Many of the mineral metabolite abnormalities encountered in chronic kidney disease (CKD) patients were found also associated with acute kidney injury (AKI). In the last decade, sclerostin was found to intimately affect bone mineral metabolism in CKD patients. Nothing is known about sclerostin in AKI.
ObjectiveWe looked for serum level of sclerostin in AKI patients in comparison to normal control subjects and if there is an impact on metabolic derangement, endothelial function or clinical outcome.
Cases and methodsThis is a cross sectional case control observational study of 219 AKI cases (group I) beside 219 age matched normal control subjects (group II). All cases of group I were in the intensive care because of sepsis; 86 had acute on CKD (group Ib), while 133 had de novo AKI (group Ia). All studied subjects underwent estimation of serum sclerostin, parathyroid hormone (PTH), 25 hydroxy vitamin D (25 OH vit D), fibroblast growth factor 23 (FGF23), C-reactive protein (CRP), interleukin 6 (IL6), Homeostatic Model Assessment for Insulin Resistance (Homa IR), beside the routine CBC, kidney and liver function tests, serum calcium, and phosphorus, and flow mediated vasodilation of brachial artery (FMD). Follow-up of group I cases was done till they recovered or passed away.
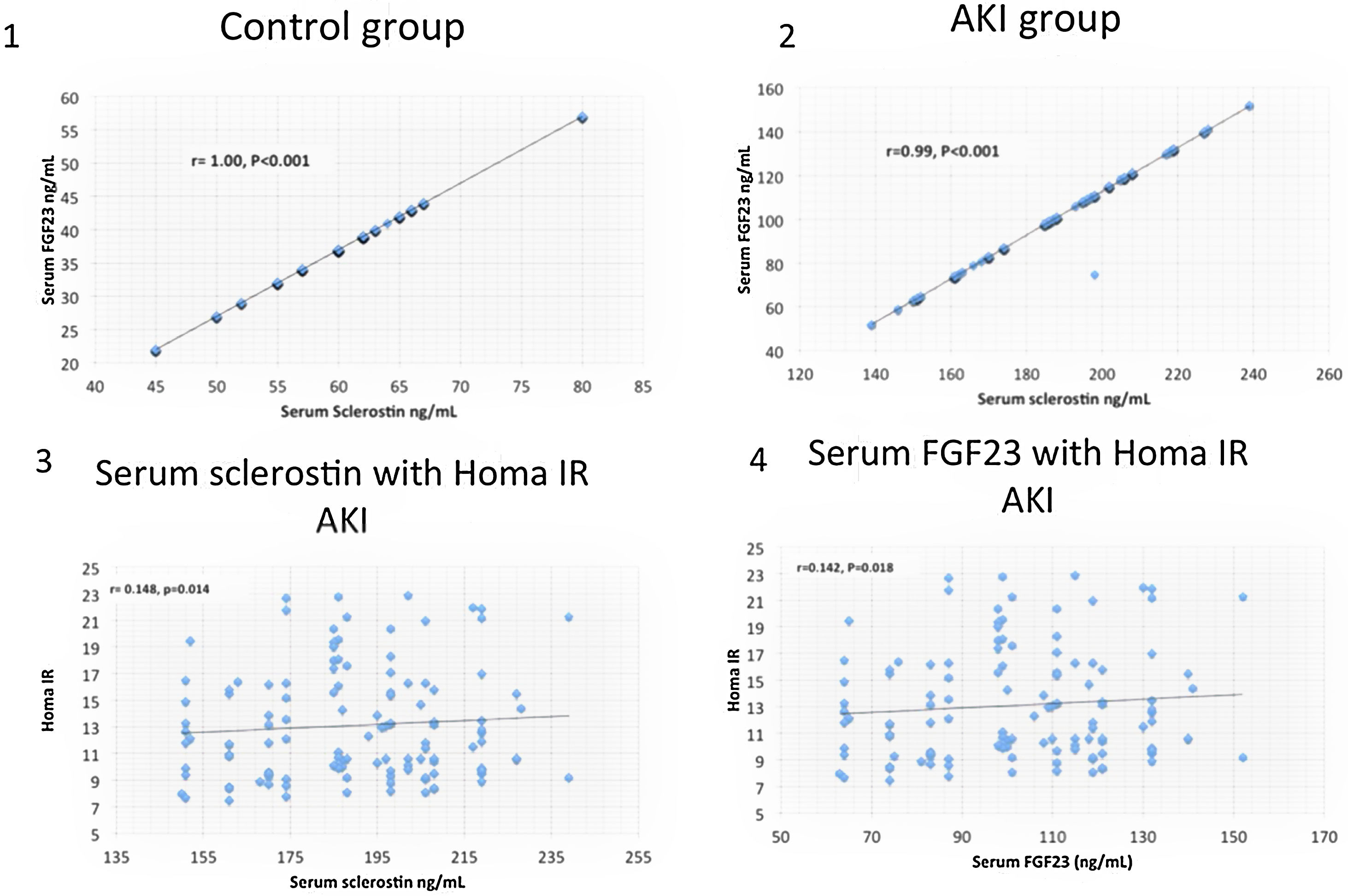
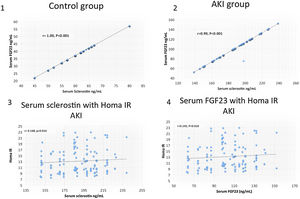
ResultsSerum sclerostin, PTH, FGF23, phosphorus, CRP, IL6, HOMA IR, creatinine, urea, uric acid, ALT, AST and white blood cell count (WBC) were significantly higher while serum calcium, 25 OH vit D, hemoglobin, platelet count and FMD were significantly lower in group I compared to group II (P<0.001 in all). On the other hand, there was no significant difference in serum sclerostin, PTH, FGfF23, 25 OH vit D, CRP, IL6, Homa IR and FMD between group Ia and Ib. Survivors were younger in age (median 55.5 vs. 60 years, P<0.04), had lower AST (30.5 vs. 58 units, P<0.001), had higher platelet count (206 vs 162×109/L, P<0.001), otherwise, there was no significant difference in any of the other parameters between survivors and patients that were lost. Serum sclerostin had strong correlation with FGF23 in group I (r=0.99, P<0.001) and group II (r=1, P<0.001). Homa IR had positive correlation with serum sclerostin (r=0.148, P=0.014) and serum FGF23 (r=0.142, P=0.018) in group I.
