Sodium-glucose co-transporter-2 inhibitors (SGLT2i) and aldosterone inhibitors show promise for treating chronic kidney disease (CKD). This systematic review and meta-analysis explored the efficacy and safety of aldosterone inhibitors plus SGLT2i combination compared to their effects. We searched PubMed, Scopus, Web of Science, Cochrane Central Register of Controlled Trials [CENTRAL], and EBSCOhost. We reported dichotomous outcomes as pooled relative ratios and continuous outcomes as standardized mean differences with a 95% confidence interval. Three studies were included in this meta-analysis. The combination therapy was associated with a significantly higher rate of 30% reduction of urine albumin creatinine ratio (UACR) compared to SGLT2i (RR=2.38, 95% CI, 1.46–3.46, P<0.001; I2=0%, P=0.54) and compared to MRA (RR=1.34, 95% CI, 1.12–1.60, P=0.001; I2=13%, P=0.28). It also showed a significant reduction in the UACR compared to SGLT2i (SMD=−1.47, 95% CI, −2.25 to −0.68, P=0.0003; I2=78%, P=0.03) but no significant reduction compared to aldosterone inhibitors (SMD=−0.10, 95% CI, −0.38 to 0.19, P=0.51; I2=67%, P=0.05). The pooled data showed no significant difference in the incidence of serious adverse events between the combination therapy and SGLT2i (RR=1.01, 95% CI, 0.72–1.41, P=0.96; I2=0%, P=0.58) or MRA (RR=1.01, 95% CI, 0.79–1.30, P=0.92; I2=0%, P=0.90). In conclusion, combining SGLT2i and aldosterone inhibitors may offer a promising approach for managing albuminuria and potentially slowing kidney disease progression in CKD patients.
We registered the protocol in PROSPERO CRD42024511675.
Los inhibidores del cotransportador de sodio-glucosa tipo 2 (iSGLT2) y los inhibidores de la aldosterona muestran resultados prometedores en el tratamiento de la enfermedad renal crónica (ERC). Esta revisión sistemática y metanálisis exploró la eficacia y la seguridad de la combinación de inhibidores de la aldosterona e iSGLT2 en comparación con sus efectos. Se realizaron búsquedas en PubMed, Scopus, Web of Science, el Registro Cochrane Central de Ensayos Controlados [CENTRAL] y EBSCOhost. Los resultados dicotómicos se presentaron como razones relativas agrupadas y los resultados continuos como diferencias de medias estandarizadas con un intervalo de confianza del 95%. Se incluyeron tres estudios en este metanálisis. La terapia combinada se asoció con una tasa significativamente mayor de reducción del 30% del cociente albúmina-creatinina en orina (UACR) en comparación con SGLT2i (RR = 2,38, IC del 95%, 1,46: 3,46, P < 0,001; I2 = 0%, P = 0,54) y en comparación con MRA (RR = 1,34, IC del 95%, 1,12: 1,60, P = 0,001; I2 = 13%, P = 0,28). También mostró una reducción significativa en el UACR en comparación con SGLT2i (SMD=-1,47, IC 95%, -2,25: -0,68, P= 0,0003; I2= 78%, P=0,03) pero ninguna reducción significativa en comparación con los inhibidores de la aldosterona (SMD=-0,10, IC 95%, 0,38: 0,19, P= 0,51; I2= 67%, P=0,05). Los datos agrupados no mostraron diferencias significativas en la incidencia de eventos adversos graves entre la terapia combinada y los inhibidores del SGLT2 (RR = 1,01; IC del 95%, 0,72-1,41; p = 0,96; I2 = 0%, p = 0,58) o la ARM (RR = 1,01; IC del 95%, 0,79-1,30; p = 0,92; I2 = 0%, p = 0,90). En conclusión, la combinación de inhibidores del SGLT2 e inhibidores de la aldosterona podría ofrecer un enfoque prometedor para el manejo de la albuminuria y, potencialmente, ralentizar la progresión de la enfermedad renal en pacientes con ERC.
