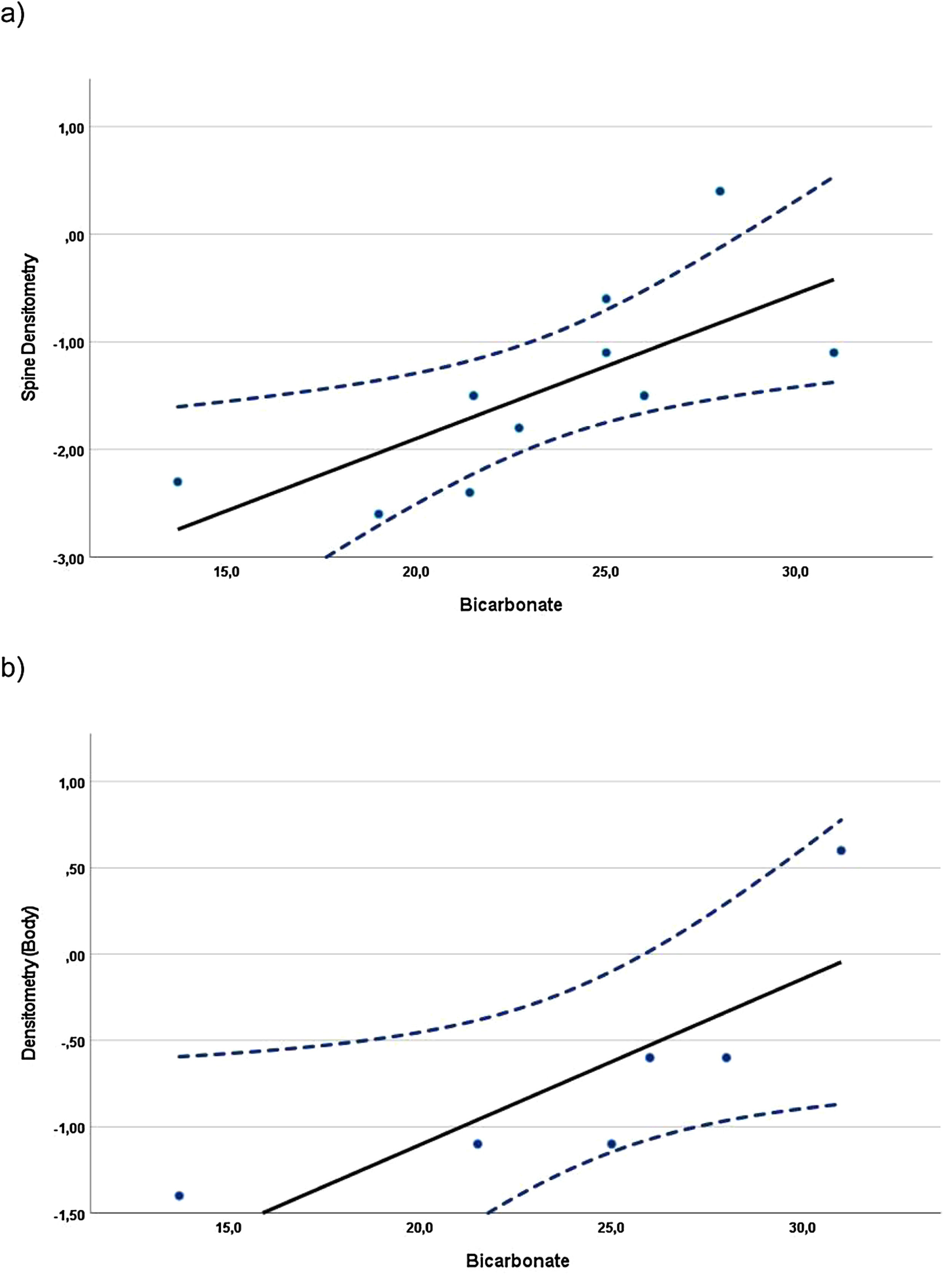
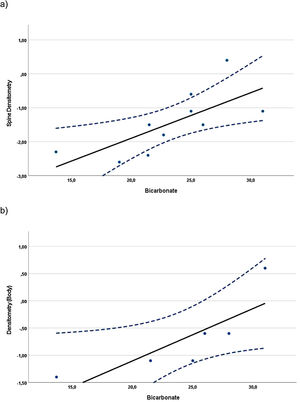
Correlations were found when evaluating the relationship between BMD z-scores and blood and urine parameters, including all data available from the evaluations at baseline and at the 24-month visit. In children, whole-body BMD z-scores (n=10) showed a statistically significant direct (positive) correlation with the UCi/UCr ratio (rS=0.745, p=0.013). In adolescents, the whole-body densitometry z-scores (n=12) showed a statistically significant indirect (negative) correlation with UCa/UCr ratio (rS=−0.585, p=0.046). In the case of adults (Fig. 1), spine (n=10) and whole-body (n=6) BMD z-scores, showed statistically significant direct correlation with plasma bicarbonate levels (rS=0.817, p=0.004 and rS=0.971, p=0.001, respectively).
