C3 glomerulopathy (C3G) is a rare and progressive kidney disorder characterized by dysregulation of the alternative complement pathway and predominant C3 deposition in the glomeruli.1,2 It comprises two major subtypes: C3 glomerulonephritis (C3GN) and dense deposit disease. Approximately 50% of patients progress to end-stage kidney disease within ten years.3 Current therapeutic approaches include immunosuppressants and complement inhibitors, though no universally effective strategy exists.4 Recently, iptacopan, a selective factor B inhibitor, has shown promising results and received FDA approval.5 Bortezomib, a proteasome inhibitor, was reported effective in a patient with anti-factor H antibody-mediated C3G.6
We present an 18-year-old male diagnosed with C3GN who failed to respond to corticosteroids, mycophenolate mofetil (MMF), eculizumab, plasmapheresis, and ultimately bortezomib. The patient presented in October 2022 with macroscopic hematuria, proteinuria of 4.4g/day, and low serum C3 (13.4mg/dL) (Table 1). Renal biopsy confirmed C3GN with intense C3 deposition along glomerular capillary walls (Fig. 1). Initial treatment with MMF and standard-dose prednisone showed no improvement. Eculizumab was administered for three months without clinical benefit. Genetic screening revealed no pathogenic variants and C3 nephritic factor was negative. Testing for other complement-related autoantibodies could not be performed due to unavailability. Based on the possibility of undetected nephritic factors (e.g., anti-C4 or C5 antibodies), the patient underwent five sessions of plasma exchange followed by continued eculizumab therapy. However, significant hypoalbuminemia, edema, and proteinuria persisted.
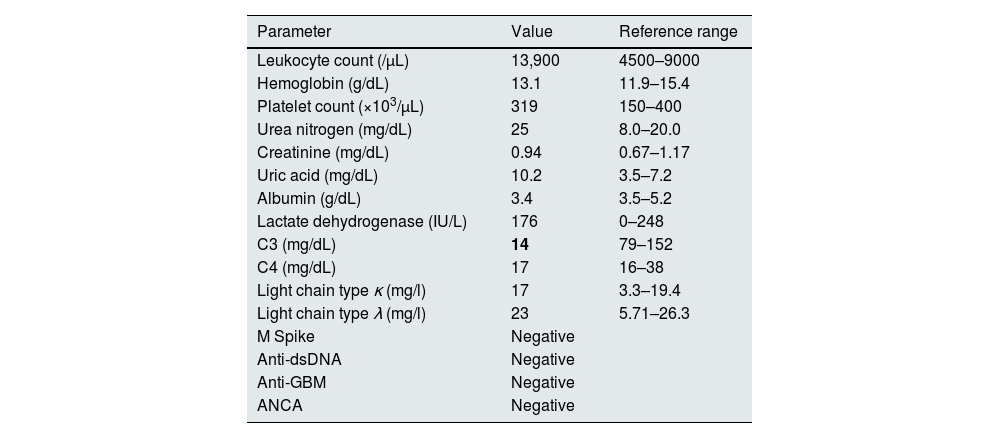
Baseline laboratory findings.
| Parameter | Value | Reference range |
|---|---|---|
| Leukocyte count (/μL) | 13,900 | 4500–9000 |
| Hemoglobin (g/dL) | 13.1 | 11.9–15.4 |
| Platelet count (×103/μL) | 319 | 150–400 |
| Urea nitrogen (mg/dL) | 25 | 8.0–20.0 |
| Creatinine (mg/dL) | 0.94 | 0.67–1.17 |
| Uric acid (mg/dL) | 10.2 | 3.5–7.2 |
| Albumin (g/dL) | 3.4 | 3.5–5.2 |
| Lactate dehydrogenase (IU/L) | 176 | 0–248 |
| C3 (mg/dL) | 14 | 79–152 |
| C4 (mg/dL) | 17 | 16–38 |
| Light chain type κ (mg/l) | 17 | 3.3–19.4 |
| Light chain type λ (mg/l) | 23 | 5.71–26.3 |
| M Spike | Negative | |
| Anti-dsDNA | Negative | |
| Anti-GBM | Negative | |
| ANCA | Negative |
C3, complement component 3; C4, complement component 4; Anti-dsDNA, anti-double stranded DNA antibodies; Anti-GBM, anti-glomerular basement membrane antibodies; ANCA, anti-neutrophil cytoplasmic antibodies.