Chronic kidney disease (CKD) is defined as decreased kidney function (GFR <60mL/min per 1.73m2), or by markers of kidney damage, or both, for at least 3 months. While many conditions can lead to CKD, hypertension, and diabetes are the main causes globally.1,2 Around 800 million individuals globally have CKD.3 The prevalence of CKD is steadily rising over time, now affecting about 10% of people worldwide. There is an increase in the prevalence of CKD which makes it a major public health issue that requires attention.4 The first-line medications for treating individuals with CKD are angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). It has been demonstrated that the use of ACEI or ARBs has a greater impact on reducing proteinuria and delaying the course of renal disease than placebo treatment. The effectiveness of ARB and ACEI is comparable.5 In recent years, several clinical trials have demonstrated the effectiveness of various medications for kidney protection. Sodium-glucose co-transporter-2 inhibitors (SGLT2i) are among these drugs.6–9
SGLT2i were initially proposed as glucose-lowering and weight-lowering drugs in patients with type 2 diabetes mellitus (DM).10,11 SGLT2i reduces the reabsorption of filtered glucose from the tubular lumen, decreases the renal threshold for glucose (RTG), and promotes urinary glucose excretion by inhibiting the activity of SGLT2 proteins that are located in the proximal convoluted tubules of the kidneys.12–14 However, recent cardio-renal trials have demonstrated that the benefits of SGLT2i go beyond managing type 2 DM. These medications are effective in preventing cardiovascular events and slowing the progression of CKD.14,15 One of these trials is the DAPA-CKD trial, which concluded that compared to a placebo, dapagliflozin significantly reduces the risk of a composite outcome in patients with CKD, regardless of DM status. This includes a persistent reduction in estimated glomerular filtration rate (eGFR) of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes.7
Aldosterone expedites the progression of chronic renal disease.16,17 Aldosterone exerts harmful effects through many mechanisms, including inflammation and fibrosis, which culminate in glomerular, tubulointerstitial, and vascular damage to the kidney.18 The recent discovery of aldosterone inhibitors such as non-steroidal mineralocorticoid receptor antagonists (MRAs) represents a significant advancement in cardio-renal disease treatment.8,9 Similar to steroidal MRAs, non-steroidal MRAs have positive effects on inflammation, remodeling, and fibrosis in the kidneys, heart, and vasculature. However, non-steroidal MRAs distribute equally between the kidneys and heart, showing better suppression of mineralocorticoid receptor (MR) co-regulator recruitment and fibrosis than steroidal MRAs.19,20 Recent trials, including FIGARO-DKD and FIDELIO-DKD, disclosed that finerenone reduced the risk of cardiovascular and major kidney events in patients with type 2 DM and CKD.9,21 Additionally, aldosterone synthase inhibitors directly reduce aldosterone synthesis, potentially increasing treatment efficacy.18,22
We designed this systematic review and meta-analysis to study the safety and efficacy of SGLT2i and aldosterone inhibitors either MRAs or aldosterone synthase inhibitors combination in CKD. Additionally, we aimed to check whether this dual therapy is superior to either agent alone.
MethodsStudy protocol and registrationFollowing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), we carried out this systematic review and meta-analysis of randomized clinical trials.23 We followed the instructions provided in Cochrane's Handbook of Systematic Reviews of Interventions for every step we took.24 We registered the protocol in PROSPERO CRD42024511675.
Search strategy and data collectionWe searched four electronic databases: PubMed, Scopus, Web of Science, Cochrane Central Register of Controlled Trials [CENTRAL], and EBSCOhost. We searched for all studies published until January 2024. Details of the search strategy are mentioned in Table S1.
We assessed all retrieved studies for our eligibility criteria in two steps: title and abstract screening, then full-text screening. Studies that met our eligibility criteria are included. We manually screened the references to the previous reviews and our included studies. Two separate authors did all the screening steps, and a third author resolved all conflicts.
Eligibility criteriaWe included randomized controlled trials (RCTs) and observational studies, including retrospective and prospective studies reported in English that fulfilled the illustrated PICO criteria as follows: P: Patients suffering from CKD either diabetic or non-diabetic. I: SGLT2i plus aldosterone inhibitors “MRAs or aldosterone synthase antagonist” combinations. C: SGLT2 inhibitor monotherapy, aldosterone inhibitors either MRAs or aldosterone synthase inhibitors monotherapy, conventional therapeutic methods, or placebo. O: Any of the following:
- •
The incidence of a 30% reduction of urine albumin-to-creatinine ratio (UACR)
- •
The incidence of a 50% reduction of UACR
- •
Changes in UACR
- •
Changes in blood pressure
- •
Changes in eGFR
- •
Changes in measured glomerular filtration rate (mGFR)
- •
Serious adverse events
- •
Any adverse events
- •
The incidence of hyperkalemia
- •
The incidence of patients having potassium more than 5mmol
Two authors extracted data using Excel in three sheets: 1 – summary of the included trials’ settings; 2 – baseline characteristics of the participants in the included trials; 3 – data of the reported outcomes, then any conflict between both authors was resolved by a third author.
