Sickle cell disease, is a genetic disorder caused by a mutation in the HBB gene, affecting the β-globin and resulting in the formation of sickle hemoglobin. The disease can affect the kidney through various mechanisms, including vaso-occlusion, ischemia–reperfusion, endothelial dysfunction, and hemolysis-induced renal injury. This article describes the case of a 41-year-old woman with sickle cell disease who presented with a hemolytic crisis, developed acute kidney injury (AKI), 8g of proteinuria in 24h, and required renal support therapy. A renal biopsy was performed, revealing focal segmental glomerulosclerosis (FSGS), severe acute tubular necrosis, interstitial fibrosis with moderate tubular atrophy, and pigment casts. Thus, histochemistry was performed with a positive reaction for iron with evidence of intraluminal and cytoplasmic granular deposits in the proximal tubules compatible with hemosiderin and negative for hemoglobin. The case of this patient with sickle cell disease and AKI illustrates the importance of considering hemoglobin cast nephropathy as a potential cause.
Sickle cell disease is a genetic disorder with an autosomal recessive inheritance pattern. It is produced by mutation of the HBB gene that affects the β subunit of globin, resulting in the substitution of an amino acid Glu6Val and the formation of a sickled protein (HbS) that undergoes polymerization upon exposure to critical levels of deoxyHbS.1,2 HbS polymers harden red blood cells and can deform them into a crescent shape.
Sickle cell disease can alter the function and structure of the kidney through several mechanisms such as vaso-occlusion phenomena, ischemia–reperfusion, endothelial dysfunction, glomerular hypertrophy, disruption of the endothelium–podocyte/pericyte integrity and renal injury induced by hemolysis. Furthermore, the conditions of the renal medulla generate the propensity of red blood cells to deform and lyse due to conditions of hypoxia, hyperosmolarity and acidosis.2
Sickle cell disease is one of the most common inherited hematological diseases in the world. Renal involvement negatively affects life expectancy among people with sickle cell disease, and it is estimated that approximately 16–18% of global mortality is secondary to chronic kidney disease (CKD).2
Case presentation41-Year-old woman with a history of sickle cell disease with three hemolytic crises in the last 6 months, dilated cardiomyopathy of unclear etiology with ejection fraction 44%, secondary arterial hypertension, CKD G4 with baseline creatinine 3.3mg/dL and proteinuria 1000mg in 24h; under treatment with hydroxyurea 500mg every 12h with adequate pharmacological adherence. She was admitted to the emergency room due to a 3-day clinical of abdominal pain associated with emesis, asthenia, adynamia and headache. On physical examination, blood pressure was 185/90mmHg, grade I edema of the lower limbs, and pain on palpation of the right upper quadrant. Labs showing severe anemia with Hb 4.4g/dL, MCV 98 pg, CHbCM 34, reticulocyte count 4%, LDH 1503mg/dL, total bilirubin 2.2mg/dL, indirect bilirubin 2.0mg/dL, BUN 45mg/dL, serum creatinine 3.8mg/dL, urinalysis with proteins 500mg/dL, dysmorphic erythrocytes 3–5 XC and abdominal ultrasound that showed hepatomegaly and findings suggestive of chronic kidney disease.
Sickle cell disease was considered with criteria of acute hemolysis, to rule out decompensated acute heart failure due to signs of systemic congestion and known dilated cardiomyopathy. A transthoracic echocardiogram was taken, and described dilated cardiomyopathy with a pattern highly suggestive of cardiac amyloidosis, deterioration of left ventricular systolic function with an ejection fraction of 47% and pericardial effusion of 120mL, without hemodynamic repercussion.
She was evaluated by cardiology with a NT pro-BNP result of 36,400 and decided that it was important to exclude Fabry disease and amyloidosis with cardiac involvement as differential diagnoses, so protein electrophoresis with immunofixation in serum and urine, kappa light chains and lambda, biopsy of subcutaneous cellular tissue with Congo red staining and GLA (galactosidase alpha) genetic test. The complementary studies ordered by cardiology were negative.
