We aim to adapt the International Consortium for Health Outcomes Measurements standard set for chronic kidney disease (CKD) patients to the Spanish setting and supplement it with those variables agreed upon through initiatives proposed by the Spanish Society of Nephrologists (S.E.N.).
Material and methodsThe working group defined a first standard set of variables based on a literature review. The S.E.N. members then assessed the suitability of each variable for inclusion (Consensus≥75%). A second draft of the standard set was generated and evaluated by the Patient advocacy group Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER). Lastly, the working group established the final standard set of variables (Consensus≥75%).
ResultsThe standard set targets patients with very high-risk CKD (G3a/A3 and G3b/A2-G5) in pre-end-stage kidney disease (pre-ESKD), hemodialysis (HD), peritoneal dialysis (PD), kidney transplantation (KT) or conservative care (CC). The essential follow-up variables agreed for all patients (All) were patient survival, hospitalizations, cardiovascular events, smoking status, health-related quality of life, pain, fatigue, physical function, daily activities, depression, renal function and hemoglobin. Additionally, it was agreed to collect PD survival (in PD patients), peritonitis (PD), infection/bacteremia (PD, HD, KT), vascular access type (HD), vascular access survival (HD), acute rejection (KT), post-transplant cancer (KT), albuminuria (KT) and kidney allograft survival (KT). The optional variables agreed were phosphorus (All), potassium (All), diabetes control (All with diabetes), and albuminuria (pre-ESKD).
ConclusionsThis standard set may constitute a highly efficient tool allowing the evaluation of patient outcomes and helping to define strategies to enhance CKD patients’ quality of care in the Spanish healthcare system.
El objetivo del estudio es adaptar el conjunto de variables de resultados del International Consortium for Health Outcomes Measurements para pacientes con enfermedad renal crónica al ámbito español y complementarlo con aquellas variables consensuadas en iniciativas de la Sociedad Española de Nefrología.
Material y métodosEl grupo de trabajo definió un primer conjunto de variables a partir de una revisión bibliográfica. Seguidamente, los miembros de la Sociedad Española de Nefrología valoraron la idoneidad de cada variable para su inclusión (consenso≥75%). Posteriormente, se generó un segundo borrador que fue evaluado por la asociación de pacientes Federación Nacional de Asociaciones para la lucha contra las enfermedades del riñón. Por último, el grupo de trabajo estableció el conjunto de variables final (consenso≥75%).
ResultadosEl conjunto de variables se dirige a pacientes con enfermedad renal crónica y muy alto riesgo de progresión (G3a/A3 y G3b/A2-G5) en estadios previos al tratamiento renal sustitutivo, hemodiálisis (HD), diálisis peritoneal (DP), trasplante renal (TR) o tratamiento conservador. Las variables esenciales de seguimiento acordadas para todos los pacientes fueron la supervivencia del paciente, las hospitalizaciones, los eventos cardiovasculares, el hábito tabáquico, la calidad de vida relacionada con la salud, el dolor, la fatiga, la función física, las actividades diarias, la depresión, la función renal y la hemoglobina. Además, se acordó recoger la supervivencia en DP (en pacientes en DP), peritonitis (DP), infección/bacteriemia (DP, HD, TR), tipo de acceso vascular (HD), supervivencia del acceso vascular (HD), rechazo agudo (TR), cáncer postrasplante (TR), albuminuria (TR) y supervivencia del aloinjerto renal (TR). Las variables opcionales acordadas para todos los pacientes fueron los niveles de fósforo y potasio y el control de la diabetes (en pacientes con diabetes). Además, se recomendó recoger la albuminuria (pretratamiento renal sustitutivo).
ConclusionesEste conjunto de variables puede constituir una herramienta muy eficaz para evaluar los resultados de los pacientes y ayudar a definir estrategias para mejorar la calidad asistencial de los pacientes con enfermedad renal crónica en el sistema sanitario español.