Quality assessmentWe assessed the methodology of the included studies using the Cochrane Risk of Bias Assessment Tool (ROB-II).25 We also used the ROB-II tool for crossover studies.26 The authors rated each domain as low, high risk, or unclear. We used Risk-of-bias Visualization (Robvis) software to visualize ROB figures.27
Data analysisWe used the Review Manager software (RevMan 5.4). Continuous outcomes were analyzed by mean difference (MD), standardized mean difference (SMD), and 95% confidence interval (CI) throughout the entire record.
Dichotomous data were analyzed as risk ratio (RR) and 95% CI. Statistical heterogeneity among the studies was assessed by visual inspection of the forest plot, besides using I-squared (I2) and chi-squared (Chi2) statistics. I2 values of 50% were indicative of significant heterogeneity. Subgroups or subsets will be applied for the outcomes eligible for that.
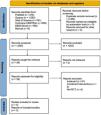
ResultsStudy selectionA literature search through PubMed, Scopus, Web of Science, Cochrane CENTRAL, EBSCOhost, and the manual search has revealed 4066 articles with 2383 duplicates. Title and abstract screening were done on 1683 articles, and 1252 were excluded. Full-text screening was performed on 39 studies. Finally, three studies were included in this systematic review and meta-analysis. The study flow diagram is shown in Fig. 1.
Study characteristicsThis systematic review and meta-analysis includes three randomized clinical trials (RCTs).28–30 One of them was conducted in three clinical trial centers in Italy and Spain,29 and the other two studies were conducted in multiple centers across various countries.28,30 There is a randomized controlled crossover trial that included 46 patients in the trial. Patients have been treated with eplerenone 50mg once daily, dapagliflozin 10mg once daily, or a combination of eplerenone 50mg once daily and dapagliflozin 10mg once daily in random order during three consecutive open-label crossover treatment periods of four weeks each, with four-week washout periods in between each active treatment period.23,29 The second study examined three dosages of BI 690517, with or without the SGLT2i empagliflozin, combined with ACE inhibitors or ARB.28 The last one included 5674 participants who were divided into two groups. Of them, 259 patients were on an SGLT2i at baseline. These patients were divided into two groups: 135 and 124 in the intervention and control groups respectively. The remaining participants, 5415 patients, did not receive SGLT2i at baseline. These patients were divided into two groups: 2709 and 2706 in the intervention and control groups respectively.30 The mean age of the included patients ranged from 61.8 to 65.8. Most of the included studies in the intervention and control group are males (69.9%), and white ethnicity (63.09%). The summary and baseline characteristics of the included studies are shown in Tables 1 and 2, and Table S2.
Summary of the included studies.
| Study ID | Study arm | Sample size | Study design | Country/region | Combined medications used | Treatment dose | Frequency | Treatment duration | Follow-up duration | Main outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Provenzano et al., 2022 | Dapagliflozin | 46 | Prospective, randomized, open-label crossover trial | Italy and Spain (3 sites) | Eplerenone 50mg OD, dapagliflozin 10mg OD, or combination | Dapagliflozin 10mg; eplerenone 50mg | Once daily | Three 4-week treatment periods with 4-week washouts | Until end of study | Correlation between 24-h UACR changes from baseline with dapagliflozin, eplerenone, and combination |
| Eplerenone | ||||||||||
| Dapagliflozin+eplerenone | ||||||||||
| Rossing et al., 2022 | Finerenone+SGLT2i | 124 | RCT | 48 countries | ACEi, ARB, β-blocker, diuretics, statins, potassium-lowering agents, glucose-lowering agents, insulin, GLP-1RA, SGLT2i | Not specified | Not specified | N/A | N/A | Positive outcomes with finerenone in CKD and T2D, regardless of SGLT2i use |
| Placebo (with SGLT2i) | 135 | |||||||||
| Finerenone+no SGLT2i | 2709 | |||||||||
| Placebo (no SGLT2i) | 2706 | |||||||||
| Tuttle et al., 2024 | BI 690517 placebo+empagliflozin | 74 | RCT | Multinational (27 countries) | ARB, ACEi, GLP-1RA; background empagliflozin or placebo | BI 690517: 3mg, 10mg, 20mg | N/A | 14 weeks | Follow-up at week 18 (4 weeks after washout) | Reduction in UACR and eGFR, especially with BI 690517+empagliflozin |
| BI 690517 3mg+empagliflozin | 76 | 3mg | ||||||||
| BI 690517 10mg+empagliflozin | 74 | 10mg | ||||||||
| BI 690517 20mg+empagliflozin | 74 | 20mg | ||||||||
| BI 690517 placebo+placebo | 73 | – | ||||||||
| BI 690517 3mg+placebo | 71 | 3mg | ||||||||
| BI 690517 10mg+placebo | 72 | 10mg | ||||||||
| BI 690517 20mg+placebo | 72 | 20mg | ||||||||
Abbreviations: UACR: urine albumin to creatinine ratio; SGLT2i: sodium-glucose transport protein 2 (SGLT2) inhibitor; ACEi: angiotensin convertase enzyme inhibitor; ARB: angiotensin receptor blocker; GLP-1RA: glucagon-like peptide-1 receptor agonist; eGFR: estimated glomerular filtration rate; BI 690517: potent, highly selective aldosterone synthase inhibitor; CKD: chronic kidney disease; T2D: type 2 diabetes mellitus; OD: once daily; RCT: randomized controlled trial.