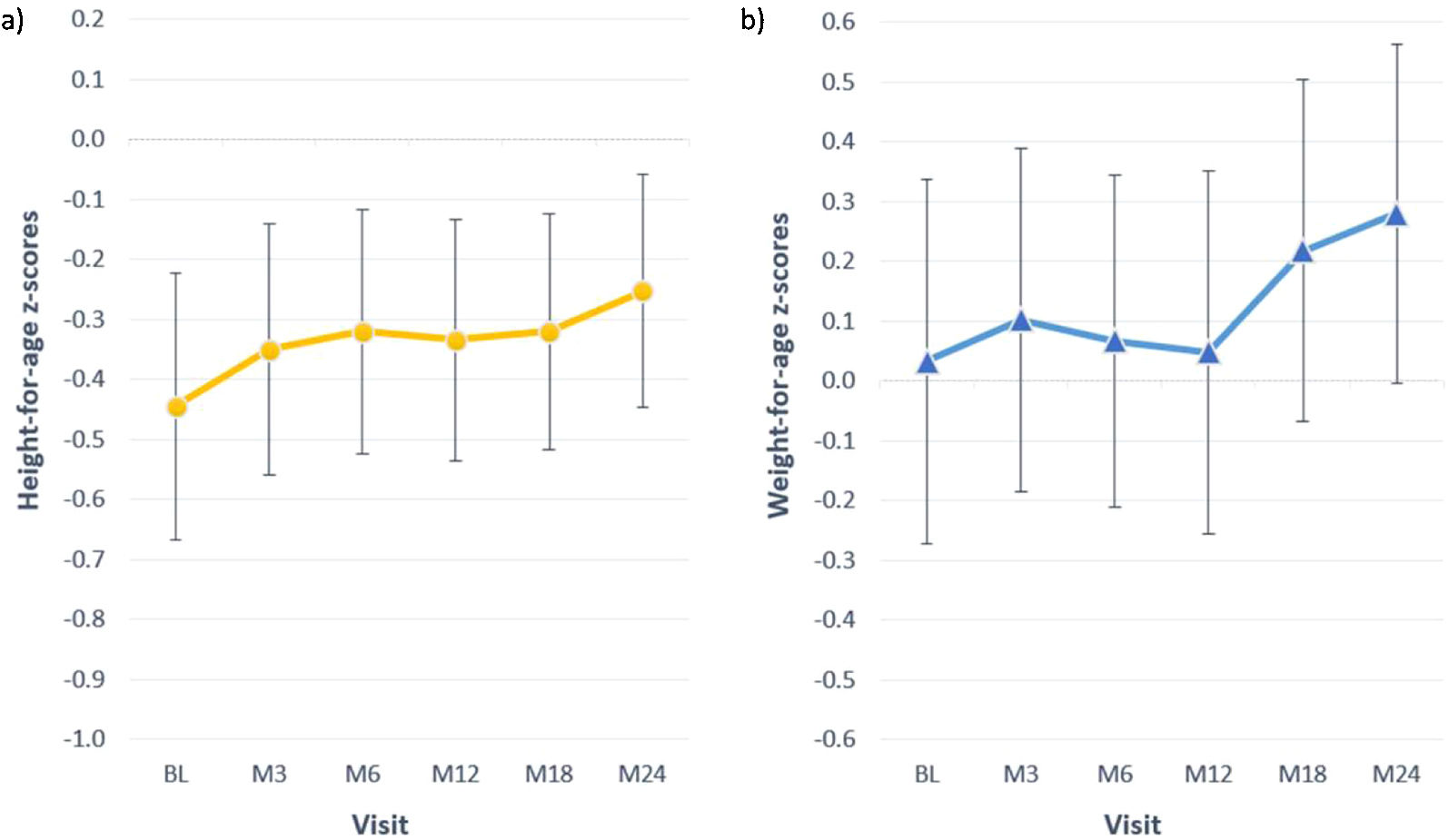
Growth in paediatric patients and young adultsBetween the first and the third month of the study, 5/24 (21%) children presented height velocity values below the 3rd centile and by the end of the study all patients were above the 3rd centile and 18 of them (75%) were above the 25th centile. The mean growth velocity in the overall paediatric population (Table 3) did not show statistically significant changes throughout the study (ANOVA p=0.522).