Chronic kidney disease (CKD) is a clinical pathology affecting 1/10 of individuals in Spain (predominantly males, of advanced age or suffering from cardiovascular disease).1
Early stages are usually asymptomatic; manifestations of symptoms tend to occur in the advanced stages of the disease. Health-related quality of life (HRQoL) in CKD patients is greatly affected in all aspects,2 worsening progressively and significantly according to renal function and CKD stages.2–4
The aims of CKD care are to maintain kidney function, prevent or delay progression to advanced CKD and preserve or restore HRQoL.5 In advanced stages of kidney disease, specific care aims to manage uremia through specialized treatments such as hemodialysis (HD), peritoneal dialysis (PD), kidney transplantation (KT) or conservative care (CC).6
In Spain, costs associated to CKD treatments are estimated to reach more than 800 million euros per year.7 One of the major components of health-related costs is renal replacement therapy. Although these patients represent only 0.1% of the population, they constitute 2.5% of the National Health Service budget.7 Therefore, CKD has been a major issue facing the Spanish National Health System in recent decades. Value-based health care is being increasingly promoted. This strategy aims to improve health care based on those outcomes that matter the most to patients while driving cost effectiveness within health service quality.5 To this end, clinical variables and patient reported outcomes (PROs) should be collected.8 The use of standardized clinical variables and PROs in routine clinical practice allows for the comparison of results, favors information exchange, increases knowledge, and improves patient-healthcare professional relationships. Still, novel approaches need to be taken as the use of PROs in routine practice is not currently widespread, added to the fact that standardization is lacking in the collection of both clinical and, in particular, PRO-related variables.
A minimum standard set (including clinical variables and PROs) for CKD has been proposed by the International Consortium for Health Outcomes Measurements (ICHOM)5 to achieve three main objectives: (1) improve quality assistance, (2) facilitate decision making, and (3) reduce healthcare costs. The standard set targets patients with very high-risk CKD, corresponding to KDIGO (Kidney Disease: Improving Global Outcomes) classification stages G3a/A3 and G3b/A2-G5. Besides, as specific treatments may induce variations in the outcomes, the following substitutive kidney treatments were considered: management of pre-end-stage kidney disease (pre-ESKD), CC, HD, PD and KT.
Alongside the international initiatives, nationally, the Spanish Society of Nephrology (S.E.N.) works toward improving CKD patient care.9 Among other initiatives, the Quality Accreditation Project of the Advanced Chronic Kidney Disease Units (ACERCA) has proposed several standards and indicators of quality10 for expert accreditation of advanced CKD-specific program processes.
To ensure that a standard set is implemented among institutions within the same country, several conditions must be met: healthcare professionals and patients should be familiarized with the variables to be included, measurement tools should be made available, and the target population should be defined according to each country's clinical needs.11 An additional consideration in Spain is the complexity of the healthcare system due to the decentralization of competencies, which fall to the autonomous regions, and the heterogeneity of healthcare services and management.12 Hence, the present work aims to adapt the ICHOM standard set for CKD patients to the Spanish setting and supplement it with those variables agreed upon by the S.E.N. initiatives.
Material and methodsThis project was led by a working group formed by seven experts on CKD: five physicians, one hospital manager and one patient advocate.
The study included five successive steps: (1) identification of potential outcomes, (2) standard set design, (3) external input by a panel of S.E.N. experts, (4) patient's review, and (5) final standard set.
Identification of potential outcomesThe objective of the literature review was to identify clinical variables and PROs associated to CKD patients. The literature review conducted by the ICHOM5 was updated, spanning the September 2016–March 2020 period. International PubMed/MedLine databases were consulted following the specific strategy based on a search of terms and inclusion/exclusion criteria previously defined by ICHOM.5 Additionally, a review was undertaken of the principal documents published by the S.E.N. describing the quality indicators in CKD.13
Standard set designAccording the ICHOM, the target population defined in the project framework included high-risk CKD patients: pre-ESKD, HD, PD, KT and CC.5
Based on the results obtained from the literature review, the working group defined a first standard set of variables according to the following selection criteria: (i) feasibility for routine use, (ii) ability to be modified by interventions, (iii) correspondence with morbi-mortality, and (iv) degree of patient-centricity.