Internal medicina requested evaluation by nephrology due to deterioration of kidney function with a result of proteinuria of 8g in 24h. This way, taking of a renal biopsy is ordered, and is subjected to light microscopy, immunofluorescence and electron microscopy, with a report of podocyte lesion of 70% associated with glomerulosclerosis. focal and segmental (FSGS), severe acute tubular necrosis, interstitial fibrosis with moderate tubular atrophy and pigment casts. Thus, histochemistry was performed with a positive reaction for iron with evidence of intraluminal and cytoplasmic granular deposits in the proximal tubules compatible with hemosiderin and is negative for hemoglobin. During follow-up, she developed severe hyperkalemia refractory to medical management, requiring a single session of renal replacement therapy with hemodialysis. It was determined that the deterioration of renal function was evidenced by pigment cast nephropathy related to sickle cell disease.
DiscussionWe present the case of a patient with sickle cell disease and AKI, a complication that has been reported in the literature in a proportion that varies between 2.3% and 13.6% in relation to a sickle cell crise due to hemolysis. Likewise, up to 4–13.6% of hospitalized patients with sickle cell disease have been reported.3 The causes of AKI are diverse and range from poor renal perfusion, mainly at the level of the medulla, vascular stasis, endothelial dysfunction, podocyte damage, hepatorenal syndrome and hemolysis that generates free hemoglobin and, ultimately, tubular damage.3
Among the clinical manifestations of kidney injury in sickle cell disease, is alteration in urine concentration; that is, hyposthenuria, increase in proximal tubule function that generates greater sodium absorption, involvement of the distal nephron with alteration in urine acidification and potassium excretion. This patient had hyperkalemia refractory to medical management requiring hemodialysis, microscopic hematuria with dysmorphic red blood cells, and proteinuria in the nephrotic range. It is estimated that approximately 4% of patients with sickle cell disease present proteinuria in the nephrotic range, which causes an unfavorable clinical prognosis. The pathophysiological mechanism is related to free hemoglobin secondary to hemolysis and podocyte injury, which is an important component of the glomerular filtration barrier.2–4