Baseline characteristics of the included studies.
| Study ID | Study arm | n | Age (Y), mean (SD) | Male, n (%) | Race (n) | DM, n (%) | HbA1c %, mean (SD) | SBP mmHg, mean (SD) | DBP mmHg, mean (SD) | CRP mg/L, mean (SD) | BMI kg/m2, mean (SD) | eGFR mL/min/1.73m2, mean (SD) | UACR mg/g, mean (SD) | Co-interventions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Provenzano et al., 2022 | Dapagliflozin | 46 | 69.5 (7.6) | 35 (76.1%) | White: 45; other: 1 | 32 (69.6%) | 48.9 (1.7) | 136.3 (9.0) | 80 (7.5) | NA | 30.8 (6.2) | 58.1 (18.6) | 401 (101) | ACEi: 17; ARB: 29; β-blocker: 17; CCB: 24; thiazide: 12; loop: 11; insulin: 11; statin: 38 |
| Eplerenone | 49.5 (1.7) | |||||||||||||
| Dapagliflozin+eplerenone | 50.0 (1.7) | |||||||||||||
| Rossing et al., 2022 | Finerenone+SGLT2i | 124 | 63.9 (9.3) | 92 (74.2%) | White: 91; Asian: 25; Black: 1 | NA | 8.02 (1.15) | 135.4 (14.4) | 74.9 (10.2) | 3.96 (5.21) | 31.8 (5.8) | 51.8 (11.3) | 772 (671.3) | ACEi: 36; ARB: 88; β-blocker: 64; metformin: 89; insulin: 89; statin: 102; GLP-1RA: 17; K+-lowering: 4 |
| Placebo+SGLT2i | 135 | 62.3 (9.9) | 96 (71.1%) | White: 89; Asian: 33; Black: 8 | NA | 7.98 (1.26) | 134.1 (14.5) | 76.3 (9.2) | 3.46 (3.87) | 31.8 (5.5) | 50.3 (12.5) | 737 (663.2) | ACEi: 41; ARB: 94; β-blocker: 73; metformin: 93; insulin: 84; statin: 121; GLP-1RA: 3; K+-lowering: 1 | |
| Finerenone+no SGLT2i | 2709 | 65.5 (8.9) | 1861 (68.7%) | White: 1686; Asian: 692; Black: 139 | NA | 7.65 (1.33) | 138.2 (14.3) | 75.9 (9.7) | 4.57 (9.01) | 31.1 (6.0) | 44.0 (12.5) | 978 (883.4) | ACEi: 914; ARB: 1791; β-blocker: 1398; metformin: 1162; insulin: 1754; statin: 2003; GLP-1RA: 172; K+-lowering: 66 | |
| Placebo+no SGLT2i | 2706 | 65.8 (9.1) | 1934 (71.5%) | White: 1726; Asian: 690; Black: 116 | NA | 7.67 (1.36) | 138.2 (14.4) | 75.8 (9.7) | 4.66 (9.32) | 31.1 (6.0) | 44.0 (12.5) | 1005 (895.2) | ACEi: 951; ARB: 1752; β-blocker: 1433; metformin: 1146; insulin: 1710; statin: 1989; GLP-1RA: 174; K+-lowering: 65 | |
| Tuttle et al., 2024 | BI 690517 placebo+empagliflozin | 74 | 63.4 (10.3) | 43 (58.1%) | Asian: 19; Black: 10; White: 42 | 51 (68.9%) | 7.0 (1.3) | 132.7 (13.9) | 76.9 (10.7) | N/A | 29.5 (5.2) | 49.6 (17.5) | 492.7 (632.1) | ACEi: 24; ARB: 49; GLP-1RA: 5 |
| BI 690517 3mg+empagliflozin | 76 | 65.4 (11.2) | 49 (64.5%) | Asian: 17; Black: 9; White: 49 | 67 (88.2%) | 7.1 (1.2) | 134.4 (16.4) | 76.4 (9.2) | 30.3 (5.8) | 50.3 (16.7) | 576.3 (609.8) | ACEi: 23; ARB: 55; GLP-1RA: 10 | ||
| BI 690517 10mg+empagliflozin | 74 | 64.4 (12.3) | 44 (59.5%) | Asian: 27; Black: 6; White: 35 | 46 (62.2%) | 6.9 (1.4) | 131.7 (13.1) | 77.1 (9.4) | 29.6 (5.3) | 50.2 (17.9) | 467.7 (475.6) | ACEi: 18; ARB: 55; GLP-1RA: 6 | ||