Variables selected by at least 50% of the members of the working group were included in the draft as essential variables. Those variables voted for by at least one of the members but that fell short of the established cut-off were included as optional variables.
External input by a panel of S.E.N. expertsAn electronic questionnaire including the first draft of the standard set was emailed individually to S.E.N. members. A total of 62 nephrologists, with an average experience of 24 years and geographically distributed throughout Spain, participated in the external input. This expert panel evaluated the suitability of each variable for inclusion into each patient CKD profile (dichotomous response Yes/No). Consensus was established when at least 75% of the panelists reached agreement on inclusion/exclusion. Based on these results, a second draft of the standard set was generated.
Patient's reviewThe second draft of the standard set was presented to CKD patients through the Spanish patient advocacy group Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER). A qualitative evaluation was then performed according to patients’ experience, values and preferences. Then, a third draft of variables was generated.
Final standard setThe third draft was then re-evaluated by the working group for final ratification. The same predetermined criteria were applied for analysis and selection. Consensus was reached when at least 75% agreed on their inclusion in the standard set. Finally, the definitive catalog integrating the standard set of outcomes variables for CKD patients was defined.
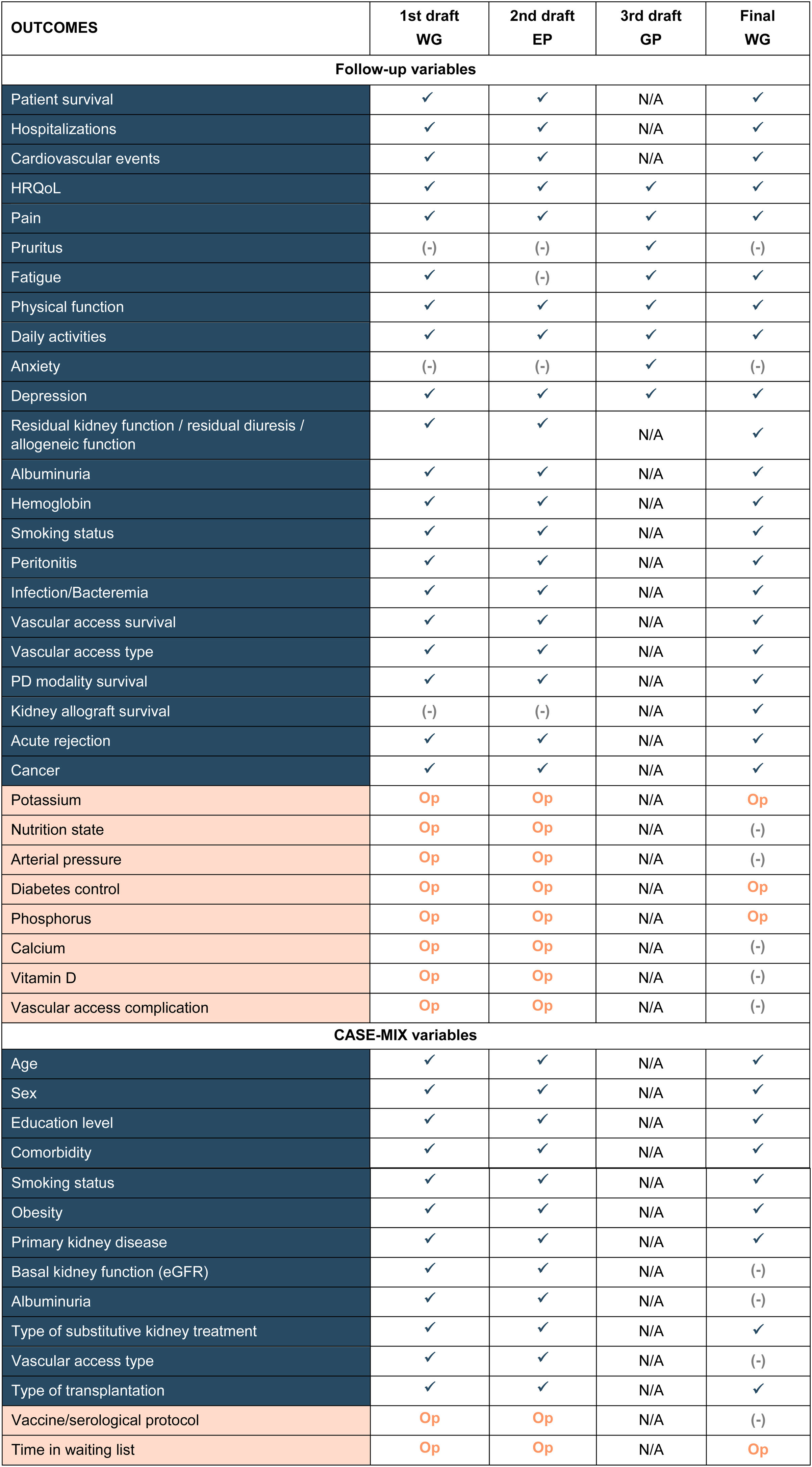
ResultsBased on the results of the literature review and the working group meeting, 42 potential CKD-related variables were included in the first draft of the standard set (Table 1). After external input given by the panel of S.E.N. experts (Tables S1 and S2) and patients’ review, 34 variables comprised the final standard set (Table 1).
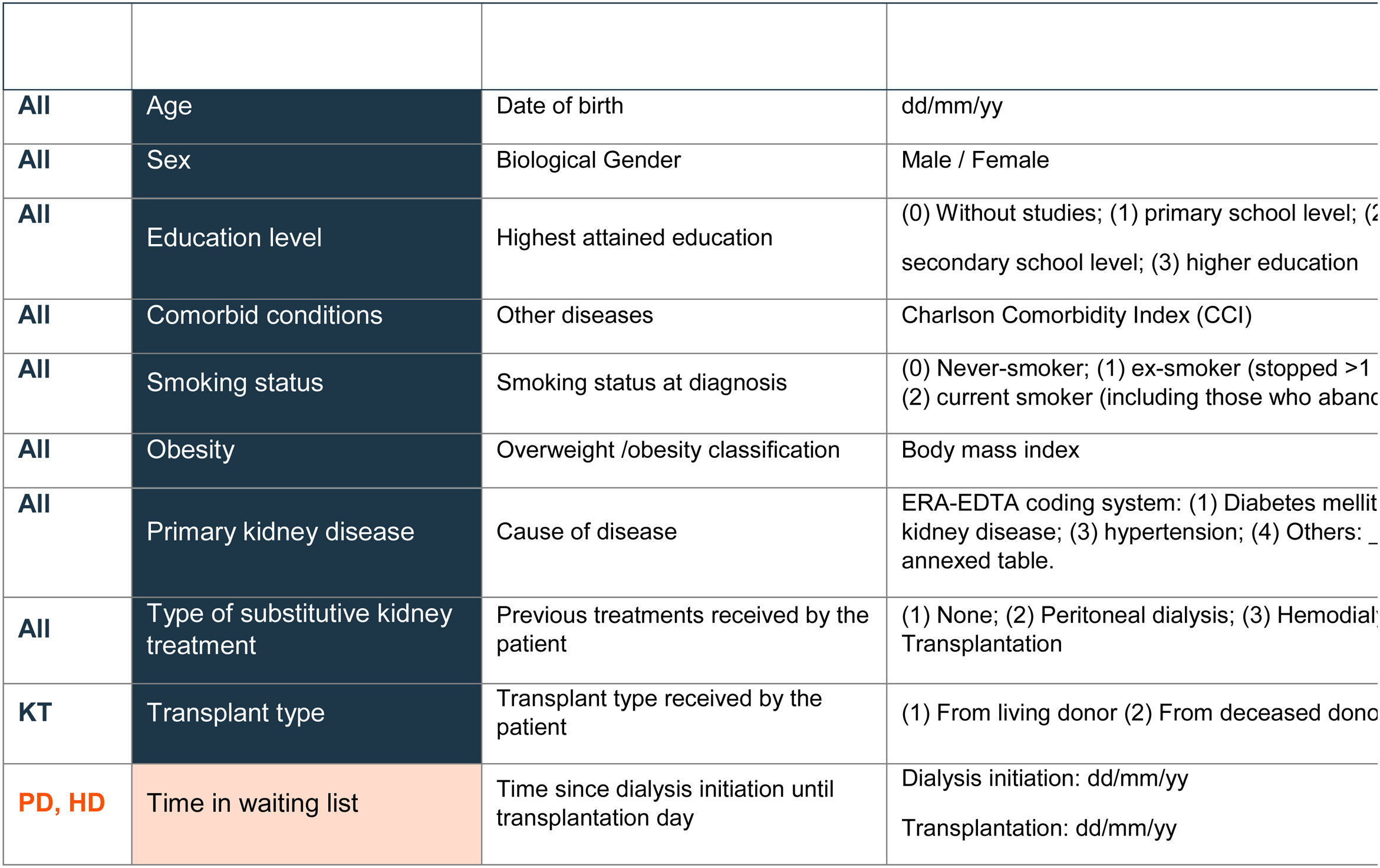
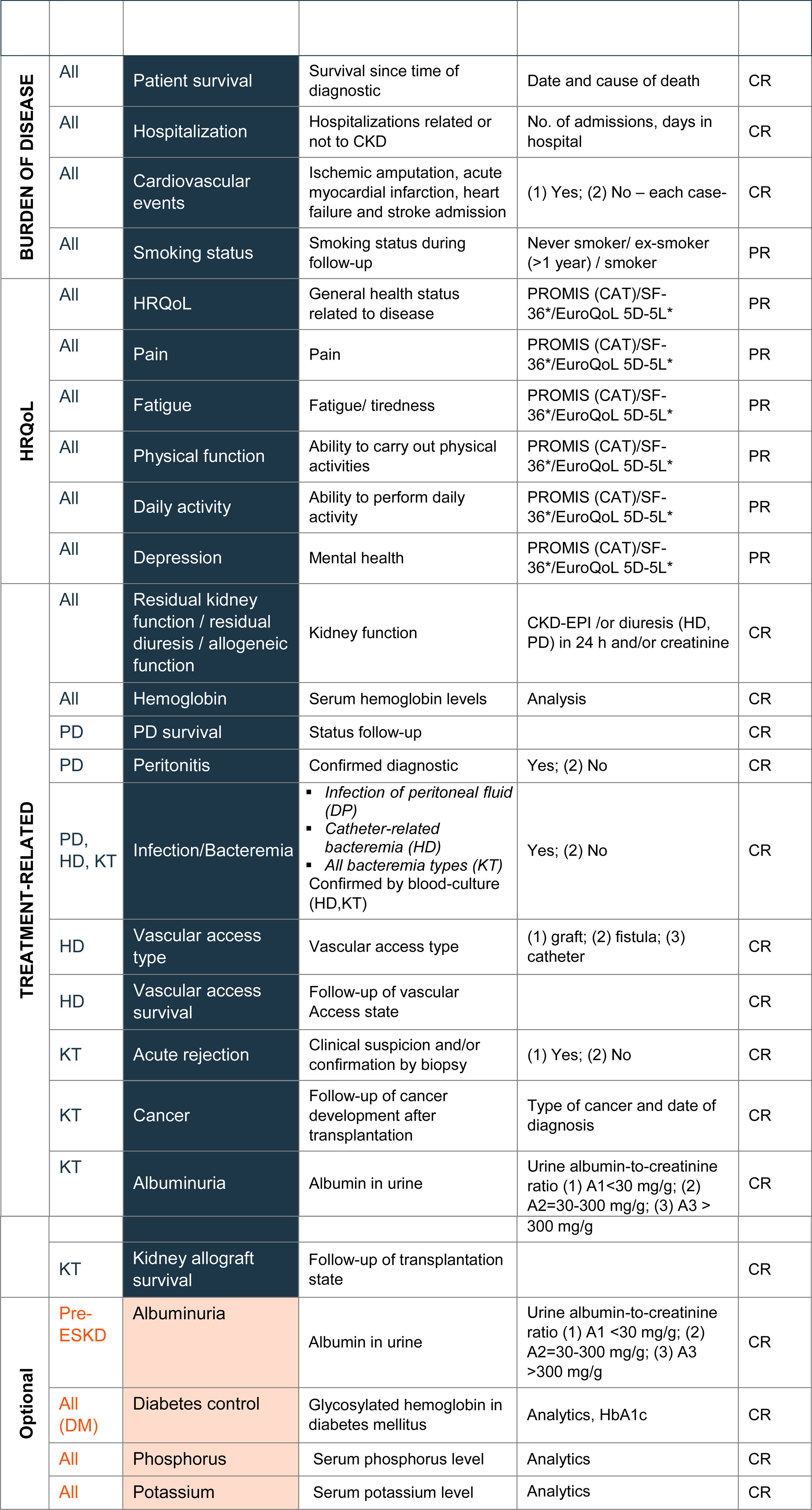
In line with the ICHOM proposal, variables of the final standard set were divided into two main groups: 10 case-mix adjustment variables and 24 follow-up variables. Case-mix variables enable patients to be classified into different