Urinary epidermal growth factor (uEGF) is primarily produced by the kidney, and alterations of it have been associated with several kidney diseases. The aim of this review was to describe uEGF levels in presence or progression of kidney diseases. We conducted a systematic review of observational studies with uEGF determination, patients with acute kidney injury, chronic kidney disease, primary or secondary nephropathy, or renal cancer were included. Studies were searched in Medline, Google Scholar, Science Direct, and EBSCO up to August 2, 2021. Participants and measurements characteristics from which uEGF were determined as the specificity, sensitivity, and the area under the ROC curve, whenever available, were gathered. 53 studies were included, the most frequent kidney diseases studied were acute kidney injury, chronic kidney disease, and diabetic nephropathy. In most studies, uEGF levels were lower in cases than in controls. Studies showed that uEGF levels can predict presence or progression of acute kidney injury, chronic kidney disease, and nephropathy. Heterogeneity in the reported uEGF values can be attributed to the different techniques, sampling, and ways of reporting uEGF values.
Although uEGF values are lower in patients with almost all kidney diseases and their progression, uEGF evaluation methods should be standardised to be used as a biomarker in clinical practice.
El factor de crecimiento epidérmico urinario (uEGF) es producido principalmente por el riñón, y sus alteraciones se han asociado con varias enfermedades renales. El objetivo de esta revisión fue describir los niveles de uEGF en presencia o progresión de enfermedades renales. Realizamos una revisión sistemática de estudios observacionales con determinación de uEGF en la que se incluyeron pacientes con insuficiencia renal aguda, enfermedad renal crónica, nefropatía primaria o secundaria, o cáncer renal. Se realizaron búsquedas de estudios en Medline, Google Scholar, Science Direct y EBSCO hasta el 2 de agosto de 2021. Se extrajeron las características de los participantes y de las mediciones del uEGF, así como la especificidad, la sensibilidad y el área bajo la curva ROC, siempre que estuvieran disponibles. Se incluyeron 53 estudios, y las enfermedades renales más frecuentes estudiadas fueron la insuficiencia renal aguda, la enfermedad renal crónica y la nefropatía diabética.
En la mayoría de los estudios los niveles de uEGF fueron más bajos en los casos que en los controles. Los estudios demostraron que los niveles de uEGF pueden predecir la presencia o la progresión de la lesión renal aguda, la enfermedad renal crónica y la nefropatía. La heterogeneidad en los valores de uEGF informados se puede atribuir a las diferentes técnicas, muestreo y formas de informar los valores de uEGF.
Aunque los valores de uEGF son más bajos en pacientes con casi todas las enfermedades renales y su progresión, los métodos de evaluación de uEGF deben estandarizarse para ser utilizados como biomarcadores en la práctica clínica.
Epidermal growth factor (EGF), formed from pro-pre-EGF and pre-EGF, is a polypeptide of 53 amino acids of 6.2kDa with multiple biological functions such as cell proliferation, transformation, and migration.1 Synthesised in various tissues including the kidney, EGF exerts its actions through the EGF receptor.
The EGF has been identified in various species including humans, and its renal production is present in both apical and basal membranes of the epithelial cells of the proximal tubules, the loop of Henle, and the distal tubules2; and the function exerted by EGF in the kidney is associated with electrolyte homeostasis and proliferation and repair of cell damage also. Kidney diseases that cause acute kidney injury (AKI) and/or chronic kidney disease (CKD) are currently one of the main causes of morbidity and mortality worldwide.3 Epidermal Growth Factor has been identified in different situations as a biomarker of kidney function: the alteration of EGF urine levels (generally its decline) has been associated with the presence of nephropathy, AKI, and CKD, or progression towards these states, as well as the presence of kidney cancer, in a population at risk. With new techniques to measure EGF including in urine, the evidence regarding its capacity as a biomarker has been increasing, the alteration in EGF levels usually precedes the alterations in creatinine and blood urea nitrogen levels, albumin-to-creatinine ratio or uresis, which could represent a therapeutic window for kidney disease; yet despite this, there is no consensus on its use in daily clinical practice, mainly due to a lack of specific cut-off points for each scenario. The objective of this systematic review was to describe the urinary EGF (uEGF) levels for the presence or progression of kidney diseases (primary or secondary nephropathy, AKI, CKD, and/or renal cancer).
MethodsStudy designThe rationale, objective and search strategy of this systematic review were registered in the International Prospective Registers of Systematic Reviews (PROSPERO) under the registration number CRD42021271501.