ConclusionSclerostin is intimately related to FGF23. Sclerostin level increases in AKI patients. Both sclerostin and FGF23 might increase insulin resistance but have no impact on FMD. Neither sclerostin nor FGF23 interfere with AKI outcome.
Muchas de las anomalías de los metabolitos minerales que se encuentran en el riñón de los pacientes con enfermedad renal crónica (ERC) también se asociaron con lesión renal aguda (IRA). En la última década, se ha descubierto que la esclerostina afectaba íntimamente al metabolismo mineral óseo en pacientes con ERC. No se sabe nada sobre la esclerostina en la LRA.
ObjetivoBuscamos el nivel sérico de esclerostina en pacientes con IRA en comparación con los niveles normales en sujetos de control, y si hay un impacto en el trastorno metabólico, la función endotelial o el resultado clínico.
Casos y métodosEste es un estudio observacional transversal de casos y controles de 219 casos de IRA (grupo I) además de 219 sujetos de control normales de la misma edad (grupo II). Todos los casos del grupo I se hallaban en cuidados intensivos por sepsis; 86 tenían ERC aguda (grupo Ib), mientras que 133 tenía de novo AKI (grupo Ia). Todos los sujetos estudiados se sometieron a una estimación de la esclerostina sérica, hormona paratiroidea (PTH), 25 hidroxi vitamina D (25 OH vit D), factor de crecimiento de fibroblastos 23 (FGF23), proteína C reactiva (CRP), interleucina 6 (IL6), evaluación del modelo homeostático para resistencia a la insulina (Homa IR), además del hemograma completo de rutina, pruebas de función renal y hepática, suero calcio y fósforo, y vasodilatación de la arteria braquial (FMD) mediada por flujo. El seguimiento de los casos del grupo I se realizó hasta que se recuperaron o fallecieron.