Considering the presence of proteinuria in the nephrotic range, the decision was made to perform a renal biopsy, the report of which revealed cortical infarcts due to obstruction of the arterioles and FSGS according to light microscopy. Among the glomerular lesions detected in this patient, FSGS was found to be the most common in patients with sickle cell disease. In a study that included 18 patients, FSGS was confirmed to be the most prevalent histopathological diagnosis, present in 43.7% of cases. Furthermore, collapsing variants with signs of ischemia, membranoproliferative glomerulonephritis in 5 cases and glomerular hypertrophy with or without mesangial hypercellularity and thrombotic microangiopathy (TMA) in 3 patients were reported.5,6
In this particular case, the presence of pigment casts with a negative result for hemoglobin in immunohistochemistry was identified, which is why histochemistry was performed with positive staining for hemosiderin, indicating the presence of a pigment derived from hemoglobin. Pigment cast nephropathy was diagnosed, a condition in which renal function deteriorates due to the toxic effect of pigments containing the heme group. This condition can be caused by various causes, such as rhabdomyolysis (myoglobin pigment), cholestasis (bile acid pigment), and, as in this case, intravascular hemolysis (hemoglobin pigment). It is important to highlight that the presence of hemoglobin casts is rare, and what is described here is the accumulation of hemosiderin in the proximal tubule, as occurs in sickle cell crisis, where there are frequent and persistent episodes of hemoglobinuria.7,8 Furthermore, findings compatible with acute tubular necrosis (ATN) were observed in this patient. It is relevant to note that when ATN occurs alone, without cast nephropathy, special stains in immunohistochemistry do not detect hemoglobin and myoglobin, as happened in this case. It is important to keep in mind that, in case series of patients with pigment cast nephropathy, 100% of cases present acute tubular injury.9–11