| BI 690517 20mg+empagliflozin | 74 | 61.8 (12.2) | 54 (73.0%) | Asian: 20; Black: 7; White: 45 | 54 (73.0%) | 7.1 (1.3) | 131.8 (16.0) | 75.6 (8.6) | 29.4 (5.9) | 51.5 (17.1) | 499.7 (446.8) | ACEi: 24; ARB: 49; GLP-1RA: 11 | ||
| BI 690517 placebo+placebo | 73 | 62.3 (11.4) | 55 (75.3%) | Asian: 16; Black: 10; White: 44 | 49 (67.1%) | 7.0 (1.4) | 133.9 (15.9) | 80.2 (11.4) | 30.4 (6.0) | 54.6 (18.7) | 503.3 (532.4) | ACEi: 25; ARB: 49; GLP-1RA: 7 | ||
| BI 690517 3mg+placebo | 71 | 64.4 (11.8) | 51 (71.8%) | Asian: 23; Black: 3; White: 42 | 48 (67.6%) | 6.8 (1.2) | 134.8 (18.7) | 78.5 (9.1) | 30.5 (5.0) | 51.8 (16.4) | 602.7 (750.7) | ACEi: 29; ARB: 41; GLP-1RA: 4 | ||
| BI 690517 10mg+placebo | 72 | 64.8 (9.9) | 49 (68.1%) | Asian: 15; Black: 9; White: 45 | 52 (72.2%) | 6.9 (1.3) | 135.0 (15.1) | 75.9 (8.7) | 30.3 (5.2) | 56.1 (20.9) | 453.7 (427.4) | ACEi: 14; ARB: 56; GLP-1RA: 4 | ||
| BI 690517 20mg+placebo | 72 | 64.3 (10.7) | 45 (62.5%) | Asian: 19; Black: 9; White: 40 | 47 (65.3%) | 6.9 (1.3) | 136.1 (16.2) | 77.4 (9.0) | 29.5 (5.3) | 51.6 (16.0) | 554.0 (448.6) | ACEi: 21; ARB: 51; GLP-1RA: 3 | ||
Abbreviations: SBP: systolic blood pressure; DBP: diastolic blood pressure; DM: diabetes mellitus; UACR: urine albumin to creatinine ratio; SGLT2i: sodium-glucose transport protein 2 (SGLT2) inhibitor; ACEi: angiotensin convertase enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; CRP: C-reactive protein; HbA1c: hemoglobin A1C; GLP-1RA: glucagon-like peptide-1 receptor agonist; eGFR: estimated glomerular filtration rate; BI 690517: potent, highly selective aldosterone synthase inhibitor; CKD: chronic kidney disease; T2D: type 2 diabetes mellitus.
The follow-up duration in the study by Provenzano et al. was short, consisting of three 4-week treatment periods (dapagliflozin, eplerenone, and their combination), each separated by 4-week washout periods, for a total duration of approximately 12 weeks.29 This design allowed for the assessment of immediate effects on albuminuria and GFR following treatment initiation. Similarly, the study by Tuttle et al. had a short follow-up period comprising an 8-week run-in, a 14-week treatment phase, and a 4-week post-treatment follow-up, totaling around 18 weeks.31 This study focused on short-term changes in UACR, eGFR, and serum potassium levels. In contrast, the study by Rossing et al. featured a long-term follow-up with a median duration of 2.6 years, enabling a comprehensive evaluation of finerenone's sustained effects on GFR decline, kidney failure, and cardiovascular outcomes.30 Among the three included studies, only the FIDELIO-DKD trial provides long-term data, whereas ROTATE-3 and BI 690517 offer insights into early, short-term changes. This distinction has been emphasized in the revised manuscript to more accurately contextualize the findings related to GFR changes.