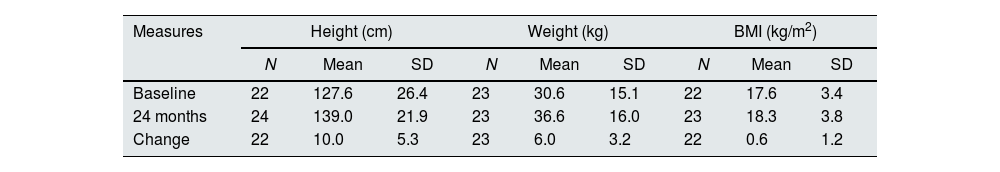
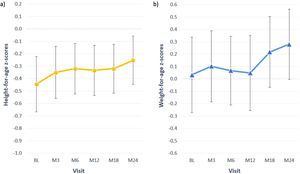
At baseline most children presented z-scores within the ±2SD range for weight, height and BMI. After completion of 24 months of study, the change in z-score for height was 0.2±0.5; for weight the change was also 0.2±0.5 (Table 4). Among children with available data height and weight-for-age z-scores increased>0.5 units in 4 (18%) and 8 (36%), respectively. Fig. 2 shows the evolution of mean height and weight z-scores throughout the study. Particularly interesting was the positive evolution of growth of a girl (4.5 years old at study entry), who presented stunting and failure to thrive (z-scores<−2) at baseline. Her height and weight-to-age z-scores were both>−2 by the end of the study (they increased by 1.8 and 0.8 units, respectively).
Evolution of anthropometric data in paediatric patients.
| Measures | Height (cm) | Weight (kg) | BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Baseline | 22 | 127.6 | 26.4 | 23 | 30.6 | 15.1 | 22 | 17.6 | 3.4 |
| 24 months | 24 | 139.0 | 21.9 | 23 | 36.6 | 16.0 | 23 | 18.3 | 3.8 |
| Change | 22 | 10.0 | 5.3 | 23 | 6.0 | 3.2 | 22 | 0.6 | 1.2 |
| z-Scores | Height-for-age z-score | Weight-for-age z-score | BMI-for-age z-score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Baseline | 22 | −0.4 | 1.0 | 23 | 0.0 | 1.5 | 22 | 0.2 | 1.6 |
| 24 months | 24a | −0.3 | 1.0 | 23 | 0.3 | 1.4 | 23 | 0.2 | 1.5 |
| Change | 22 | 0.2 | 0.5 | 23 | 0.2 | 0.5 | 22 | −0.1 | 0.5 |
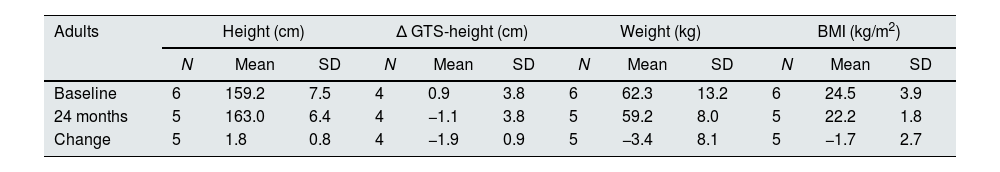
Considering the 5 adults with available data (19–21 years old) mean height increased by 1.8±0.8cm after the 2 years of treatment (Table 5). While at baseline their mean height was 0.9cm below the GTS, they caught up and exceeded GTS by 1.1cm at the end of the treatment.