groups and can help to explain differences in the results observed in the health outcomes (Table 2). Follow-up variables facilitated the evaluation of each patient's health status and treatment response and were grouped in three categories: burden of disease (n=4), quality of life (n=6), and treatment-specific (n=14) (Table 3).
Spanish standard set of patient-centered outcomes in CKD.
Blue, Essential variables; Orange, optional variables; All, all patients; pre-ESKD, advanced stage with High Risk of Progressive CKD; CC, conservative care; PD, peritoneal dialysis; HD, hemodialysis; KT, kidney transplantation; CH, clinician reported; PR, patient reported; DM, diabetes mellitus.
*Alternatively.
Additionally, variables were classified as essential (n=30), necessary to evaluate the results accurately, and optional (n=4), improving the overall information but with no obligation to be registered (Tables 2 and 3).
Agreement was made to collect the variables included in the CKD standard set at least once per year.
Case-mix variablesThe essential case-mix variables included in the final standard set were age, sex, education level, comorbid conditions, smoking status, obesity, primary kidney disease, and type of substitutive kidney treatment. In the case of patients with kidney transplantation, agreement was reached to collect the type of transplant (from a living or deceased donor).
Time on the waiting list was included as an optional variable for PD and HD patients due to its correlation with the survival of the kidney graft and the patient him/herself.21
The standard set proposed by the ICHOM also comprises renal function/allograft function, albuminuria, and vascular access type as case-mix variables. Since in our study these variables were included as follow-up variables and recorded at baseline, it was considered not to include them as case-mix variables to avoid redundancy. Similarly, the “vaccine/serological protocol” proposed by the S.E.N. panel was not incorporated in the final standard set as the working group considered it was not sufficiently relevant for inclusion (Table 2).
There was agreement to register the case-mix variables in the first visit at the time of diagnosis and/or before the beginning of each treatment phase (pre-ESKD, HD, PD, KT or CC).