ResultadosEsclerostina sérica, PTH, FGF23, fósforo, PCR, IL6, HOMA IR, creatinina, urea, ácido úrico, ALT, AST y recuento de glóbulos blancos (WBC) fueron significativamente más altos mientras que el suero calcio, 25 OH vit D, hemoglobina, recuento de plaquetas y fiebre aftosa fueron significativamente más bajos en el grupo I en comparación con el grupo II (p<0,001 en total). Por otro lado, no hubo diferencia significativa en suero de esclerostina, PTH, FGfF23, 25 OH vit D, CRP, IL6, Homa IR y FMD entre grupos Ia y Ib. Los supervivientes eran más jóvenes (mediana 55,5 frente a 60 años, p<0,04), tenían AST más bajo (30,5 vs 58 unidades, p<0,001) y un recuento de plaquetas más alto (206 vs 162 × 109 / L, p<0,001); no hubo diferencia significativa en ninguno de los otros parámetros entre los supervivientes y los pacientes que fallecieron. La esclerostina sérica tuvo una fuerte correlación con FGF23 en el grupo I (r=0,99, p<0,001) y en el grupo II (r=1, p<0,001). Homa IR tuvo correlación positiva con suero esclerostina (r=0,148, p=0,014) y FGF23 sérico (r=0,142, p=0,018) en el grupo I.
ConclusiónLa esclerostina está íntimamente relacionada con FGF23. El nivel de esclerostina aumenta en pacientes con IRA. Tanto la esclerostina como el FGF23 pueden aumentar la resistencia a la insulina, pero no tienen ningún impacto sobre la fiebre aftosa. Ni la esclerostina ni el FGF23 interfieren con el resultado de la LRA.
Sclerostin is a small 24-kDa glycoprotein expressed and secreted by osteocytes to inhibit the Wingless-type mouse mammary tumor virus integration site (Wnt) pathway. By antagonizing Wnt, sclerostin inhibits osteogenesis.1 The earliest report of sclerostin in CKD patients was in dialysis patients. Elevated level in these patients was negatively correlating with osteoblast number.2 Sclerostin was negatively correlating with glomerular filtration rate among pre-dialysis CKD patients.3 Pelletier et al. 2013 demonstrated elevated level of sclerostin in stage 3 CKD.4 Low serum sclerostin was associated with better patient survival and lower prevalence of cardiovascular events in CKD patients maintained on peritoneal dialysis, but no similar relationships in hemodialysis patients. Serum sclerostin level did not correlate with coronary artery calcification.5 However, previous studies reported conflicting results. Some disclosed significant positive correlation between serum sclerostin and calcification of coronary artery and abdominal aorta in CKD patients.6,7 On the other hand, another study disclosed that high serum sclerostin is associated with lower aortic calcification scores.8 Serum sclerostin was associated with decreased flow mediated vasodilation, fatal and nonfatal cardiovascular events in pre-dialysis CKD patients.9 Nevertheless, it failed to show significant correlation with brachial artery flow mediated vasodilatation in rheumatoid arthritis patients.10 In CKD patients treated with kidney transplantation, sclerostin was associated with increased overall mortality.11
No studies have looked for impact of increased serum sclerostin on insulin resistance in CKD patients. In women with polycystic ovary, serum sclerostin was associated with increased insulin resistance.12
In spite of the plethora of data about sclerostin in CKD Patients before dialysis, during dialysis and after kidney transplantation, the literature lacks any studies in AKI patients.
Aim of workThe aim of this work is the study of serum sclerostin level in AKI patients and its association with endothelial function, insulin resistance and mortality among these patients.