Evolution of anthropometric data in adult patients.
| Adults | Height (cm) | Δ GTS-height (cm) | Weight (kg) | BMI (kg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Baseline | 6 | 159.2 | 7.5 | 4 | 0.9 | 3.8 | 6 | 62.3 | 13.2 | 6 | 24.5 | 3.9 |
| 24 months | 5 | 163.0 | 6.4 | 4 | −1.1 | 3.8 | 5 | 59.2 | 8.0 | 5 | 22.2 | 1.8 |
| Change | 5 | 1.8 | 0.8 | 4 | −1.9 | 0.9 | 5 | −3.4 | 8.1 | 5 | −1.7 | 2.7 |
GTS=genetic target stature.
After the phase II/III the serum bicarbonate was normal in 63% of patients and it remained normal in most patients (69 to 86%) throughout the 24 months of treatment. At 24 months, overall mean±SD plasma bicarbonate levels were 22.8±2.9mmol/L. Clinically significant metabolic acidosis was reported in one adult patient presenting bicarbonate levels of 19.0mmol/L at the two last visits. With the exception of two cases of occasional hypercalciuria, the UCa/UCr ratios were normal throughout the study, while 9 to 17 patients presented low UCi/UCr levels at the different visits. Approximately half of the patients presented UCa/UCi excretion ratios below the threshold considered for an increased risk of lithogenesis throughout the study.
PTH and bALP levelsPTH and bALP plasma levels were available in 15 patients at baseline and in 24 at the end of the study. Mean (95% CI) PTH was 15.6 (11.1, 20.2) ng/L at baseline and 22.6 (17.4, 27.8) ng/L after 24 months of treatment. There were no significant changes in PTH throughout the study (Kruskal–Wallis p=0.267).
Mean (95% CI) bALP was 57.5 (41.4, 73.6) μg/L at baseline and 53.5 (40.0, 67.0) μg/L after 24 months of treatment without significant modifications observed throughout the study (Kruskal-Wallis p=0.786).
Vitamin D levelsNo significant changes in 25-hydroxyvitamin D and 1.25-dihydroxyvitamin D were observed throughout the study (Kruskal–Wallis p=0.349 and 0.131, respectively).
Mean±SD 25-hydroxyvitamin D was 51.5±33.7nmol/L at baseline (n=10) and 58.9±20.8nmol/L at the 24-month visit (n=21). The mean±SD serum level of 1,25-dihydroxyvitamin D was 153±48pmol/L at baseline (n=15) and 135±30pmol/L at the end of the study (n=25).
Blood levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were normal in 60% and 80% of the patients at baseline and in 43% and 100% of the patients after 24 months, respectively. Clinically significant low levels of 25-hydroxyvitamin D were measured at different time points in six paediatric patients. Occasional vitamin D supplementation was given to 60% of patients.
Safety and tolerabilityA total of 104 adverse events in 27 patients were reported, among which only 9 in 5 patients were confirmed treatment related: three cases of diarrhoea affecting one patient, two cases of abdominal pain in two children and single events of dyspepsia, upper abdominal pain, gastrointestinal disorder and gastrointestinal pain. They were all of mild or moderate severity and no discontinuation of treatment was needed.22
DiscussionAmong the long-term effects of dRTA in children are failure to thrive, stunted growth, osteopenia and rickets4,32; osteopenia and osteomalacia are observed mainly in adults.5
In these patients, alkaline therapy is believed to increase bone mass through the restoration of bone mineral balance and the improvement of osteoblast function.33 In general, the prognosis of dRTA is favourable in most patients on alkali therapy. Treatment has been shown to improve radiological abnormalities of rickets,32,34 increase growth,4,17,35,36 and improve bone structure and bone metabolism.33,37
Adherence to treatment is shown to be key to correct bone abnormalities.38 Reduced growth rates in dRTA children have been associated with sporadic parental noncompliance in administering alkali therapy.4
Additionally, current treatments are not effective in all cases. In a UK cohort of 24 patients with inherited dRTA, growth retardation (height-for-age z-score<−2) was found in 10 patients at presentation (42%) and persisted in 3 (13%) once treated.39 Similarly, in a cohort of 31 dRTA patients with a median follow up of 77 months, severe growth retardation at the time of diagnosis was observed in 14 (45%) patients and at their last visit catchup growth was not achieved in six (19%) of them.19 As illustrated by observations in recent cohorts of paediatric patients, significantly improved height-for-age z-scores are obtained with adequate metabolic control.19,20
As our cohort was previously treated with alkali therapy (in most cases potassium citrate, sodium bicarbonate or a combination of both), most patients were already within the±2SDs range for height and weight before starting ADV7103. However, a positive effect of ADV7103 treatment on the height and weight of patients who were below that range at baseline was shown. Additionally, several paediatric patients presenting growth z-scores within the±2SDs range, but most frequently<0, benefited from increases>0.5 units in their z-scores. In young adults, mean GTS was exceeded, showing a potential benefit on growth also in this population.