A systematic review of observational studies with uEGF determination was performed. Studies were searched in Medline, Google Scholar, Science Direct, and EBSCO up to August 2, 2021. Inclusion criteria: the presence of kidney disease, presence of urinary cancer, presence of kidney disease risk factors; in cross-sectional, case-control, or cohort studies, with uEGF determined by enzyme immunoassay (ELISA or EIA) or multiplex magnetic bead-based assay; language, and with availability of the full text. Exclusion criteria: reviews, clinical trials, pre-clinical studies, letters to the editor, or conference posters were excluded. Studies that did not report uEGF levels as means or medians were also excluded (for example, studies that showed data by tertile only).
Setting & study populationsThe search strategy structure adopted was based on a PICO-style approach: Problem: human kidney disease; Intervention or prognostic factor: uEGF; Comparison: healthy or without kidney disease risk factors participants; Outcome: presence, absence, or progression of kidney disease.
We consider kidney disease as any primary or secondary nephropathy, ureteropelvic dysfunction or hydronephrosis, renal cancer, AKI, or CKD. Different clinical settings included ICU, hospitalised, or outpatient milieu.
Search strategy & sourcesThe electronic search strategy for Medline was carried out with the following terms:
((((((((((EGF [Title/Abstract]) OR EPIDERMAL GROWTH FACTOR [Title/Abstract]) OR EPITHELIAL GROWTH FACTOR [Title/Abstract])) OR EPIDERMAL GROWTH FACTOR [mesh]))) OR (EGF [MeSH Major Topic]) AND (((((KIDNEY [Title]) OR NEPHROPATHY [Title/Abstract]) OR RENAL [Title])) OR ((((NEPHROPATHY [MeSH Major Topic]) AND RENAL DISEASE [MeSH Major Topic]) AND KIDNEY DISEASE [MeSH Major Topic]))))) NOT REVIEW[Publication Type] AND (humans[Filter])) NOT (cells[Title]), and the search strategy for other databases is presented in the supplementary material. Reference lists, similar articles or those cited by another article of identified articles, as well as other review studies, were also reviewed manually to identify additional articles. The MOOSE and PRISMA guidelines for reporting systematic reviews were followed4,5 and quality assessment was performed to assess potential risk of bias for each included study according to the NIH/NHLBI Quality Assessment Tools6; depending on the methodological design as cross sectional/cohort, or cases and controls, the respective NIH/NHLBI Quality Assessment Tool was applied; and, the methodological design as indicated by the authors was considered, whereas if it was not specified, or was only mentioned as a prospective study, the methodological design corresponding to the methodology described in the study was identified. The quality of the studies was classified as good, fair, or poor (see supplementary material).
Study selection processAll reviewers are researchers or students from the health area. One person extracted the data, and another person checked the extracted data. Disagreement was discussed and consensus was reached using a third opinion. Two reviewers independently assessed potential risk of bias and were blinded to each other. Disagreement was discussed and a consensus reached using a third opinion. Studies in languages other than English or Spanish were translated using an online Google translator.
Data extractionStudies were grouped according to the authors’ declaration as CKD, AKI, or a specific nephropathy, the latter could present different degrees of renal function. An Excel datasheet was used for data extraction. The variables extracted included: age, sex, sample size, settings, biological material in which uEGF was measured (spot or 24-h urine), uEGF measurement technique, sample storage, uEGF values, and its units of measurement. Also, specificity, sensitivity, area under the ROC curve (AUC), and hazard ratio (HR) with 95% confidence interval (CI) were extracted whenever they were available.
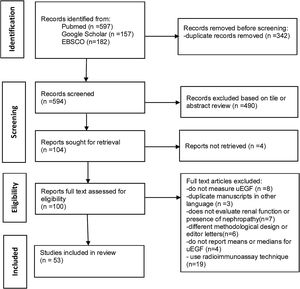
ResultsStudy characteristicsThe initial results of the bibliographic search identified 936 articles, from which 342 were eliminated because they were duplicates, 494 were excluded based on title or abstract review or not retrieved and 19 for using radioimmuno-assay. The main reason for excluding articles after reading the full text was that they did not evaluate uEGF levels. After reading the full articles, 53 studies were ultimately included.7–59 The flowchart for the selection process was according to PRISMA guidelines, and Fig. 1 shows a flowchart of the study's selection process. The characteristics of studies included are presented in Supplementary Tables 1–4.
Quality assessmentSupplementary Table 5 shows the evaluation of the quality of the selected articles. The most frequently found risks of bias were the lack of justification of the sample size power description or variance and effect estimates provided, the lack of a specified and defined study population, and the lack of measurement and analysis of key potential confounding variables. None of the studies reported whether there was blinding to the exposure status of participants of outcome assessors. From the included studies in this review, 30 (56.6%) were identified as “good” quality, 22 (41.5%) were classified as “intermediate”, and 1 (1.8%) were classified as “poor” quality.
