Serum 25-hydroxyvitamin D (25(OH)D) negatively correlates with serum phosphorus level of stage 3a-5 chronic kidney disease (CKD) patients. So far, no explanation has been provided for this negative association.
ObjectiveTo confirm this negative association and determine if this relationship is mediated through other known co-morbid factors.
Cases and methodsOne hundred (57 male and 43 female) pre-dialysis stage 3a-5 CKD patients were selected. Estimated glomerular filtration rate (eGFR), serum calcium (Ca), phosphorus (P), 25(OH)D, parathyroid hormone (PTH), and intact fibroblast growth factor-23 (FGF23) were assessed. A correlation analysis between serum 25(OH)D and the different parameters studied was performed. Multivariate linear regression analysis was carried out to determine predictors of 25(OH)D.
ResultsThe negative association between serum 25(OH)D and serum P was confirmed in univariate and multivariate correlation analysis. On the other hand, we failed to detect a significant association between 25(OH)D and serum FGF23. Serum P is the most important independent predictor of 25(OH)D in these patients (partial R2=0.15, p<0.0001).
ConclusionSerum P is likely to have a direct negative impact on serum 25(OH)D. Further studies are needed to determine the underlying mechanism.
La 25-hidroxivitamina D (25[OH]D) sérica se correlaciona negativamente con el nivel de fósforo sérico en pacientes con enfermedad renal crónica (ERC) en estadio 3a-5. Hasta la fecha, no se dispone de ninguna explicación sobre esta asociación negativa.
ObjetivoConfirmar la asociación negativa y averiguar si esta relación está mediada por otros factores de comorbilidad conocidos.
Casos y métodosSe seleccionaron 100 pacientes (57 varones y 43 mujeres) con ERC en estadio 3a-5 prediálisis. Se evaluaron la tasa de filtración glomerular estimada (TFRe), el calcio sérico (Ca), el fósforo (P), la 25(OH)D, la hormona paratiroidea (HPT) y el factor de crecimiento de fibroblastos 23 intacto (FGF23). Se realizó un análisis de correlación entre la 25(OH)D sérica y los distintos parámetros estudiados. Se llevó a cabo un análisis de regresión lineal multivariable para determinar los factores pronósticos de 25(OH)D.
ResultadosSe confirmó la asociación negativa entre la 25(OH)D sérica y el P sérico en análisis de correlación univariable y multivariable. Por otro lado, no detectamos ninguna asociación significativa entre la 25(OH)D y el FGF23 sérico. El P sérico es el factor predictivo independiente más importante de la 25(OH)D en estos pacientes (R2 parcial=0,15; p<0,0001).
ConclusiónEs probable que el P sérico tenga un impacto negativo directo sobre la 25(OH)D sérica. Es necesario realizar más estudios para averiguar el mecanismo subyacente.
Vitamin D is metabolized in two steps, first to 25 hydroxyvitamin D (25(OH)D) using CYP2R1 as the most important 25-hydroxylase, then to 1,25-dihydroxyvitamin D (1,25(OH)2D) using CYP27B1 as the key 1-α hydroxylase.1 The liver is the major source of 25(OH)D production from vitamin D. It seems that CYP2R1 is the major contributor to 25(OH)D production; however, other unknown enzymes are contributing to this hydroxylation and to 25(OH)D circulating levels.2 In general, the regulation of vitamin D 25-hydroxylation was not of major concern. Hence, the circulating levels of 25(OH)D are considered as a useful marker of vitamin D nutrition status.1 Both 25(OH)D and 1,25(OH)2 D are catabolized by CYP24A1as 24 hydroxylase.3 CYP24A1 prevents the eventual activation of 25(OH)D to 1,25(OH)2D and/or degrades the hormone 1,25(OH)2D within its target cells to terminate its biological activity.4 Serum inorganic P plays direct feedback regulatory role on 1-α hydroxylase and 24 hydroxylase activities. Low serum P directly stimulates 1-α hydroxylase and inhibits 24 hydroxylase and vice versa.5 On the other hand, low serum Ca stimulates PTH secretion that in turn stimulates 1-α hydroxylase and inhibits 24 hydroxylase.5 Progressive elevation of FGF23 is a feature of CKD.6,7 Beside many other factors, P retention, increased Ca intake, vitamin D treatment, hyperparathyroidism, and Klotho deficiency are common causes of increased FGF23 in CKD.8 FGF23 inhibits Cyp27b1 activity and thus decreases synthesis of 1,25(OH)2D. It also stimulates CYP24A1, and thus increases catabolism of both 25(OH)D and 1,25(OH)2D. Via stimulation of CYP24A1, FGF23 can reduce serum 25(OH)D and 1,25(OH)2D levels.9,10 However, when serum concentrations of 24,25-(OH)2D; a product of Cyp24a1 hydroxylation of 25(OH)D was measured in patients with CKD of variable severity, there was no support for FGF23-mediated catabolism of vitamin D metabolites in chronic kidney disease. In addition, there was no significant relationship between FGF23 and either 25(OH)D or 1,25(OH)2 in this CKD patient cohort.11
The ability of the small intestine to actively absorb P is hormonally regulated and occurs from the ileum. The sodium-dependent P co-transporter type IIb (NPT IIb) is localized to the brush border of ileum.12 1,25(OH)2 stimulates while FGF23 reduces intestinal NPT IIb transport activity.12,13
In 2 previous studies, a negative correlation between serum 25(OH)D and Serum P was observed in pre-dialysis CKD patients.14,15 This finding is additionally supported by another study of the current authors that is not yet published. So far, there is no clear explanation for this negative association. In this study, we revised this negative association and looked for a possible correlation between serum FGF23 and 25(OH)D.