Similarly, BMD scores were normal in most patients throughout the study but a significant improvement in mean spine z-scores of the cohort was observed. Additionally, there was a positive correlation between adequate control of metabolic acidosis and bone parameters in some age groups, although this was difficult to observe with our product because most parameters were near or within the normal range, and information describing the effect of acidosis was scarce.
Despite treatment, low citrate/creatinine urine ratios were observed in one third of the patients or more at the different visits. A potential remaining intracellular acidosis could suggest that alkali amounts for these patients should be increased for an optimal adaptation to their needs as they grow. In children there was a positive correlation between citrate/creatinine ratio in urine and whole-body densitometry, suggesting that normal citraturia, an indicator of good metabolic control, is linked to a higher body BMD. In adolescents, there was a negative correlation between calciuria and body densitometry, indicating that patients losing more calcium in urine are those with worst body densitometry, as expected. In adults, there was a strong positive correlation between both spine and whole-body densitometry and plasma bicarbonate levels, again substantiating that adequate metabolic control in patients with dRTA has a positive impact on BMD. These results corroborate those published in a cohort of 10 naive adult patients with dRTA showing increased plasma bicarbonate levels and BMD after treatment with potassium citrate for one year.18 As demonstrated, there is an improvement of BMD in patients with dRTA that had been previously treated with other alkalizing agents. Although we have reported an increase in spine BMD z-score, which can be explained by a sustained metabolic control of plasma bicarbonate levels achieved with ADV7103, a final conclusion with the highest level of evidence cannot be provided due to the absence of a control arm. However, it is remarkable that such an improvement is achieved in patients that were considered adequately treated before switching to ADV7103.
In conclusion, ADV7103 was well tolerated and provided a sustained control of metabolic acidosis in patients with dRTA, which could explain the improvement of growth and spine BMD after 2 years of treatment.
Authors’ contributionsAurélia Bertholet-Thomas has been involved in Investigation and Writing – review & editing. Maria A. Manso-Silván has been involved in Writing – original draft Writing – review & editing. Victor Navas-Serrano has been involved in Writing – original draft Writing – review & editing. Catherine Guittet, has been involved in Conceptualization Methodology Writing – review & editing. Sophie Joukoff, has been involved in Project administration Supervision Writing – review & editing. Justine Bacchetta has been involved in Writing – review & editing. Olivia Boyer has been involved in Investigation and Writing – review & editing. Mariano Rodriguez has been involved in Conceptualization Writing – original draft Writing – review & editing. Luc-André Granier has been involved in Conceptualization Methodology Writing – review & editing.
Ethics approvalThis study was approved by regional independent ethics committees and national regulatory health authorities, and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
Consent to participateWritten consent was obtained from all adult patients, and from the parents or legal guardians of all children. When possible, the assent from paediatric patients themselves was also obtained.
Consent for publicationNot applicable.
FundingThis study was funded by Advicenne.
Conflicts of interestA. Bertholet perceived support from Advicenne for traveling to meetings and/or funding for lectures.
C. Guittet, M.A. Manso-Silván and L.A. Granier are employees of Advicenne and hold stock options or shares in the company. S. Joukoff and V. Navas-Serrano are also employees of Advicenne.
The authors acknowledge L. Archambeaud and the teams of the investigators in the B22CS study and their corresponding Clinical Investigation Centers for their involvement in conducting the clinical trial.