Overall summary of uEGF in different types of kidney diseaseSeveral studies reported more than one type of kidney disease: the most frequently studied diseases were AKI (20.7%), CKD (18.8%), diabetic nephropathy (9.4%), and reflux or obstructed nephropathy (NPT) (7.5%); while other kidney diseases were: glomerulonephritis, IgA NPT, post-transplantation renal tumour, polycystic kidney disease (PKD), congenital anomalies, lupus nephritis, carcinoma of the bladder, renal amyloidosis, Henoch-Schönlein purpura nephritis, renal pelvic or calyceal stone, Alport syndrome, Wilms tumour, human immunodeficiency virus (HIV) NPT, and unspecified origin NPT.
The methodological design reported by the authors or identified by the reviewers were, according to inclusion criteria, 34 studies with cohort design, 15 cross-sectional, and 4 with case-control design. The uEGF assays used in the studies were Enzyme Immunoassay/Enzyme-Linked Immunosorbent Assay (ELISA), and multiplex assay. The oldest studies were those using radioimmunoassay (RIA); most of them with a low-quality assessment, so they were not included in the review, while the most recent studies used both ELISA and multiplex assay (a type of immunoassay to simultaneously measure multiple analytes in a single sample). Regarding the units reported for uEGF levels, 39 studies reported uEGF adjusted to urinary creatinine, 9 studies reported uEGF in weight units over volume units (ej, pg/mL), 3 studies presented the levels in weight units over 24h (ej. pg/24-h), one study reported as a rate (mg/mL/min), and one study did not specify units.
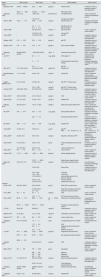
Urinary EGF findings in studies of nephropathy or CKDThe uEGF levels were analysed in 39 studies in both primary and secondary kidney diseases as well as in CKD; they can be seen in Table 1. According to the age of the patients studied, 13 studies were performed on children and adolescents, and 26 on adults and older adults, one of them did not mention the age of the participants.7 Among the studies that reported Cr-adjusted uEGF values, the lowest values in patients with kidney disease were found in adult patients by Stangou et al., in IgA NPT and in pauci-immune focal segmental necrotising glomerulonephritis12,13; however, the controls of these patients presented with the lowest levels of uEGF reported where, in both cases, values equal to or less than 0.00014ng/mg Cr were reported; while the highest values in patients with kidney disease were 80ng/mg Cr in paediatric patients with obstructive nephropathy,33 and the highest values for controls were 120.6ng/mg Cr in adults.19
uEGF levelsa in studies reporting nephropathy (NPT) or chronic kidney disease (CKD).
| Author | uEGF controls | uEGF cases | Units | Kidney disease | uEGF outcome |
|---|---|---|---|---|---|
| Adults | |||||
| Gesualdo, 19957 | 7242.6b±1530.3 | 2145±762.7 | pg/mg Cr | PKD with CKD | A reduction may be a prognostic marker of renal dysfunction |
| 9183.7±1049 | 10,335.5±1273.6 | PKD without CKD | |||
| Jørgensen, 19958 | 2 (1.4–3.4) | 1.8 (0.6–2.3) | pmol/h/mL/min | Transplants donors | Lower in cases than controls (pre-operatively donors) |
| 1.2 (0.5–3.3) | Transplants recipients | ||||
| Ranieri, 19969 | 12.96±11.15 | 20.05±2.64 | ng/mg Cr | IgA NPT grade 1–2 | Progressive decreases according to the degree of NPT. |
| 7.6±1.7 | IgA NPT grade 3–4 | ||||
| 3.14±0.71 | IgA NPT grade 5 | ||||
| Torffvit, 199810 | 0.47 (0.05–2.51) | nmol/24h | Glomerular NPT | Lower in cases than normal controls. | |
| 0.13 (0.03–1.08) | Tubular NPT | ||||
| Torres, 200811 | – | 18.35 (8.03–44.5) | ng/mg Cr | IgA NPT | A reduction may be a prognostic marker of renal dysfunction |
| Stangou, 200912 | 0.15±0.08 | 0.05±0.05 | pg/mg Cr | IgA NPT | A reduction may be a prognostic marker of renal dysfunction |