Patients and methodsThis is a cross sectional case control observational study that took place in the intensive care units of Cairo University and Theodor Bilharz research Institute Located in Guiza governerate, Egypt between June 2016 and July 2020. During this period, there were 418 cases suffering AKI as a consequence of severe sepsis, 219 of them were included in this study after obtaining a written consent from each patient or his next of kin (group I). Diagnosis of AKI was according to the Acute Dialysis Quality Initiative first proposed the Risk, Injury, Failure, Loss and End-Stage Renal Disease (RIFLE) criteria for diagnosis and classification of acute impairments in kidney function13 and acute on chronic CKD was settled when a patient has an increase of inpatient serum creatinine greater than the last preadmission serum creatinine by ≥50% or if the patient needs dialysis during hospitalization.14 According to RIFLE criteria, 60 (27.4%) of patients were in class R, 96 (43.8%) were in class I, and 63 (28.8%) were in class F. In addition, 219 age-matched normal medical and paramedical personnel were included as control group (group II). After initial clinical assessment and getting consent, all subjects were investigated for body mass index (BMI), complete blood count (CBC), serum alanine transaminase (ALT), aspartate transaminase (AST), creatinine, urea, uric acid, calcium, phosphorus, fasting blood sugar level, insulin level, CRP, IL6, PTH, 25 OH vit D, FGF23, beside serum sclerostin and FMD. In addition follow up of the outcome of group I patients till they recover or get lost was done. Group I patients were divided into two subgroups according to absence or presence of underlying CKD into group Ia and group Ib respectively. Group Ia patients had multiple comorbid conditions including acute leukemia, bronchopneumonia, aspiration pneumonia, chronic obstructive airway disease, liver cirrhosis, hepatocellular carcinoma, diabetic ketoacidosis, non-Hodgkin lymphoma, post cardiopulmonary resuscitation, tumor lysis syndrome, cardiogenic shock, puerperal sepsis, severe vaginal bleeding, ischemic heart disease, heart failure, infective endocarditis, septic shock, and hypovolemic shock. Comorbid conditions in group Ib include lobar pneumonia, bronchopneumonia, respiratory failure, infective endocarditis, diabetic ketoacidosis, lupus encephalitis, heart failure, acute coronary insufficiency, lupus nephritis, severe sepsis, cerebrovascular stroke, post cardiopulmonary resuscitation, ventricular arrhythmias, and acute gastrointestinal bleeding.
Serum sclerostin was assayed using Quantikine ELIZA according to the manufacturer's instructions. Enzyme amplified sensitivity immunoassay was used to estimate iPTH (Roche Diagnostics, Indianapolis, IN). Serum 25 OH vit D was measured using high-performance liquid chromatography (HPLC). Serum FGF23 was assayed using a two-site (NH2-terminal/C-terminal) enzyme-linked immunosorbent assay (ELISA) (Immutopics, San Clemente, CA). Serum IL-6 levels were identified immediately after blood sampling, using ELISA (eBioscience, ESP). FMD was estimated bedside for group 1 patients and in the Doppler lab for group II cases by an expert sonographer using B-mode SIEMENS ACUSON X300 ultrasonographer.
Statistical analysisThe data collected were analyzed using IBM Statistical Package for Social Science (SPSS) Statistics 22. When data were tested for normality, most for the data are not normally distributed. Values are expressed, accordingly, as median and interquartile range (IQR). Mann–Whitney test was used to compare groups. Spearman univariate analysis was used to find out the possible significant associations of serum sclerostin with the other studied parameters. For qualitative data, bivariate associations were examined using chi-square test.
ResultsTables 1–3 and Fig. 1 summarize the results of this study. Serum sclerostin, FGF23, PTH, CRP, IL6, serum creatinine, urea, uric acid, phosphorus, WBC, and Homa IR are significantly higher while serum calcium, 25 OH vit D, hemoglobin concentration (Hb), platelet count, and FMD are significantly lower in AKI cases in comparison to the normal control group (P<0.001 in all, Table 1). The patients that developed acute on chronic renal failure are significantly younger than those with de novo AKI (P=0.0012). De novo AKI had significantly higher serum ALT, AST, uric acid, Hb (P<0.001 in all), and WBC (P=0.034), significantly lower serum urea (P=0.0024), creatinine (P<0.001), and phosphorus (P=0.0168) compared to cases of acute on chronic renal failure, while there was no significant difference in serum sclerostin, PTH, FGF23, 25 OH vit D, serum calcium, CRP, IL6, platelet count, Homa IR, and FMD between the two groups (Table 2). Moreover, Patients that recovered have younger age (P<0.04), had higher platelet count (P<0.005), and lower AST (<0.0001), otherwise, there was no significant difference in serum sclerostin, FGF23, PTH, 25 OH vit D, CRP, IL6, urea, creatinine, uric acid, calcium, phosphorus, Hb, WBC, Homa IR, or FMD between survivors and non survivors. Out of 133 cases in group Ia, 69 (52%) recovered and left the ICU while 64 (48%) were lost while in group Ib, out of 86 cases, 53 (61.6%) survived and 33 (38.4%) were lost, 95% confidence interval (CI) of mortality is −3.7% to 22.5%, P=0.157.