Follow-up variablesBurden of diseasePatients with CKD are at high risk of suffering cardiovascular events, hospitalizations and mortality, especially in the advanced stages of kidney disease.14 Hence, inclusion of these variables was considered essential among the burden of disease variables (Table 3). Agreement was made to register cardiovascular events suffered by patients during follow up, including ischemic amputation, acute myocardial infarction, heart failure, and stroke admissions. Hospitalization (number of admissions and days spent in hospital), consequence or not of CKD, is collected as an indicator of healthcare burden and to acquire knowledge for management optimization. Mortality is recorded as the patient's survival (from date of diagnosis to date of death) as well as the cause of death.
In addition to these variables, there was a consensus to record smoking status during follow-up (not included in the ICHOM standard set) as this modifiable factor directly influences, and is involved in, the progression of kidney disease.15
Health-related quality of LifeBoth CKD and its associated treatment have a negative impact on patients’ HRQoL.2 In line with ICHOM, six PROs related to HRQoL have been included in the final standard set, namely, general quality of life, pain, fatigue, physical function, daily activity, and depression (Table 3).
Several tools were evaluated by the working group to collect the selected PROs: PROMIS (electronic version and PROMIS-29),16,17 SF-36,18 and EuroQoL 5D-5L.19 All options were considered suitable. However, the use of the electronic version of PROMIS (computer adaptive test, CAT) was recommended. On the one hand, this tool is available in Spanish and has been validated for different renal substitutive treatments, such as renal transplantation.20–23 Other main advantages include the reduced number of items, the automation, and the comparability between different quality of life states. In addition, further PROs, such as anxiety or pruritus, may be included. On the other hand, there are associated costs and specific technical support is required.
As an alternative to PROMIS, the SF-36 questionnaire18 was suggested for use by those centers with the license. In that event, the equivalence with PROMIS-29 will be calculated through PROsetta® tool.24 Additionally, as requested during patient review, agreement was made to use the EuroQoL 5D-5L tool,19 in line with the Nephrol Dial Transplant consensus meeting.25
Treatment-specific variablesAgreement was reached to collect several health outcomes for all patients, as well as some specific outcomes according to the treatment received by the patient (pre-ESKD, PD, HD, KT and CC).
Two essential variables were considered for all CKD patients: kidney function (including residual kidney function, residual diuresis and allogenic function) and hemoglobin (Table 3). To assess kidney function, the use of the glomerular filtration rate given by the equation CKD-EPI (CKD Epidemiology Collaboration) and/or the urine albumin-to-creatinine ratio were established. Since anemia is a frequent complication in CKD and is associated with increased morbi-mortality and disease progression,26,27 agreement was made to include hemoglobin in the final standard set, in line with the ACERCA initiative.10
Nutritional state, calcium, and vitamin D, included in the first draft, were finally discarded. Although nutritional state was considered important, it was discarded due to the complexity of its evaluation and the lack of standardization in clinical practice. Calcium was also discarded, due to insufficient scientific evidence for its association with morbi-mortality in CKD. Similarly, vitamin D was not included given the lack of evidence for a relationship between vitamin D deficiency and CKD progression.
For patients initiating PD, it was agreed to include the survival of peritoneal dialysis, peritonitis and infection of peritoneal fluid as essential variables. The latter was chosen because it usually causes serious morbidity in PD patients and increases the risk of dead28 (Table 3).
For patients initiating HD, it was considered essential to collect three variables: bacteremia (catheter-related bacteremia), vascular access type (graft, fistula or catheter), and vascular access survival. The ICHOM standard set includes vascular access type as a case-mix variable.5 However, because it may evolve during the follow-up of HD patients, the working group considered it more suitable to include this as a follow-up variable (Table 3).
For patients requiring kidney transplantation (KT), the collection of five essential variables was proposed: acute rejection (clinically suspected and/or biopsy-proven), kidney allograft survival, presence of cancer (malignancies post-transplantation), albuminuria, and bacteremia (Table 3). Patients with KT have an increased risk of developing malignancies.29 Thus, agreement was made to record malignancies post-transplantation. Albuminuria (measured by urine albumin-to-creatinine ratio) was considered for inclusion since it predicts adverse renal outcome in kidney transplant recipients30,31 (Table 3).