| Stangou, 201213 | 0.14±0.07 | 0.15±0.3 | pg/mg Cr | Pauci-immune FSNGN | A reduction may be a prognostic marker of histological damage and response to treatment |
| Harskamp, 201514 | 32,939 (26,049–63,420) | 11,345 (345–26,367) | ng/24h | Autosomal dominant PKD | Lower in cases than normal controls. |
| Ju, 201515 | – | 2.5±1.1 | Log2 ng/mg | CKD stages I–IV | Independent risk predictor of CKD progression |
| 3±1.3 | Primary proteinuric glomerular disease | Progressive decrease according to the degree of NPT | |||
| 3.5±1 | IgA NPT | ||||
| Betz, 201616 | 10.17 (5.06–16.46) | 6.42 (3.29–12.969) | μg/mmol Cr | Diabetic NPT | A reduction may be a prognostic marker of renal dysfunction |
| Worawichawong, 201617 | 11.7 (7.5–18.8) | 4.4 (2.4–7.6) | ng/mg Cr | Primary GN | Lower in cases than normal controls, associated with tubular atrophy and interstitial fibrosis |
| Segarra-Medrano, 201718 | 21.3 (14.5–26) | 12.6 (6.3–18) | ng/mg Cr | IgA NPT T1 Oxford criteria | A reduction may be a prognostic marker of interstitial fibrosis |
| 3.2 (1.7–4.89 | ng/mg Cr | IgA NPT T2 Oxford criteria | |||
| Chanrat, 201819 | 120.6 (58.3–192.4) | 59. (150.0–87.2) | ng/mg Cr | Not remission in primary GN | A reduction may be a prognostic marker of renal dysfunction and complete remission |
| Dincer, 201820 | 3.66 (1.84–5.60) | 2.74 (1.12–6.21) | ng/mg Cr | CKD | Lower in cases than normal controls. |
| Nowak, 201821 | 13.1 (8.7–18.6) | 10.5 (8.1–15.0) | ng/mg Cr | Diabetic NPT | A reduction may be a prognostic marker of renal dysfunction |
| Satirapoj, 201822 | 42.8 (23.4–65.1) | 19.5 (11.1–36.3) | ng/mg Cr | Rapid loss function diabetic NPT | Lower in rapid renal progression group than non-rapid renal progression group |
| Wu, 201823 | 4.34±0.76 | 2.04±1.41 | Log2 ng/mg | Active patients with AAV | Progressive decrease according to the degree of NPT, |
| 2.63±1.31 | Remission patients with AAV | A reduction may be a prognostic marker of resistance to treatment and renal dysfunction | |||
| Wu, 202024 | 3.08±1.12 | 2.94±0.98 | Log2 ng/mg | Diabetic NPT | Lower in diabetic with NPT than diabetic without NPT controls. |
| Yang, 202025 | 7.6 (6.0–10.1) | 3.8 (2.9–5.1) | μg/g Cr | IgA NPT GFR<60mL/min/1.73m2 | Lower in the <60mL/min/1.73m2 and associated with progression |
| Zheng, 202026 | 8.2 (6.5–10.2) | 8.3 (6–12.6) | ng/mg Cr | Idiopathic membranous NPT | No statistically significant difference between cases vs controls |
| Ascher, 202127 | 14.7 (9.4–20.7) | 9.2 (5.9, 13.4) | ng/mLb | Incident CKD/baseline women with HIV | A reduction may be a prognostic marker of incident CKD |
| 13.7 (9.2–20.6) | 9.2 (5.2, 12.0) | Incident CKD/follow up | |||
| Hefny, 202128 | 50.7±0.9 | 30.2±16.7 | ne | Lupus nephritis | Lower in cases than normal controls. |
| Heidari, 202129 | 1717.2±482.2 | 1146.8±585.3 | pg/mg Cr | Kidney allograft rejection | A reduction may be a prognostic marker of antibody mediated rejection |
| 1671.5±695.6 | Stable kidney allograft function | ||||
| Mejía-Vilet, 202130 | 16.8 (16.0–17.9) | 10.9 (6.7–15.4) | ng/mg Cr | Active lupus nephritis first flare | Urine EGF levels correlate with histologic kidney damage. |
| 5.3 (2.6–9.3) | Second flare | A reduction may be a prognostic marker of renal dysfunction | |||
| 3.5 (1.4–8.6) | Third flare | ||||
| 1.8 (1.1–2.8) | Fourth flare | ||||
| 19.9 (16.6–25.7) | Inactive/mildly active SLE no previous nephritis | ||||
| 8.9 (6.0–17.8) | Previous nephritis | ||||
| 18.2 (10.8–27.5) | Systemically active SLE | ||||
| Pediatrics | |||||
| Konda, 199731 | 36.5 (22.7–58.6a) | 23.8 (10.5–54) | μg/g Cr | Reflux NPT normal function | Lower in cases than normal controls. |
| 18.5 (9.5–36.2) | Reflux NPT unilateral low function | ||||
| 3 (1.1–8.4) | Reflux NPT total low function | ||||
| Tsau, 199932 | 15.2±6.5 | 6.9±3 | ng/mg Cr | CKD | Lower in cases than normal controls. |