Quality assessmentAll included studies showed an overall low risk of bias. One study was assessed by the ROB-II tool for crossover studies,24 and the other two studies were assessed by the ROB-II tool (Fig. 2a and b).
Primary outcomesIncidence of reduction in urine albumin creatinine ratio (UACR)Two studies reported the effect of SGLT2i combined with aldosterone inhibitors on the incidence of a 30% reduction in UACR compared to SGLT2i alone. The pooled data demonstrated a significant difference favoring the combination therapy (RR=2.38, 95% CI, 1.64–3.46, P<0.00001; I2=0%, P=0.54; Fig. 3a). Similarly, when compared to MRAs alone, the combination therapy also showed a significant advantage (RR=1.34, 95% CI, 1.12–1.60, P=0.001; I2=13%, P=0.28; Fig. 3b). Additionally, for a 50% reduction in UACR, the combination therapy was favored over SGLT2i (RR=2.60, 95% CI, 1.72–3.93, P<0.00001; I2=0%, P=0.75; Fig. 3c) and MRAs (RR=1.34, 95% CI, 1.11–1.61, P=0.002; I2=16%, P=0.28; Fig. 3d).
(a) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on incidence of 30% reduction in urine albumin creatinine ratio compared to SGLT2i. (b) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on incidence of 30% reduction in urine albumin creatinine ratio compared to MRAs. (c) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on incidence of 50% reduction in urine albumin creatinine ratio compared to SGLT2i. (d) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on incidence of 50% reduction in urine albumin creatinine ratio compared to MRAs.
CI: Confidence Interval; I2: I-squared statistic (measuring heterogeneity); M-H: Mantel-Haenszel.
Two studies reported that the combination of SGLT2i and aldosterone inhibitors resulted in a significant reduction in UACR when compared to SGLT2i alone (SMD=−1.47, 95% CI, −2.25 to −0.68, P=0.0003; I2=78%, P=0.03; Fig. 4a). However, when compared to aldosterone inhibitors, the pooled data from three studies did not show a significant reduction in UACR (SMD=−0.10, 95% CI, −0.38 to 0.19, P=0.51; I2=67%, P=0.05; Fig. 4b).
Change of urine albumin creatinine ratio. (a) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on change of urine albumin creatinine ratio compared to SGLT2i. (b) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on change of urine albumin creatinine ratio compared to aldosterone inhibitors.
CI: Confidence Interval; IV: Inverse Variance (method); I2: I-squared statistic (measuring heterogeneity).
Two studies indicated that the combination therapy resulted in a significant reduction in eGFR compared to SGLT2i alone (MD=−1.94, 95% CI, −3.24 to −0.63, P=0.004; I2=25%, P=0.25; Fig. 5a). Conversely, when compared to MRAs, the combination therapy did not result in a significant reduction in eGFR (MD=0.26, 95% CI, −0.06 to −0.57, P=0.12; I2=0%, P=0.35; Fig. 5b).
Change of estimated glomerular filtration rate. (a) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on change of estimated glomerular filtration rate compared to SGLT2i. (b) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on change of estimated glomerular filtration rate compared to MRAs.
CI: Confidence Interval; IV: Inverse Variance (method); I2: I-squared statistic (measuring heterogeneity).
Three studies reported that the combination therapy significantly reduced SBP compared to SGLT2i (MD=−8.86, 95% CI, −12.79 to −4.93, P<0.00001; I2=59%, P=0.12; Fig. 6a) and compared to aldosterone inhibitors (MD=−3.00, 95% CI, −5.50 to −0.50, P=0.02; I2=96%, P<0.00001; Fig. 6b).
Change of systolic blood pressure. (a) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on the change of systolic blood pressure compared to SGLT2i. (b) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on the change of systolic blood pressure compared to aldosterone inhibitors.
CI: Confidence Interval; IV: Inverse Variance (method); I2: I-squared statistic (measuring heterogeneity).
Three studies reported the incidence of serious AEs associated with combination therapy compared to SGLT2i and aldosterone inhibitors. The pooled data showed no significant difference in the incidence of serious AEs between the combination therapy and SGLT2i (RR=1.01, 95% CI, 0.72–1.41, P=0.96; I2=0%, P=0.58) or aldosterone inhibitors (RR=1.01, 95% CI, 0.79–1.30, P=0.92; I2=0%, P=0.90; Fig. 7a).