Additionally, two optional variables for all CKD patients were proposed, serum phosphorus and potassium levels. Moreover, diabetes monitoring was established for all CKD patients with diabetes mellitus. The serum phosphorus and potassium levels are relevant due to their association with a high risk of hyperphosphatemia and/or hyperkalemia.32 Diabetes monitoring and collection was recommended as it is associated with cardiovascular morbi-mortality in diabetic nephropathy.33
For pre-ESKD patients, albuminuria was proposed as an optional variable since it is a surrogate endpoint for the progression of chronic kidney disease34 (Table 3).
The complications of vascular access were considered for inclusion in the first standard set and agreed by the S.E.N panel. However, the working group finally discarded it due the lack of a standardized way to record it and because bacteremia (included in the standard set) is one of the main complications of vascular access.
DiscussionIncreased efforts to establish patient-centered care systems have been witnessed in recent years, with a substantial shift away from traditional care.35 The need to collect PROs during follow-up is widely accepted by the healthcare community as it facilitates the definition of personalized patient treatments.36 Nevertheless, the wide variety of PROs and measurement tools hamper the comparison of results among healthcare providers and regions, slowing down the acquisition of health-related knowledge and opportunities to improve.
In recent years, initiatives seeking the standardization of CKD-related outcomes have been driven by the ICHOM and S.E.N.5,9 The present work aims to integrate both initiatives and proposes a minimum standard set composed of clinical variables and PROs. The target population of the standard set defined in our project is in line with the ICHOM initiative: Patients with very high-risk CKD (stages G3a/A3 and G3b/A2-G5), including HD, PD, KT and CC.
The implementation of this standard set in routine practice envisages several actions, which should be assessed, in order to obtain successful results. These are: (i) to promote engagement of clinical leaders, (ii) to set up adequate processes, (iii) to identify current measurements and develop adapted strategies, (iv) to test strategies at pilot sites, and (v) to determine how to convey results to patients and clinical teams.5
The inclusion of essential and optional variables allows each center to incorporate the standard set gradually until reaching its full inclusion. In addition, to facilitate implementation, different questionnaires covering all defined PROs have been considered. Due to its advantages, we recommend the use of the PROMIS questionnaire. However, in order to foster the engagement of healthcare professionals and centers that are not technically prepared to use a specific HRQoL questionnaire, two other HRQoL alternatives have been proposed. All of these could be integrated through the PROSetta program to obtain standardized outputs.24
Nevertheless, implementation of a standardized and systematic register may encounter several hurdles. To guarantee successful results of this initiative, a pilot study in a small number of centers is recommended. The information gathered in the pilot study will allow for the evaluation of a further general extension, and the acquisition of useful information on hurdles found in situ and potential strategies to overcome them. Viability and sustainability of these initiatives will depend, to an extent, on their integration in routine clinical practice, rather than remaining as mere analysis tools for a particular study.
This study presents some limitations worthy of mention. First, the fact that the standard set has been established based on expert opinion and not on evidence or observational studies, second, the absence of validation of the standard set itself, and third, the focus on a specific stage of the disease, rather than the whole range of CKD patients.
In conclusion, consensus has been reached on a minimum standard set including both clinical variables and PROs for patients with CKD, with a view to their collection in routine clinical practice. This standard set may constitute a highly efficient tool facilitating the evaluation of patients’ results and helping to define strategies to enhance the quality of CKD patients’ care in the Spanish healthcare system. The perspectives of both patients and healthcare professionals involved in CKD management will be integrated thanks to the combined use of traditional clinical variables and PROs. On the one hand, patient participation will increase their empowerment while also facilitating physician-patient communication. On the other hand, systematically retrieved information on healthcare results will be extremely useful for clinicians and healthcare managers to take optimal decisions and to define improved strategies based on a patient-centered approach.
Conflict of interestsThe authors declare that they have no conflict of interest.