| 13.6±5.1 | NPT with normal renal function | ||||
| Chiou, 200433 | 681.8±113.7 | 800.2±118.3 | pg×102/mg Cr | Obstructed vs unobstructed kidney | Correlated with preservation of postoperative renal function |
| 937.41±124.98 | 577.07±154.43 | Preserved vs poorly preserved function | |||
| Li, 201234 | 50 (35–81) | 38 (20–57) | ng/mg Cr | Hydronephrosis | A reduction may be a prognostic marker of need of surgery |
| Madsen, 201335 | 4 (1.2–60.2) | 7.4 (1.2–13.8) | ng/mg Crb | Ureteropelvic junction obstruction | Higher in cases than normal controls |
| Pastore, 201736 | 790±190 | 681±277 | pg/mL | Vesico-ureteral reflux | Lower in cases than normal controls. |
| Ledeganck, 201837 | 67.4 (17.9–218.8) | 7.0±1.1 | ng/mL | Transplanted/Calcineurin inhibitor | Correlated with renal function |
| 11.5±2.4 | CKD | ||||
| 35.4±6 | Nephrotic syndrome/Calcineurin inhibitor | ||||
| 47.7±6.6 | Nephrotic syndrome | ||||
| Li, 201838 | 54.17±22.84 | 10.59±6.863 | ng/mg Cr | Progressors Alport syndrome | Lower in cases than normal controls. |
| 30.87±9.37 | 27.83±12.67 | Non-progressors Alport syndrome | A reduction may be a prognostic marker of renal disfunction | ||
| 22.06±5.78 | |||||
| Azukaitis, 201939 | – | 3.46 (1.92–6.47) | ng/mg Cr | CKD | A reduction may be a prognostic marker of progression in children with CKD. |
| Bartoli, 201940 | 515±168 | 754±4335 | pg/mL | Hypoplastic | Lower in cases than normal controls. |
| 628±252 | Agenesic | ||||
| 794±243 | Multicystic | ||||
| 408±201 | Nephrectomy | ||||
| Gipson, 201941 | 71.4 (40.0–91.3a) | 39.9 (27.3–55.69) | ng/mg Cr | Minimal change disease GN | A reduction may be a prognostic marker of renal dysfunction |
| 24.9 (11.4–41.29 | Focal segmental glomerulosclerosis | ||||
| Srivastava, 202042 | 18,637 (15,298–25,622) | 20,098 (13 238–30 263) | pg/mgCr | Solitary functioning kidney | No statistically significant difference between cases vs controls |
| Ledeganck, 202143 | 46 (23.1–121) | 32.8 (6.2–96.3) | ng/mg Cr | Diabetic NPT | Lower in cases than normal controls. |
Use multiplexing technique. To convert nmol/mmol Cr to ng/mg Cr, multiply by 52.694. AAV=antineutrophil cytoplasmic antibody-associated vasculitis, FSNGN=pauci-immune focal segmental necrotising glomerulonephritis, GFR=estimated glomerular filtration rate, GN=glomerulonephritis, PKD=polycystic kidney disease, SLE=systemic lupus erythematosus.
Table 2 shows the studies that analysed and reported uEGF levels in children or adults patients with AKI. Values reported in these cases were from 1.7ng/mg Cr in adults45 to 18.8ng/mg Cr in children hospitalised in the ICU.47 In the 4 studies regarding AKI, all of them reported lower levels of uEGF in cases versus healthy or exposed controls.
uEGF levelsa in studies reporting acute kidney injury (AKI).
| Author | uEGF controls | uEGF cases | Units | Specific kidney disease | Settings | uEGF outcome |
|---|---|---|---|---|---|---|
| Adults | ||||||
| Di Paolo, 199344 | 12.96±1.15 | 6.28±1.52 | ng/mg Cr | Stable graft function | ns | Lower than normal controls |
| 3.09±0.68 | Acute rejection | |||||
| 5.23±0.92 | Acute tubular damage | |||||
| Kwon, 201045 | 9549.81 (5758.75–20,271.5) | 1705.58 (814.57–2924.97) | pg/mg Crb | Ischaemic | Hospitalized | Lower than normal controls, a reduction may be a prognostic marker of recovery and mortality |
| Singal, 201846 | 4253 (2517–6983) | 2254 (1350–4651) | pg/mg Crb | No | Cirrhosis patients listed for liver transplantation | Lower than no AKI controls |
| Paediatrics | ||||||
| Wai, 201347 | 56,324 (26,342–142,460) | 18,889 (729–58,889) | pg/mg Cr | No | ICU/septic shock or requiring ECMO | Lower than no AKI controls |
Malignant neoplasms of the kidney or bladder were evaluated only in one of the included studies48 where paediatric Wilms tumour survivors with eGFR<90mL/min/1.73m2, uEGF levels were lower.