Patients and methodsOne hundred newly diagnosed CKD patients were selected. They were 57 male and 43 female. According to KDOQI classification, 19 of these patients are in stage 3, 79 in stage 4, and 2 cases in stage 5. None of the selected cases was prescribed vitamin D or any phosphate binder before selection. Their age ranged between 18 and 46 years (mean±S.D.=27±7.47). A written consent was obtained from all patients and was followed by clinical examination and collection of a blood sample. Body mass index was calculated for every patient. Blood samples were used for estimation of eGFR, the serum level of Ca, P, PTH, 25(OH)D, and FGF23.
eGFR was measured using MDRD equation.16 Intact PTH level was determined by enzyme-amplified sensitivity immunoassay (Roche Diagnostics, IN, USA). 25 (OH) vit D was assessed Using HPLC.17 Intact FGF23 was determined using a two-site (NH2-terminal/C-terminal) enzyme-linked immunosorbent assay (Immutopics, CA, USA). As recommended by the manufacturer, samples were collected in the morning after 12h fasting. The collected samples were centrifuged, and the plasma was separated from the cells. Samples were assayed immediately or stored at −70°C or below.
We then formed tertiles according to serum phosphorus to compare the blood levels of 25(OH)D, FGF23, and PTH within these tertiles.
IBM SPSS Statistics package was used for data analysis. Data were summarized as mean and standard deviation. Comparison between subgroups was evaluated using Student's t-test. One-way analysis of variance (ANOVA) test is used to determine whether there are any statistically significant differences between the means of more than two independent groups. Correlations between different parameters were performed. Multivariate linear regression analysis was done looking for predictors of 25(OH)D. The predictors that are included in the model are serum P, PTH, FGF23, eGFR as well as age & BMI. The adjusted R-squared of the final model was 0.75.
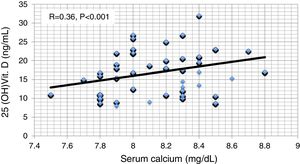
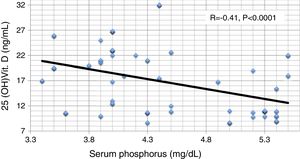
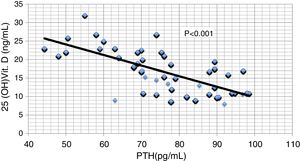
ResultsResults are summarized in Tables 1–4 and Figs. 1–4. The different studied parameters are shown in Table 1. Serum 25 (OH)D showed significant positive correlation with serum Ca and significant negative correlations with serum P and PTH (r=0.36, −0.41, and -0.71 respectively, p<0.001 in all, Figs. 1–3). On the other hand, no significant relationship was observed between serum 25 (OH)D and age, BMI, or FGF23 (Table 2). Serum P (p<0.0001), PTH (p<0.0001), and eGFR (p=0.001) were independently related to 25(OH)D in a multivariate linear regression analysis. By quantifying the relative importance of these parameters, serum P was the best predictor of 25(OH)D level (Table 3).