(a) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on incidence of serious adverse events compared to SGLT2i and aldosterone inhibitors. (b) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on the incidence of any adverse events compared to SGLT2i and aldosterone inhibitors. (c) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on incidence of hyperkalemia compared to SGLT2i and aldosterone inhibitors. (d) Effect of SGLT2 inhibitors and aldosterone inhibitors combination on the incidence of potassium more than 5mmol/L compared to SGLT2i and aldosterone inhibitors.
CI: Confidence Interval; I2: I-squared statistic (measuring heterogeneity); M-H: Mantel-Haenszel.
Three studies also reported the incidence of any AEs associated with the combination therapy compared to SGLT2i and aldosterone inhibitors. The pooled data showed no significant difference in the incidence of any AEs between the combination therapy and SGLT2i (RR=1.05, 95% CI, 0.97–1.13, P=0.26; I2=0%, P=0.92) or aldosterone inhibitors (RR=0.87, 95% CI, 0.65–1.18, P=0.38; I2=92%, P<0.00001; Fig. 7b).
HyperkalemiaThree studies reported the incidence of hyperkalemia associated with the combination therapy compared to SGLT2i and aldosterone inhibitors. The pooled data showed significant difference in the incidence of hyperkalemia between the combination therapy and SGLT2i (RR=2.29, 95% CI, 1.15–4.57, P=0.02; I2=0%, P=0.78) but associated with no significant difference compared to aldosterone inhibitors (RR=0.55, 95% CI, 0.27–1.09, P=0.08; I2=64%, P=0.06; Fig. 7c).
Two studies reported the incidence of patients who have potassium more than 5mmol associated with the combination therapy compared to SGLT2i and MRAs. The pooled data showed no significant difference in the incidence of potassium more than 5mmol between the combination therapy and SGLT2i (RR=2.67, 95% CI, 0.96–7.41, P=0.06; I2=0%, P=0.49) but associated with significant difference compared to MRAs (RR=0.34, 95% CI, 0.20–0.57, P<0.00001; I2=0%, P=0.52; Fig. 7d).
DiscussionThis systematic review and meta-analysis included three randomized controlled trials assessing the effect of SGLT2i and aldosterone inhibitors combination versus SGLT2i monotherapy and aldosterone inhibitors either MRAs or aldosterone synthase inhibitors monotherapy in CKD. The mean age range of participants ranged from 61.8 to 65.8 years, majority of participants were male (69.9%) and white ethnicity (63.09%). Pooled analysis showed that the combination therapy (SGLT2i+aldosterone inhibitors) has significant improvements compared to SGLT2i alone or aldosterone inhibitors for both 30% and 50% reductions in UACR. Combination therapy showed a significant reduction of UCAR and eGFR compared to SGLT2i alone, but no significant difference when compared to aldosterone inhibitors alone. There is also a significant reduction with combination therapy compared to both SGLT2I alone and aldosterone inhibitors alone. On the other hand, the combination therapy significantly increased the risk of hyperkalemia compared to SGLT2i alone. The low heterogeneity suggests that the included studies had consistent findings. However, no significant difference in hyperkalemia incidence was observed between the combination therapy and aldosterone inhibitors. The results are associated with moderate heterogeneity that indicates some variation among the studies; this may be a result of different types of aldosterone inhibitors, such as MRAs, either steroidal or non-steroidal, and aldosterone synthase inhibitors. According to the hyperkalemia, there is no significant difference in the incidence of potassium levels >5mmol/L observed between the combination therapy and SGLT2i. However, the combination therapy was associated with a significantly lower risk of potassium levels >5mmol/L compared to MRAs, suggesting it may be a safer option in terms of severe hyperkalemia risk. Meanwhile, no significant difference between combination therapy and either SGLT2i alone or aldosterone inhibitors alone. That indicates the combination therapy appears to be well-tolerated, with no significant increase in serious or overall adverse effects compared to monotherapy with either SGLT2i or aldosterone inhibitors.