Urinary EGF findings in studies of neonatesThe uEGF was evaluated in 9 studies carried out in neonates, in all of them reported the diagnostic accuracy analysis values and 6 also showed the specific values of uEGF (see Table 3); of which, the lowest values were those reported in neonates of term with, and without, AKI by Askenazie et al.52; yet when adjusting the values with urinary Cr levels, the values reported in neonates with AKI and with ureteropelvic obstruction were similar.
uEGF levelsa in studies in neonates.
| Author | uEGF controls | uEGF cases | Units | AKI | Specific kidney disease | Settings | uEGF outcome |
|---|---|---|---|---|---|---|---|
| Askenazi, 201249 | 17.4 (12.7–23.8) | 6.7 (4.0–11.3) | pg/mLb | Yes | No | ICU/term | Infants with AKI had lower uEGF levels |
| Mohammadjafari, 201451 | 20.06 (19.73–28.11) | 16.86 (11.76–23) | ng/mg Cr | No | Ureteropelvic junction obstruction | Outpatient | No significant differences between case and controls |
| Askenazi, 201652 | 790 (496–1200) | 468 (363–872) | pg/mLb | Yes | No | ICU/preterm | Lower than no AKI controls |
| Hanna, 201653 | 0.016 | 0.006 | μg/mLb | Yes | No | ICU/preterm | uEGF was a predictor of renal injury |
| Sweetman, 201654 | 3871.6 (1978.9–6776.3) | 585.7 (363.4–1836.7) | pg/mLb | Yes | No | ICU/perinatal asphyxia | Lower than normal and no AKI controls |
| 6193.2 (1793.3–11,033.1) | Healthy controls | ||||||
| Ahn, 202057 | 24.9 (23.4–29.6) | 16.3 (13.9–22.0) | ng/mg Crb | Yes | No | ICU/preterm | Lower than no AKI controls |
In all cases, uEGF values were lower in cases with kidney disease versus healthy controls or those without kidney disease.
Studies with diagnostic accuracy in analysis of urinary EGFOf the studies included, only 7 in adults, 1 in children and 6 in neonates reported a cut-off value: 13 of them showed the AUC of the respective cut-off value, and 11 reported sensitivity and specificity (see Table 4). In adults, the lowest cut-off values in ng/mg Cr or ng/mL were observed in CKD and in antibody-mediated kidney allograft rejection,23,29 while the highest values were described in primary glomerulonephritis.19 The highest AUC values were observed with cut-off values of 10.8ng/mg Cr to identify primary glomerulonephritis, and 5.3ng/mg Cr for lupus nephritis: these cut-off values also presented the highest sensitivities.17,30
Studies with diagnostic accuracy analysis of uEGF.
| Author | Cut-off value | Sensitivitya | Specificitya | HR | 95%IC HR | AUC | 95% IC AUC | Outcome | Kidney disease or settings |
|---|---|---|---|---|---|---|---|---|---|
| Adults | |||||||||
| Torres, 200811 | nr | nr | nr | 0.95 | 0.92–0.98 | 0.83 | 0.76–0.89 | Doubling sCr and/or ESKD | IgA NPT |
| Ju, 201515 | nr | nr | nr | 0.33 | 0.21–0.51 | 0.89 | 0.84–0.95 | CKD progression | CKD stages I-IV |
| nr | nr | nr | 0.33 | 0.21–0.52 | 0.82 | 0.73–0.91 | Primary proteinuric glomerular disease | ||
| nr | nr | nr | 0.57 | 0.46–0.70 | 0.71 | 0.64–0.77 | IgA NPT | ||
| Betz, 201616 | nr | nr | nr | 0.45 | 0.3–0.69 | 0.78 | 0.74–0.82 | Incident GFR<60mL/min per 1.73m2 and rapid decline of renal function | Type 2 diabetes |
| Worawichawong, 201617 | 10.8ng/mg Cr | 0.94 | 0.55 | 0.77 | 0.64–0.92 | 0.83 | 0.71–0.95 | Moderate to severe interstitial fibrosis and tubular atrophy | Primary glomerulonephritis |
| Segarra-Medrano, 201718 | nr | nr | nr | 0.59 | 0.36–0.96 | 0.87 | nr | Fibrosis interstitial T1 and T2 Oxford grade | IgA NPT |
| Chanrat, 201819 | 75ng/mg Cr | 0.71 | 0.66 | 2.28 | 1.08–4.84 | 0.72 | 0.60–0.84 | Complete remission | Primary glomerulonephritis |
| Satirapoj, 201822 | 29.9ng/mg Cr | 0.703 | 0.69 | 0.98 | 0.97–0.99 | 0.68 | 0.57–0.80 | Rapid GFR decline | Type 2 diabetic patients with NPT |