Differential diagnoses such as light chain nephropathy should be considered when positivity for hemoglobin and myoglobin is not found. However, this diagnosis is ruled out due to the absence of light chain restriction on immunofluorescence. When there is lysis of red blood cells, excess hemoglobin is released. Under these circumstances, it is impossible for haptoglobin to capture all the hemoglobin that is filtered by the kidneys and absorbed by the tubular cells. At the intracellular level, iron cannot be processed and the excess accumulates as hemosiderin. This increase in free hemoglobin and hemosiderin is observed in conditions where there is continuous hemolysis, as in the case of paroxysmal nocturnal hemoglobinuria, complications of cardiac valve prostheses and, as presented in this case, in patients with sickle cell disease during crises of intravascular hemolysis.9,12
Iron deposition secondary to excessive hemolysis can cause kidney damage through three different mechanisms. First is direct iron toxicity, which leads to acute tubular injury. This process involves the stimulation of lipid peroxidation, the formation of free radicals that generate damage to cell membranes, the denaturation of proteins, the alteration of some enzymes related to glycolysis, affectation of mitochondrial and cytoskeleton integrity, induction of pathways of pro-inflammatory signaling, increased release of cytokines and chemokines through the NLRP3 inflammasome pathway. Likewise, it can incite cell death through caspases and cathepsins, and promote the development of CKD.9,13-15
The second mechanism involves the formation of hemoglobin casts that obstruct the tubules, especially at the distal level of the nephron. Furthermore, hemolysis can lead to decreased renal perfusion, increasing vasoconstriction due to the sequestration of nitric oxide by free hemoglobin. On the other hand, cases of TMA associated with this process have been described.9 A relevant factor to consider is the possibility