Different studied parameters.
| Variable | CKD group (100) | |
|---|---|---|
| Range | Mean (S.D.) | |
| Age (years) | 18–46 | 27 (7.47) |
| Body mass index (kg/m2) | 17.5–28 | 23.5 (2.75) |
| eGFR (mL/min/1.73m2) | 14.3–45.4 | 23.6 (5.98) |
| Serum calcium (mg/dL) | 7.5–8.8 | 8.2 (0.3) |
| Serum phosphorus (mg/dL) | 3.4–5.5 | 4.3 (0.67) |
| Serum parathormone (pg/mL) | 44.3–98.5 | 76.9 (15.27) |
| Serum 25 hydroxyvitamin D (ng/mL) | 8.7–32 | 17 (5.63) |
| Serum FGF23 (pg/mL) | 187–287 | 235 (22.96) |
Pearson correlations between studied parameters.
| Variable | Age | BMI | eGFR | S.Ca | S.P | PTH | 25OHD | FGF23 | ACR |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 0.02 | −0.22 | 0.04 | 0.01 | 0.09 | −0.08 | 0.13 | 0.13 |
| BMI | 0.02 | 1 | 0.05 | −0.15 | 0.16 | 0.11 | −0.11 | −0.11 | 0.1 |
| eGFR | −0.22 | 0.05 | 1 | 0.08 | −0.08 | 0.01 | 0.03 | 0.01 | 0.02 |
| S.Ca | 0.04 | −0.15 | 0.08 | 1 | −0.74 | −0.26 | 0.36 | 0.05 | −0.36 |
| S.P | 0.01 | 0.16 | −0.08 | −0.74 | 1 | 0.26 | −0.41 | 0.03 | 0.41 |
| PTH | 0.09 | 0.11 | 0.01 | −0.26 | 0.26 | 1 | −0.71 | −0.14 | 0.61 |
| 25OHD | −0.08 | −0.11 | 0.03 | 0.36 | −0.41 | −0.71 | 1 | 0.1 | −0.85 |
| FGF23 | 0.13 | −0.11 | 0.01 | 0.05 | 0.03 | −0.14 | 0.1 | 1 | −0.02 |
| ACR | 0.13 | 0.1 | 0.02 | −0.36 | 0.41 | 0.61 | −0.85 | −0.02 | 1 |
Significant correlations in italic font. Two tailed probability (p) <0.05 if correlation coefficient (r) ≥0.197 and p<0.001 if r≥0.33; BMI: body mass index; eGFR: estimated glomerular filtration rate; Ca: calcium; P: phosphorus; PTH: parathyroid hormone; 25OHD: 25 hydroxyvitamin D; FGF23: fibroblast growth factor 23; ACR: urine albumin/creatinine ratio.
Multivariate linear regression for predictors of vitamin D level.
| Partial R2 | 95% conf. interval | p value | |
|---|---|---|---|
| Age | 0.0003 | −0.01 to 0.02 | 0.8 |
| BMI | 0.006 | −0.07 to 0.03 | 0.4 |
| Calcium | 0.13 | 1.05 to 2.53 | <0.0001 |
| PO4 | 0.15 | −2.38 to −1.71 | <0.0001 |
| PTH | 0.13 | −0.12 to −0.09 | <0.0001 |
| Albumin/creatinine (urine) | 0.14 | −0.11 to −0.08 | <0.0001 |
| Uric acid | 0.14 | −1.79 to −1.39 | <0.0001 |
| Albumin | 0.03 | −0.15 to 2.02 | 0.09 |
| eGFR | 0.1 | −0.06 to −0.014 | 0.001 |
BMI: body mass index; eGFR: estimated glomerular filtration rate; PO4: serum phosphorus; PTH: parathyroid hormone.
One-way ANOVA analysis of serum 25 OH vit D and FGF23 in between the three tertiles of serum phosphorus.
| Parameter | Lowest tertile Range Median±S.D. | Middle tertile Range Median±S.D. | Highest tertile Range Median±S.D. | F-ratio | p value |
|---|---|---|---|---|---|
| Log s.25 OH vit D | 1–1.43 1.31±0.14 | 0.94–1.51 1.24±0.19 | 0.94–1.34 1.04±0.13 | 15.79 | <0.0001 |
| Log s.FGF23 | 2.27–2.44 2.37±0.05 | 2.3–2.46 2.37±0.04 | 2.29–2.44 2.37±0.04 | 0.723 | 0.488 |
| Log s.PTH | 1.65–1.99 1.85±0.1 | 1.74 –1.95 1.88±0.07 | 1.7–1.99 1.93±0.08 | 7.08 | 0.00135 |
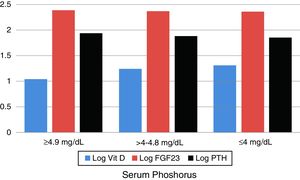
ANOVA: analysis of variance; s. 25 OH vit D: serum 25 hydroxy vitamin D; s.FGF23: serum level of fibroblast growth factor-23; lowest tertile: patients having serum phosphorus of 4mg/dL or less; middle tertile: patients having serum phosphorus above 4 and below 4.9mg/dL; highest tertile: patients having serum phosphorus 4.9mg/dL or more; PTH: parathyroid hormone.