| Wu, 201823 | 0.46 log2 uEGF/Cr | 0.63 | 0.63 | 0.88 | 0.80–0.97 | 0.66 | nr | ESKD or 30% reduction of GFR. | Antineutrophil cytoplasmic antibody-associated vasculitis |
| Yepes-Calderón, 201958 | nr | nr | nr | 0.68 | 0.59–0.78 | 0.81 | nr | Risk of graft failure | Renal Transplant Recipients |
| Wu, 202024 | nr | nr | nr | 0.66 | 0.53–0.82 | ESKD or a 30% reduction in GFR. | Type 2 diabetic patients with NPT | ||
| 0.96 | 0.95–0.96 | Discrimination of diabetic NPT | Type 2 diabetes | ||||||
| Yang, 202025 | 4.7μg/g Cr | nr | nr | 3.9a | 2.4–6.7a | nr | nr | NPT progression | IgA NPT |
| Zheng, 202026 | nr | nr | nr | 0.502 | 0.16–2.81 | nr | nr | Massive proteinuria | Idiopathic membranous NPT |
| nr | nr | nr | 2.476 | 0.94–3.35 | nr | nr | GFR decreased | ||
| nr | nr | nr | 0.748 | 0.41–2.18 | nr | nr | Interstitial fibrosis and renal tubular atrophy | ||
| Norvik, 202059 | nr | nr | nr | 1.17 | 0.89–1.53 | nr | nr | per 1μg/mmol lower uEGF GFR decline>3.0mL/min/1.73m2/year | Subjects without diabetes or established CKD (Norway cohort) |
| 1.32 | 1.13–1.54 | Subjects without diabetes or established CKD (Netherlands cohort) | |||||||
| Ascher, 202127 | nrc | nr | nr | 0.61b | 0.50–0.75 | nr | nr | Incident CKD | Women living with HIV |
| Heidari, 202129 | 1199.9pg/mL | 0.77 | 0.68 | nr | nr | 0.74 | nr | Early diagnosing of rejection | Antibody mediated kidney allograft rejection |
| Mejía-Vilet, 202130 | 5.3ng/mg | 0.81 | 0.77 | 0.88b | 0.77–0.99 | 0.82 | nr | Progress to ESKD | Lupus nephritis |
| Paediatrics | |||||||||
| Li, 201234 | 43ng/mg Cr | 0.667 | 0.75 | nr | nr | 0.69 | 0.47–0.91 | Surgery in the first 6 months of life | High-grade hydronephrosis |
| Azukaitis, 201939 | nr | nr | nr | 0.76 | 0.69–0.84 | nr | nr | Incident CKD | Children with several kidney diseases |
| Gipson, 201941 | nr | nr | nr | 2# | 1.1–2.9 | nr | nr | Incident CKD | Children with Nephrotic Syndrome, |
| Neonates | |||||||||
| Askenazi, 201249 | nrc | nr | nr | nr | nr | 0.81 | nr | AKI | Newborns |
| Hoffman, 201350 | 45,000pg/mg Cr | 0.73 | 0.82 | nr | nr | 0.77 | nr | AKI | Critically ill neonates |
| 3179pg/mL | 0.64 | 0.84 | nr | nr | 0.73 | nr | |||
| Mohammadjafari, 201451 | 300.485ng/L | 0.6 | 0.53 | nr | nr | 0.56 | nr | Needed surgery | Ureteropelvic junction obstruction |
| 16.8554ng/mg Cr | 0.71 | 0.77 | nr | nr | 0.72 | nr | |||
| Askenazi, 201652 | 590pg/mLc | nr | nr | nr | nr | 0.68 | nr | AKI | Very low-birth-weight infants |
| Hanna, 201653 | nrc | nr | nr | nr | nr | 0.97 | nr | Stage I AKI | Preterm |
| nr | nr | nr | nr | nr | 0.86 | nr | Stage II/III AKI | Preterm | |
| Sweetman, 201654 | 2923.2pg/mLc | nr | nr | nr | nr | 0.91 | nr | AKI | Neonatal encephalopathy |
| De Freitas, 201655 | 3ng/mLc | 0.85 | 0.42 | nr | nr | 0.79 | 0.65–0.93 | GFR<30mL/min/1.73m2 | Preterm and Term newborns |
| Gupta, 201656 | 1.75pg/mL | 0.7 | 0.75 | nr | nr | 0.75 | 0.53–0.91 | AKI | Treated with hypothermia for hypoxic ischaemic encephalopathy |
In neonates, the lowest cut-off value (1.75pg/mL) was observed in patients with AKI treated with hypothermia for hypoxic ischaemic encephalopathy,56 and when the uEGF was adjusted for the level of urinary Cr, the cut-off values in neonates with AKI were higher, reaching up to 45ng/mg Cr; where this last value was the one that reported the highest AUC as well as sensitivity and specificity in neonates.50
No cut-off points were identified for studies related to AKI, or kidney or bladder cancer, in children or adults.
DiscussionSince its identification in the early 1960s, numerous articles on EGF have been published.