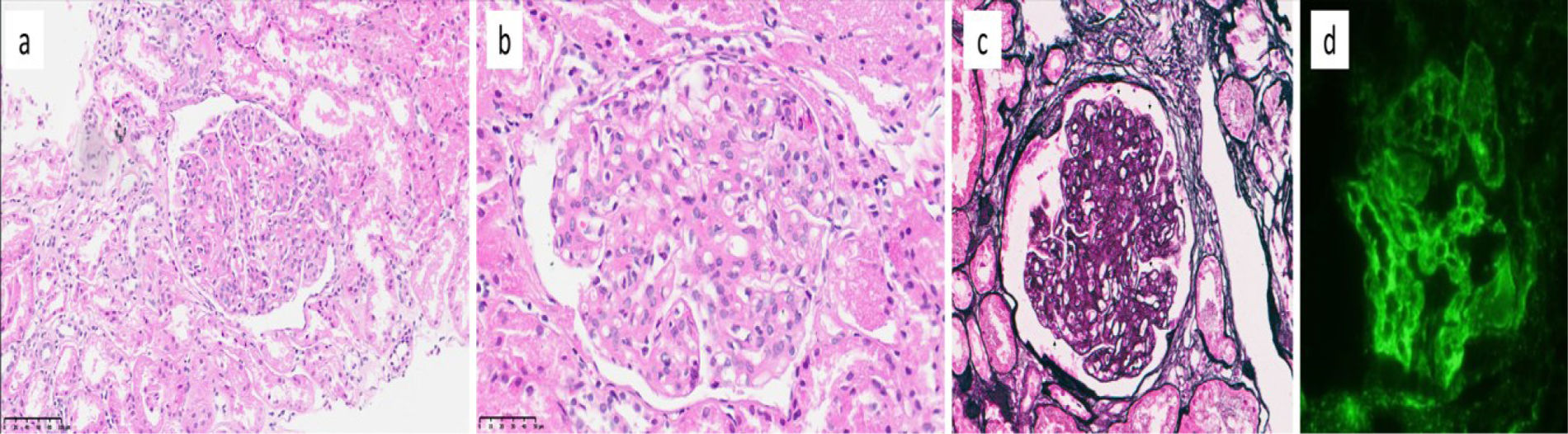
Histopathologic and immunofluorescence findings in C3 glomerulopathy. (a, b) Glomeruli show increased lobulation, endocapillary hypercellularity, and mesangial matrix expansion (a. H&E, ×200; b. H&E, ×400). (c) Double-contour formation is noted along the glomerular capillary walls (arrows) (Jones, ×400). (d) Immunofluorescence reveals diffuse, global, and strong (3+) deposition of C3 in glomerular capillary walls and mesangium (C3, ×400).
As salvage therapy, bortezomib was administered subcutaneously at 1.3mg/m2 (2.5mg) weekly for four weeks, combined with dexamethasone, followed by a one-week break and an additional four-week course. No adverse effects, including neuropathy, were noted. Despite treatment, proteinuria remained>4g/day and serum C3 levels did not improve. The patient is currently maintained on supportive care.
This is, to our knowledge, the second published report on bortezomib use in C3G. Hui et al. described clinical remission in a C3G patient with anti-factor H autoantibodies who responded well to bortezomib after failing conventional treatments.6 In contrast, our patient—who shared a comparable clinical profile—did not respond.
Bortezomib induces plasma cell apoptosis and is commonly used in multiple myeloma.7 It has also demonstrated efficacy in antibody-mediated kidney disorders, such as renal transplant rejection,8 monoclonal gammopathy-associated dense deposit disease,9 and treatment-resistant membranous nephropathy.10 The lack of response observed in our patient might have several explanations. First, the duration of bortezomib treatment, limited by reimbursement restrictions, might have been insufficient to achieve clinical efficacy. Second, the absence of detectable genetic mutations suggests the involvement of pathogenic mechanisms potentially distinct from those targeted by proteasome inhibition or antibody clearance.
This case highlights the complex pathophysiology of C3G and its variable response to targeted therapies. While complement inhibition and plasma cell depletion are rational strategies, individualized approaches remain essential. Future research should aim to define biomarkers for therapy selection and determine the role of bortezomib in C3G through prospective studies.
ORCID IDsUlver Derici ID: 0000-0002-9741-6779
Galip Güz ID: 0000-0002-9146-2133
Betül Ogüt ID:0000-0002-1385-7324
Ozant Helvacı ID: 0000-0002-1382-2439
Ethical approvalAll procedures performed in this case report were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent to participateWritten informed consent was obtained from the patient for publication of this case report.
FundingNo funding was obtained for this case report.
Conflict of interestsOH discloses that he is currently serving as a Principal Investigator for Study of Efficacy and Safety of Iptacopan in Patients With C3 Glomerulopathy. (APPEAR-C3G) (NCT04817618). The other authors declare that they have no competing interests to disclose.
Data availability statementThe datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.