Comparative analysis of AKI patients (group I) versus control (group II).
| Variables | Group I (n=219) | Group II (n=219) | U value | Z score | P-Value |
|---|---|---|---|---|---|
| Age (Years) Median (IQR) | 57 (27) | 56 (13) | 6146 | 1.35 | 0.061 |
| BMI (kg/m2) Median (IQR) | 23.5 (3.2) | 24 (3) | 20927 | 1.3 | 0.055 |
| ALT (U/L) Median (IQR) | 28 (58) | 18 (6) | 17440 | 4.94 | <0.001 |
| AST (U/L) Median (IQR) | 37 (75) | 20 (8) | 9500 | 10.9 | <0.001 |
| S urea (mg/dL) Median (IQR) | 140 (100) | 13 (5) | 438 | 17.7 | <0.001 |
| S. creatinine (mg/dL) Median (IQR) | 4.7 (3.8) | 0.7 (0.1) | 15 | 18.1 | <0.001 |
| S.uric acid (mg/dL) Median (IQR) | 8 (4.8) | 4.5 (1.1) | 4506 | 14.6 | <0.001 |
| S. Calcium (mg/dL) Median (IQR) | 7.9 (1.4) | 8.9 (0.5) | 6720 | 13 | <0.001 |
| S.Phosphorus(mg/dL) Median (IQR) | 5.46 (2.9) | 3.6 (0.3) | 9972 | 10.6 | <0.001 |
| S.25 (OH) vit D (ng/mL) Median (IQR) | 16.8 (10) | 37.5 (5.6) | 66 | 18.1 | <0.001 |
| S.PTH (Pg/mL) Median (IQR) | 77.8 (20.4) | 47.8 (4.3) | 155 | 16.9 | <0.001 |
| S.FGF23 (ng/mL) Median (IQR) | 101 (36) | 39 (8) | 18 | 18.1 | <0.001 |
| S.sclerostin (ng/mL) Median (IQR) | 188 (36) | 62 (8) | 0 | 18.1 | <0.001 |
| Homatest IR Median (IQR) | 11.8 (5.8) | 4 (0.4) | 0 | 18.1 | <0.001 |
| CRP (mg/dL) Median (IQR) | 56 (102) | 0.8 (0.9) | 0 | 18.1 | <0.001 |
| IL6 (Pg/mL) Median (IQR) | 25 (6.6) | 6.4 (1.9) | 0 | 18.1 | <0.001 |
| Hemoglobin Conc. (gm/dL) Median (IQR) | 9.1 (3.6) | 12.8 (0.4) | 4945 | 14.4 | <0.001 |
| WBC count (×109/L) Median (IQR) | 12.5 (9) | 6.4 (1.3) | 6979 | 12.8 | <0.001 |
| Platelet count (×109/L) Median (IQR) | 184 (187) | 357 (87) | 7553 | 12.4 | <0.001 |
| Flow mediated dilation (%) | 7.3 (1.1) | 11.8 (7.9) | 0 | 18.1 | <0.001 |
BMI=Body mass index; ALT=Alanine transaminase; AST=Aspartate transaminase; 25 OH vit D=25 hydroxy vitamin D; S.PTH=serum parathyroid hormone; s.FGF23=serum fibroblast growth factor23; IR=insulin resistance; CRP=c-reactive protein; ILS=interleukin6; WBC=white blood cell count.