that this patient experiences recurrent episodes of hemolysis, since the renal injury resulting in these conditions can compromise the tubulointerstitial tissue, as observed in the patient's light microscopy. Furthermore, the increase in proximal tubule function leads to an increase in oxygen consumption and cellular hypermetabolism by multiple adenosine triphosphate (ATP)-dependent channels.2,3,15
It is interesting to note that the causes of intravascular hemolysis that result in renal compromise, as evidenced by biopsies, can vary significantly depending on the geographic region studied. Research in India has revealed that rifampicin was the leading cause of hemolysis, followed by snake bites. In contrast, in the United States, autoimmune hemolytic anemia is the leading cause in approximately 30% of cases, followed by pharmacologic causes. Hemoglobinopathies represent only 4% of cases.10,11,16 This regional variability highlights the importance of considering geographic context when evaluating potential causes of intravascular hemolysis and its effects on the kidney (Figs. 1–6).
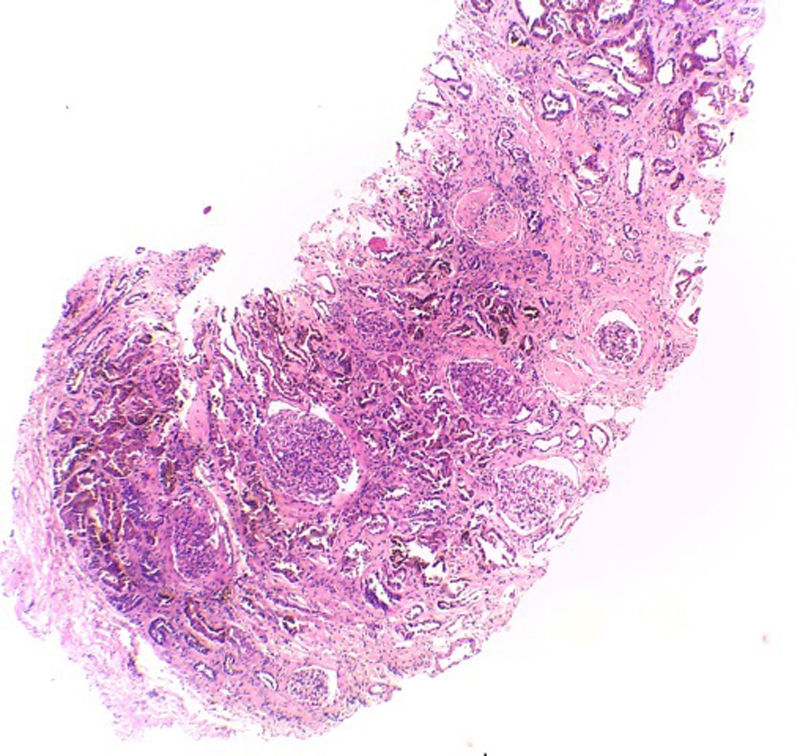
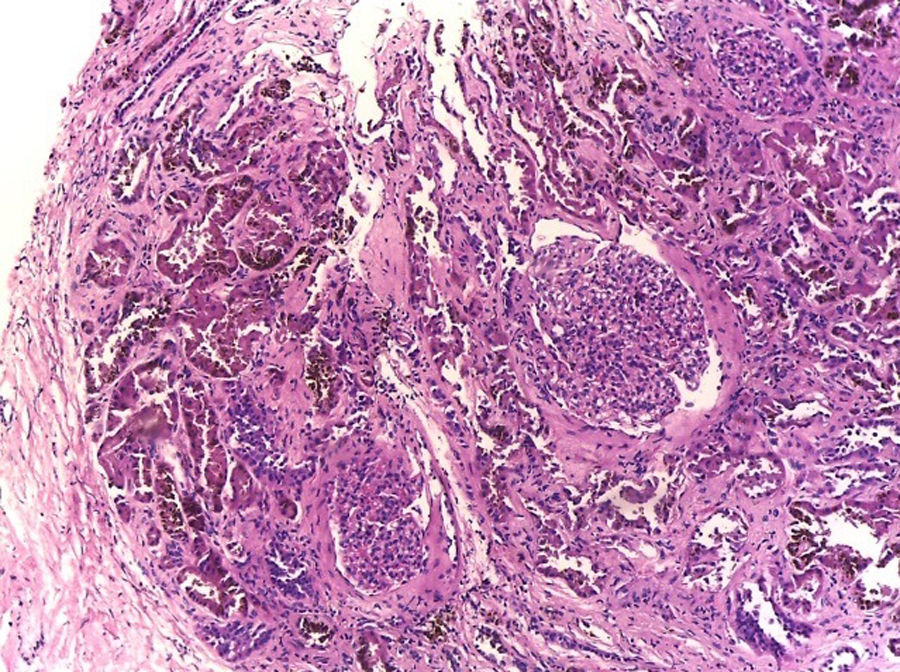
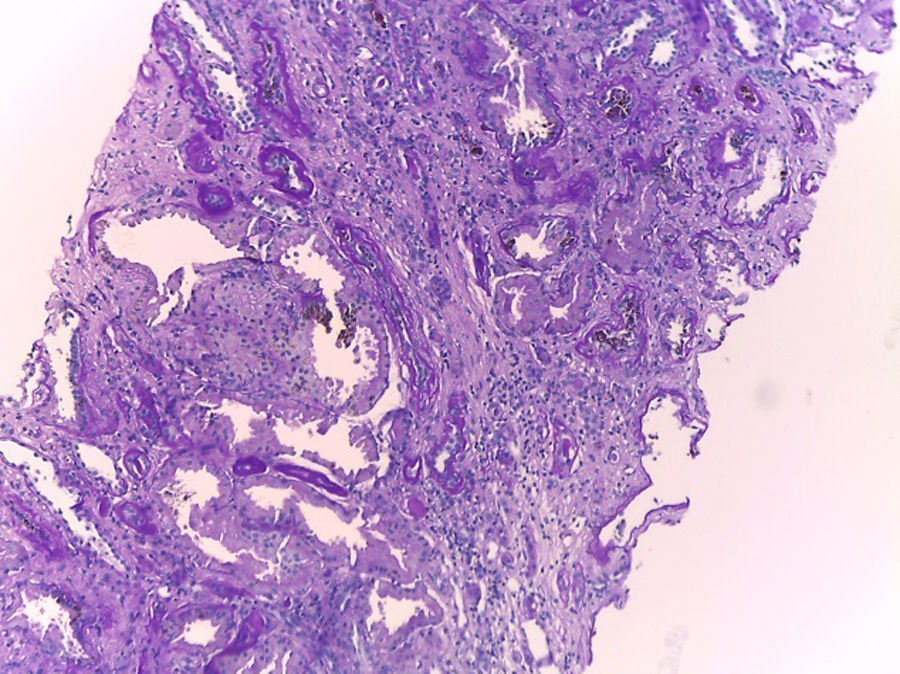
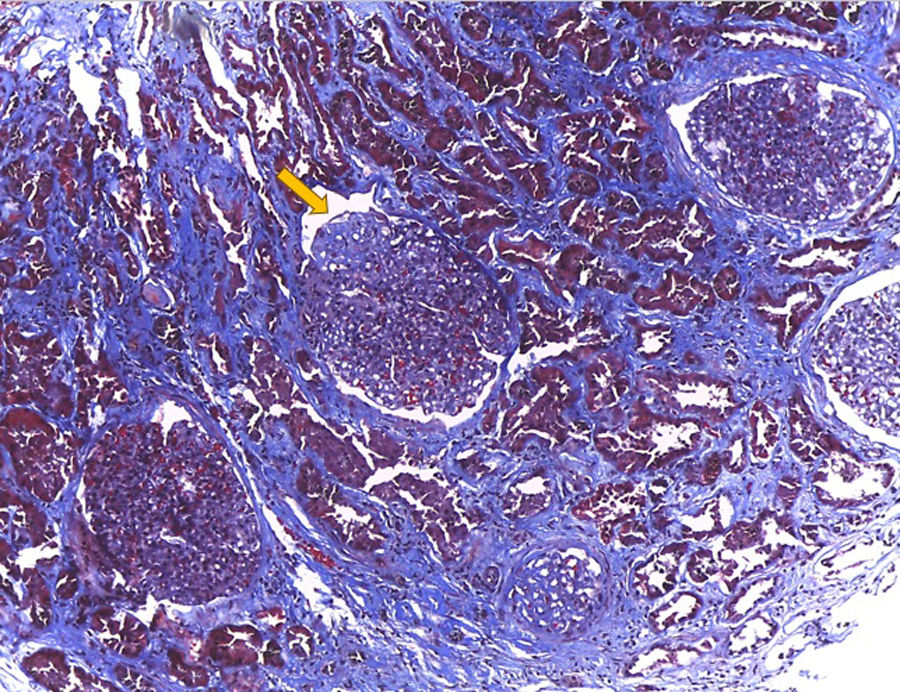
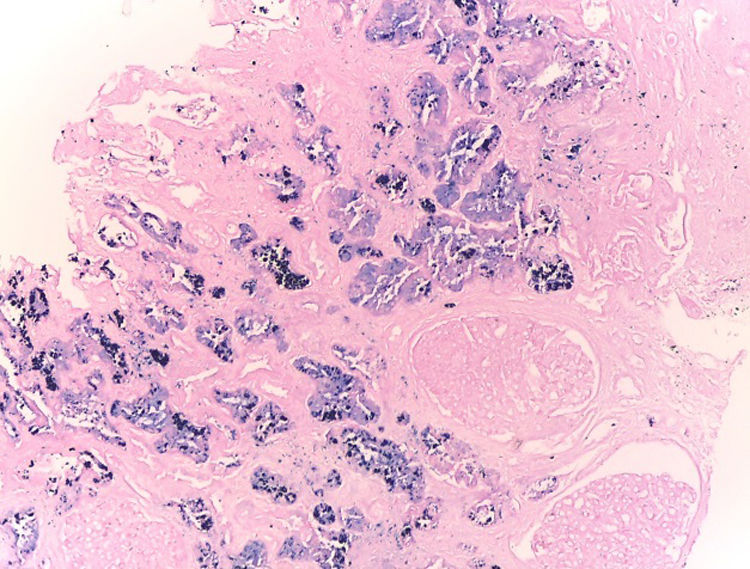
Hematoxylin–eosin stain – 4×. In the sample, 10 glomeruli were identified, 4 of them with global sclerosis (40%) and 1 with segmental sclerosis of a non-specific pattern. The evaluable glomeruli present open capillary loops, preserved mesangial matrix, marked podocyte activation, one with hyalinosis and segmental sclerosis, another with periglomerular fibrosis; without significant alterations in the glomerular basement membrane with the special stains. Casts of brown granular and intracytoplasmic epithelial material predominant in proximal tubules. Signs of acute tubular damage with loss of the brush border, luminal dilation, thinning of the lining epithelium, peeling of cells towards the lumen, in 50% of the sample. Interstitial fibrosis and tubular atrophy, moderate arteries, arterioles and peritubular capillaries, without obvious alterations.