According to our results, the combination of SGLT2i and aldosterone inhibitors has a potential synergistic effect in managing albuminuria, which is a marker of kidney damage. Our results are consistent with the results of Provenzano et al., who reported the combination of dapagliflozin (SGLT2i) and eplerenone (MRA) had a greater albuminuria-lowering effect than either drug alone in patients with CKD.23,29 The combination therapy also shows promise in reducing systolic blood pressure, which could have additional cardiovascular benefits. This is consistent with the results of the same trial that also found that the combination therapy resulted in a significant reduction in SBP compared to MRA alone.29 While the combination therapy showed a significant reduction in eGFR compared to SGLT2 inhibitors alone, this difference was not observed when compared to aldosterone inhibitors alone. The clinical significance of this finding needs further investigation. These results are consistent with the results of Rossing et al. and Provenzano et al.29,30 According to hyperkalemia, the absence of a significant difference between the combination and the aldosterone inhibitors is consistent with the results of Tuttle et al. that the hyperkalemia manifested at a frequency characteristic of a chronic kidney disease cohort; however, the majority of instances did not necessitate medical intervention or the cessation of BI 690517 (a potent, highly selective aldosterone synthase inhibitor).28 However, the combination has a significantly higher risk of hyperkalemia compared to the SGLT2i. Therefore, clinicians should be cautious about hyperkalemia risk when prescribing combination therapy, particularly when comparing it to SGLT2i alone. The choice between combination therapy and aldosterone inhibitors should consider individual patient risks, as combination therapy may reduce the likelihood of severe hyperkalemia. Further studies with larger sample sizes and standardized methodologies are needed to confirm these findings and clarify the clinical significance of potassium level changes.
Experimental studies and secondary analyses from large clinical outcome trials have suggested that SGLT2i and MRAs have complementary biological mechanisms of action that may result in significant reductions in albuminuria and clinically meaningful reductions in kidney outcomes. Empagliflozin and finerenone combination therapy produced a synergistic anti-albuminuric effect and enhanced survival compared with either medication alone, according to a preclinical rat trial in a model of cardio-renal illness.32 It is of great importance to determine the fundamental biological mechanisms that govern the response of each individual to treatment. To better customize therapy, future research may shed more light on the specific biological pathways and biomarkers that predict each patient's reaction to a certain medicine.
Strength pointsThis is the first systematic review and meta-analysis conducted to assess the safety and efficacy of SGLT2i and aldosterone inhibitors combination on patients with CKD. Our study provides promising results in the treatment of CKD and in reducing the disease progression. The included studies are low risk of bias.
LimitationsThe analysis is based on only three studies, with a small sample size, which limits the generalizability of the findings. There was significant heterogeneity in some of the analyses, particularly for changes in UACR and SBP, which suggests variability in the results across studies. Additionally, different types of aldosterone inhibitors, such as MRAs and aldosterone synthase inhibitors, are a major source of heterogeneity. Therefore, more head-to-head randomized controlled trials are needed to directly compare the efficacy and safety of SGLT2i and aldosterone inhibitor combinations versus each drug separately in CKD patients. We also need more RCTs that assess the effects of the combination for longer-term durations of follow-up. The metabolic acidosis was not assessed or reported in the trials included in this review, representing a notable limitation. This omission is clinically relevant, as metabolic acidosis can significantly influence potassium homeostasis by promoting extracellular potassium shifts and impairing renal potassium excretion. While the combination of SGLT2 inhibitors and MRAs was associated with a higher incidence of hyperkalemia across various MRA doses, this effect appeared to be independent of bicarbonate levels. The lack of data on acid-base status limits the ability to fully interpret the mechanisms underlying hyperkalemia in these patients. Future studies should consider evaluating metabolic acidosis and bicarbonate levels to better understand their potential contribution to electrolyte disturbances during combination therapy. While the results are promising, more research is needed to establish the long-term efficacy and safety of this combination therapy before it can be widely recommended in clinical practice.
ConclusionThe combination of SGLT2 inhibitors and aldosterone inhibitors may offer a promising approach for managing albuminuria and potentially slowing the progression of kidney disease in patients with CKD. Clinicians should be cautious about hyperkalemia risk when prescribing combination therapy. However, larger and longer-term studies are needed to confirm these findings and assess the impact on hard clinical outcomes such as progression to end-stage renal disease or cardiovascular events.
CRediT authorship contribution statementRS and AWH: conceptualization, validation, data curation, and original draft preparation. AKA, AH, ME, and KL: methodology. RS, LM, MA, WN, GEA, and SM: writing. RS and EIB: supervision. RS, AA, and AA: writing – reviewing and editing.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
FundingThis research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interestThe authors declare no competing interest.
Data availabilityData will be provided upon request from Reem Sayad (reem.17289806@med.aun.edu.eg).
The authors would like to thank Dr Ahmed Elgebaly, from MedDots FZC, for his support in medical writing and editorial processing of the manuscript.