Comparative analysis of native AKI patients (group Ia) versus AKI on top of chronic renal failure (group Ib).
| Variables | Group Ia (n=133) | Group Ib (n=86) | U value | Z score | P-value |
|---|---|---|---|---|---|
| Age (Years) Median (IQR) | 60 (22) | 53 (31) | 4301 | 3.24 | 0.0012 |
| BMI (kg/m2) Median (IQR) | 24 (3) | 22.8 (4) | 5187 | 1.33 | 0.18 |
| ALT (U/L) Median (IQR) | 37 (64) | 20 (28) | 4196 | 3.47 | <0.001 |
| AST (U/L) Median (IQR) | 55 (104) | 29 (25) | 3868 | 4.18 | <0.001 |
| S urea (mg/dL) Median (IQR) | 120 (86) | 160 (116) | 4204 | −3.04 | 0.0024 |
| S. creatinine (mg/dL) Median (IQR) | 3.5 (3) | 5.9 (3.7) | 3110 | −5.81 | <0.001 |
| S.uric acid (mg/dL) Median (IQR) | 8.9 (5) | 7.55 (3.5) | 4123 | 3.32 | <0.001 |
| S. Calcium (mg/dL) Median (IQR) | 8 (1.4) | 7.65 (1.5) | 5180 | 1.35 | 0.177 |
| S.Phosphorus(mg/dL) Median (IQR) | 4.7 (3) | 5.55 (2.6) | 4696 | −2.39 | 0.0168 |
| S.25 (OH) vit D (ng/mL) Median (IQR) | 16.8 (10.1) | 17.2 (11.2) | 5690 | −0.25 | 0.8 |
| S.PTH (Pg/mL) Median (IQR) | 77.8 (20.4) | 77 (19.4) | 5530 | 0.59 | 0.555 |
| S.FGF23 (ng/mL) Median (IQR) | 101 (32) | 100 (36) | 5623 | −0.39 | 0.697 |
| S.sclerostin (ng/mL) Median (IQR) | 188 (32) | 187 (36) | 5663 | −0.31 | 0.757 |
| Homatest IR Median (IQR) | 11.9 (5) | 11.7 (6.3) | 5770 | −0.07 | 0.944 |
| CRP (mg/dL) Median (IQR) | 48 (105) | 60 (86.8) | 5338 | −1 | 0.313 |
| IL6 (Pg/mL) Median (IQR) | 25 (9.6) | 25 (7) | 5383 | 0.9 | 0.363 |
| Hemoglobin Conc. (gm/dL) Median (IQR) | 10.2 (3.9) | 8.5 (2.6) | 3817 | 4.9 | <0.001 |
| WBC count (×109/L) Median (IQR) | 13 (10) | 11.5 (8.6) | 4821 | 2.12 | 0.034 |
| Platelet count (×109/L) Median (IQR) | 184 (196) | 178 (169) | 5526 | 0.6 | 0.549 |
| Flow mediated dilation (%) | 7.3 (1.2) | 7.3 (1) | 5757 | 0.1 | 0.92 |
BMI=Body mass index; ALT=Alanine transaminase; AST=Aspartate transaminase; 25 OH vit D=25 hydroxy vitamin D; S.PTH=serum parathyroid hormone; s.FGF23=serum fibroblast growth factor23; IR=insulin resistance; CRP=c-reactive protein; ILS=interleukin6; WBC=white blood cell count.
Comparative analysis of AKI patients according to outcome.