In this study, we carried out a systematic review on the levels of uEGF in kidney diseases in patients of all ages. A significant number of studies were found where a statistically significant alteration was identified in patients with various kidney pathologies, in most cases with a decrease in uEGF levels. Although recent research shows uEGF used as a biomarker of function, in the presence of kidney disease or therapeutic response,60–62 the measurement of uEGF in routine clinical practice is not performed. Numerous factors may be contributing to this situation with the main one being the lack of a universally accepted cut-off value either to establish the normality of the values or to identify a particular disease. In this review, a significant heterogeneity of uEGF levels could be identified not only in the patients studied but also in the controls, even among patients of the same age range. This problem in establishing a cut-off point may be due to the lack of uniformity in the way of reporting the levels of uEGF and to the different techniques used to measure it.
Most authors reported the levels of uEGF adjusted to the levels of urinary Cr, while another group of researchers evaluated the levels without considering this parameter, which makes it difficult to compare the results since there is no study that evaluates the correlation between the different techniques available for measuring uEGF. Another difference in the report of uEGF values was the sampling method: some authors collected the urine sample for 24h while others were spot samples. To this regard, it has been reported that there was no significant difference in uEGF according to the way the urine was collected63,64; however, in some kidney pathologies, the spot uEGF/creatinine ratio could over or underestimate the 24-h uEGF values, in addition that, in the former method, a high intra-individual variability could be found, requiring serial measurements.65,66 Age, on the other hand, is a factor that has been identified as intervening in uEGF levels; from neonatal patients where uEGF values showed differences according to gestational age,55 as well as between children, adolescents, and adults. Other characteristics that vary significantly between studies is the lack of uniformity between the timeline in prospective studies, and the criteria to consider the presence of kidney diseases; and, frequently, it is not established whether to identify its value as a diagnostic or as a prognostic factor.
Regarding the pathologies studied and the way of reporting, it is suggested that in the case of AKI, the adjustment of uEGF values with urinary Cr might not be ideal due to the changes that occur in the latter; so, in AKI, the uEGF levels could be more exact without adjusting.67
On the other hand, it was not possible to identify whether any kidney disease was associated with the lowest levels of uEGF, since, as previously commented, when the units were equivalent the age or the technique used to measure was not, so the utility of uEGF for distinguishing between different types of kidney disease is not clear.
In general, we substantiated that in most studies patients of all ages with kidney disease, including cancer, have lower levels of uEGF compared to their controls. The EGF is a growth factor that has been identified in various tissues; however, EGF measured in urine appears to be produced mainly in the kidneys, while in plasma the source of EGF may be more diverse.68 The predominantly renal origin of uEGF makes it an important marker of kidney homeostasis, and it has been shown to participate in the control of electrolytes, particularly magnesium,69 and in podocytes, provides an effect repair and protection against noxious stimuli such as hyperglycemia.70 Although the overexpression of the EGF receptor is widely described in the genesis of cancer including kidney cancer, this receptor has various ligands so a decrease in uEGF in kidney cancer could be explained, according to some authors, by the decrease in the renal production due to epithelial cell damage.71
Our study had some limitations. Firstly, we did not use a single criterion to define the various types of kidney diseases that we included in the review. Secondly, we were not able to convert all the uEGF values to a single unit of measurement to be able to make an adequate comparison between all of them since the studies did not report urinary Cr values or uresis in 24h, so we could only compare between those for which we were able to obtain the equivalent units. Thirdly, other standard early markers such as albuminuria were not reported due to most studies not reporting such findings. Fourth, our study did not include the calculation of a cut-off value for uEGF due to the great heterogeneity between the studies, and we could not establish a normal value among healthy patients. On the other hand, this review provides a reference source for the use of uEGF in the clinical practice, without established cut-off points, the comparison with the levels reported in similar populations may be useful in monitoring uEGF in patients. The prospective cohort studies included in this review show the association of low abnormal levels of uEGF with the progression of the disease and decreased function of kidney, so using the uEGF levels of patients as their own control could be a monitoring strategy in these patients.
In conclusion, uEGF values are decreased in patients with primary and secondary nephropathy, AKI, CKD, and renal or bladder carcinoma; and progression to AKI in patients with risk, or to CKD in patients with primary and secondary nephropathy, were also associated to lower levels of uEGF. It is necessary to establish criteria to standardise the way of evaluating uEGF to be able to use it as a valuable biomarker in clinical practice.
Authors’ contributionsMRS, MH and XT designed the study, JABB, II, YD and YC carried out the data collection. MRS, OMC and EMZ analyzed the data. JABB, II, YD, YC and EMZ made the figure and the tables. MRS, MH, OMC and XT wrote and reviewed the article. All authors agree and have approved this version of the manuscript. We declare that the work has not been previously published and that it is not under evaluation for publication in any other medium.
Conflicts of interestAll the authors declare no competing interest.
This research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.