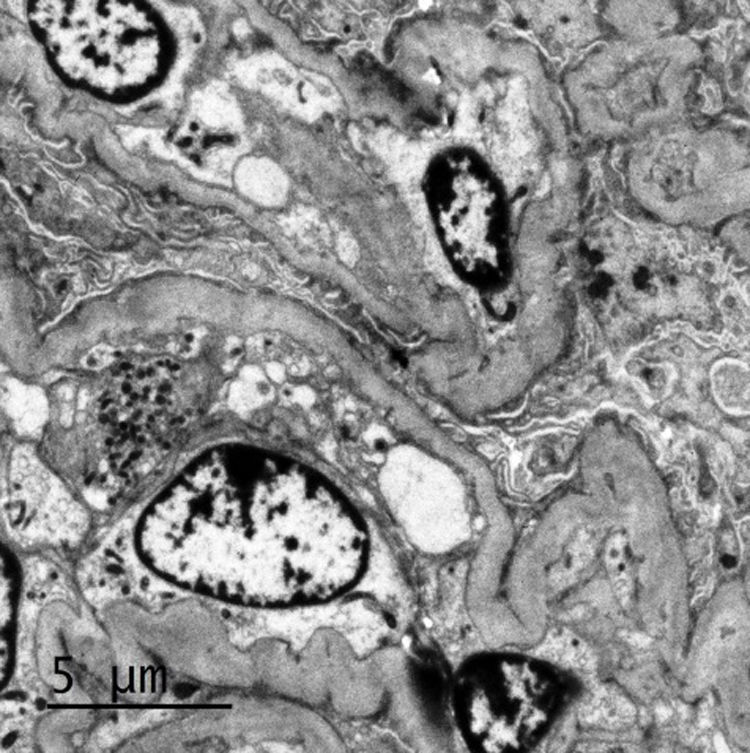
Transmission electron microscopy. Partially occluded capillary lumens with endothelial edema. Glomerular basement membranes irregular, folded, with areas of thickening and variable thickness between 327nm and 491nm. Erasure of podocyte processes associated witn microvillous degeneration.
Although the patient required hemodialysis, it is important to keep in mind that when renal deterioration is solely due to hemoglobin cast nephropathy, most cases have a good prognosis, with recovery of renal function to its baseline level. In the case of our patient, it was not necessary to continue hemodialysis upon discharge from the hospital.10,16
Pigment cast nephropathy is usually more common in cases of rhabdomyolysis. However, as in this case, the second most common cause is related to hemolysis. Iron and free hemoglobin generate direct tubular cell damage, vasoconstriction, and vascular obstruction. Therefore, in patients with sickle cell disease, in addition to the presence of FSGS, cast nephropathy should be suspected when there are frequent episodes of hemolysis accompanied by AKI, even if hemodialysis is required. The diagnosis is confirmed by the identification of pigmentary casts with positive staining for hemoglobin by immunohistochemistry. Failing that, as in this clinical case, with positive histochemistry for hemosiderin.
ConclusionThe case of this patient with sickle cell disease and AKI illustrates the importance of considering hemoglobin cast nephropathy as a potential cause. Despite the initial need for hemodialysis, the prognosis is usually favorable with appropriate management. The variability in the causes of intravascular hemolysis highlights the importance of approaching each case considering the clinical context and geographic region. Early detection and appropriate treatment are essential to improve the prognosis in patients with sickle cell disease and kidney complications.
Conflict of interestThe authors declare that they have no conflict of interest.