| Variables | Survivors (n=122) | Died (n=97) | U value | Z score | P-value |
|---|---|---|---|---|---|
| Age (Years) Median (IQR) | 55.5 (28) | 60 (21) | 4862 | 2.1 | <0.04 |
| BMI (kg/m2) Median (IQR) | 23 (3.8) | 24 (3) | 5522 | 0.85 | 0.395 |
| ALT (U/L) Median (IQR) | 23 (55) | 33 (57) | 5083 | 1.79 | 0.073 |
| AST (U/L) Median (IQR) | 30.5 (55) | 58 (93.5) | 4256 | 3.56 | <0.001 |
| S urea (mg/dL) Median (IQR) | 136 (96) | 149 (109) | 5553 | 0.19 | 0.85 |
| S. creatinine (mg/dL) Median (IQR) | 5 (4.1) | 4.4 (3.6) | 5648 | 0.58 | 0.562 |
| S.uric acid (mg/dL) Median (IQR) | 8.3 (4.5) | 7.9 (4.8) | 5376 | 0.81 | 0.418 |
| S. Calcium (mg/dL) Median (IQR) | 7.8 (1.6) | 7.8 (1.25) | 5712 | 0.44 | 0.66 |
| S.Phosphorus (mg/dL) Median (IQR) | 4.9 (2.3) | 5.3 (3.3) | 5029 | 1.9 | 0.057 |
| S.25 (OH) vit D (ng/mL) Median (IQR) | 16.4 (11) | 16.8 (10) | 5688 | 0.49 | 0.62 |
| S.PTH (Pg/mL) Median (IQR) | 78 (20) | 76 (21) | 5873 | 0.09 | 0.928 |
| S.FGF23 (ng/mL) Median (IQR) | 101 (36) | 100 (41) | 5157. | 1.6 | 0.1 |
| S.sclerostin (ng/mL) Median (IQR) | 188 (33) | 187 (41) | 5116 | 1.72 | 0.085 |
| Homatest IR Median (IQR) | 11.9 (6.1) | 11.5 (5) | 5282 | 1.36 | 0.174 |
| CRP (mg/dL) Median (IQR) | 57 (109) | 50 (76) | 5825 | 0.196 | 0.84 |
| IL6 (Pg/mL) Median (IQR) | 25 (6.6) | 25.5 (9.6) | 5430. | 0.92 | 0.36 |
| Hemoglobin Conc. (gm/dL) Median (IQR) | 9.1 (3.6) | 9.1 (3.25) | 5877 | 0.08 | 0.94 |
| WBC count (×109/L) Median (IQR) | 11.7 (9.6) | 13 (8) | 5450 | 1 | 0.32 |
| Platelet count (×109/L) Median (IQR) | 206 (187) | 162 (202) | 4601 | 2.82 | <0.005 |
| Flow mediated dilation (%) | 7.3 (1.1) | 7.3 (1.2) | 5879 | 0.08 | 0.936 |
BMI=Body mass index; ALT=Alanine transaminase; AST=Aspartate transaminase; 25 OH vit D=25 hydroxy vitamin D; S.PTH=serum parathyroid hormone; s.FGF23=serum fibroblast growth factor23; IR=insulin resistance; CRP=c-reactive protein; ILS=interleukin6; WBC=white blood cell count.
This is the first study of sclerostin in AKI. It proved that serum sclerostin level increases in parallel to FGF23 in AKI patient to levels comparable to CKD patients. Apart from the week association with insulin resistance, neither sclerostin nor FGF23 had an impact on endothelial function or survival of AKI patients. Previous studies have shown strong association of FGF23 with insulin resistance among CKD patients.15,16 The variation of this association between CKD and AKI cases is likely related to disease duration. Similar studies on sclerostin in CKD patients are lacking, but it was found associated with insulin resistance in women with polycystic ovary.12 The present study failed to disclose significant association of sclerostin with FMD as an index of endothelial function, there is no significant difference in serum sclerosin between AKI patients that recovered and those that were lost. The only study that looked on endothelial function in association with high serum sclerostin in CKD patients showed different effect. They also disclosed a significant association of sclerostin with mortality.9 This last association was found in another study in peritoneal dialysis patients but failed to show similar association among hemodialysis cases.5
The main drawbacks of this study include the cross-sectional nature; longitudinal research is more suitable for the study of biomarkers. The fluctuations in serum biomarkers in relation to the state of disease activity can be best evaluated in longitudinal studies; that are therefore more capable of identifying differences in the levels of biomarkers according to various circumstances. Another limitation that faced this work was the multiple comorbid conditions affecting individual AKI patients and may explain the increased mortality among these patients.
Authorship disputeAll authors have contributed significantly and equally in the design of this work, data acquisition, analysis, and interpretation. In addition to the writing and revising of this manuscript, all authors approved the final version before submission.
Source of fundingThis research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Conflicts of interestThe authors have declared that no conflict of interest exists.