According to serum P, patients were arranged into tertiles to compare the blood levels of 25(OH)D, FGF23, and PTH within these tertiles. The lowest tertile of CKD patients had serum P of 4mg/dL or less, the middle tertile had P between 4 and 4.8mg/dL while the highest had P of 4.9 and more. The mean log serum level of 25 (OH)D±S.D. in these 3 tertiles was 1.31±0.14, 1.24±0.19, and 1.04±0.13 respectively, p<0.0001. Similarly, serum PTH was significantly different. On the other hand, there was no significant difference in serum FGF23 in between the three tertiles, p=0.488 (Table 4 and Fig. 4).
DiscussionBeing a substrate for 1,25(OH)2D, the correlation between serum 25(OH)D and serum P should have been a positive one. 1,25(OH)2D is a strong stimulant of intestinal NPTIIb activity and thus enhances intestinal phosphate absorption. In CKD patients with impaired renal phosphate excretion, both 25(OH)D and 1,25(OH) should have a positive association with serum phosphorus. The negative association observed in previous unpublished and published studies (14,15) and confirmed in the current study raises many possible explanations. The increased FGF23 level induced by increased serum P might inhibit 25 hydroxylase or stimulate 24 hydroxylase. FGF23 was found to negatively correlate with serum 25(OH)D when studied in mice, but the same study failed to find a similar relation in human CKD patients.11 In the current study, FGF23 failed to correlate with 25(OH)D. The second possible explanation is through stimulation of PTH. Serum P can directly stimulate PTH synthesis and secretion in CKD.18 PTH is positively correlated with P in the current study. Meanwhile, PTH consistently has a negative correlation with 25(OH)D in different studies including the current study. However, this negative association is, most probably, due to the negative feedback inhibition of parathyroid by the active 1,25(OH)2D. Meanwhile, PTH is known to stimulate Cyp27b1 responsible for 1-α hydroxylase and inhibits Cyp24a1 responsible for 24 hydroxylase activity.5 These facts make suppression of 25(OH)D by PTH unlikely. The 3rd possible explanation is the impact of deteriorating kidney function on 25(OH)D. Serum 25(OH)D is significantly lower in patients with a severe decrease in eGFR compared with those with normal kidney function.19 However, the current study beside other studies denied an impact of eGFR on 25(OH)D.20–22 The 4th and most likely possibility is a direct effect of P on 25(OH)D. Serum P directly inhibits Cyp27b1 1-α hydroxylase.5 No similar effect on CYP2R1 25 hydroxylase is encountered in the literature. On the other hand, serum P correlates with 24 hydroxylase RNA.23 This relation means that high serum P can decrease serum level of 25(OH)D through its increased catabolism by the excessive activation of Cyp24a1 24 hydroxylase. In favor of this hypothesis, Kim et al., observed a lower response to vitamin D supplementation in patients having lower baseline serum levels of 25(OH)D.24
The negative association between serum 25(OH)D and SUA might be related to the recently disclosed inhibitory action of PTH on the intestinal uric acid transporter ABCG2.25 The consequent inhibition of intestinal uric acid excretion results in its retention.26
Finally, this study casts doubt on the diagnostic value of serum level of 25(OH)D in the assessment of vitamin D status of pre-dialysis CKD patients. Further studies are needed before confirming this conclusion. Till such studies are available, excess administration of vitamin D2 and/or D3, especially in CKD patients, should be used cautiously.
Limitations of the current study- 1.
This study is an uncontrolled observational cross-sectional study. The results of such studies might carry biases that are difficult to detect or correct.
- 2.
The lack of a significant association between FGF23 and serum 25 (OH) vit D in this cross-sectional study cannot completely exclude a possible inhibitory effect that necessitates longitudinal studies to detect.
- 3.
Linear regression analysis might have its limitations.
- 4.
We did not use ionized calcium instead of serum calcium. This might pause limitations especially if patients are hypoalbuminemic. However, this is not the case in this group of patients.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical committee approvalThe local ethical committee of the Internal Medicine department, School of Medicine, Cairo University, approved this work.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflict of interestsThe authors have declared that no conflict of interests exists.
Professor Usama and Dr Ahmed Fyed suggested the hypothesis and objectives of this study, Dr. Dina collected the necessary literature, Dr Mahmoud El Nokeety and Dr Ahmed Heikal collected the study subjects, Dr Ahmed Fyed, Dr. Hany Hammad, and Dr khaled Marzouk collected the samples and made the statistics Prof Usama and Prof Mona wrote the manuscript, Dr Dina made the final revision.