Ankylosing spondylitis (AS) is an autoimmune disease that emerged from the interaction of genetic tendency and environmental risk factors.1 Patients with AS are mostly diagnosed at the end of their third decade of life after approximately 10 years of insidious course.1 Membranous glomerulopathy secondary to etanercept therapy, immunoglobulin A (Ig A) nephropathy, and renal amyloidosis were reported as kidney diseases associated with AS.2–4 Anti-neutrophil cytoplasmic antibody (ANCA)-negative pauci-immune necrotizing glomerulonephritis (PING) is also one of the auto-immune diseases.5 The association of PING with AS has not been reported. Herein, we report for the first time the development of ANCA-negative PING accompanied by pulmonary hemorrhage in a patient with AS.
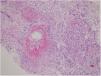
A 39-year-old male patient was diagnosed as having AS on the basis of bilateral grade 2 sacroileitis (Fig. 1) and inflammatory low back pain that had woken him up at nights for 1 year.6 He had been treated with sulfasalazine 1g twice daily and acemetacin 120mg/d for 1 year, and methotrexate 15mg once weekly, methylprednisolone 32mg/d and colchicine 0.5mg/d for 2 months in another hospital. Some of the patient's symptoms including swelling and pain of the fingers (dactylitis), and buttocks pain, did not resolve with this management. After the first year of follow-up, a skin lesion biopsy was performed for the leukocytoclastic vasculitis that appeared on his left lower leg. By that time his serum creatinine levels had increased to 4.0mg/dL from the basal levels of 0.77mg/dL in a period of 1 month. He was hospitalized at our department because of the rapid increase in his serum creatinine level and nephrotic range proteinuria (3.9g/d) with urinary dysmorphic erythrocytes. His serum complement 3 (C3) and 4 (C4) levels were normal (0.94g/L; normal range, 0.9–1.8; and 0.2g/L; normal range, 0.1–0.4, respectively), and no antinuclear antibody, anti-double-stranded deoxyribonucleic acid antibody, ANCA, anti-SS-A, anti-Smith (anti-Sm), anti-histone, and anti-nucleosome antibodies were found. Renal biopsy revealed PING, as evidenced by 8% of glomeruli having cellular crescents without immune deposition, 26% of glomeruli having fibrinoid necrosis, fibrosis, and edema in 80% of the interstitium, and subintimal fibrosis in middle-sized vessels (Fig. 2). As soon as the renal biopsy results were released, the patient received pulse (high-dose intravenous [i.v.]) methylprednisone 12.5mg/kg/d for 3 days.7 The rest of the previous medications were stopped. Meanwhile, oliguria, uremic symptoms with hypervolemia, and hemoptysis due to pulmonary hemorrhage developed, and hemodialysis and plasma exchange with albumin replacement were initiated via temporary jugular venous catheter. Subsequently, daily oral methylprednisone 1mg/kg/d with three doses of i.v. cyclophosphamide 0.5g/m2 every 2 weeks were administered to the patient. However, pulmonary hemorrhage relapsed 4 weeks after initiation of the treatment, so rituximab (i.v. 375mg/m2) four times weekly were prescribed to the patient. Renal recovery without the need for hemodialysis was observed nearly 2 months after the start of cyclophosphamide treatment and on the third week of rituximab therapy. Azathioprine, methylprednisolone, spironolactone, nifedipine, nebivolol, furosemide, pantoprazole, and calcitriol with calcium carbonate were prescribed. The patient is currently followed up in the nephrology outpatient clinic (without dialysis and pulmonary hemorrhage), with a serum creatinine level of 1.7mg/dL, serum albumin level of 3.6g/dL, and proteinuria of 1g/d.
One of the well-known etiologies of rapidly progressive glomerulonephritis is PING. ANCA plays a major role in the pathogenesis of PING. However, 10% of PING cases have been reported to occur without ANCA, similar to our case.8 The activation mechanism of the neutrophils that are the primary responsible immune cells in the development of ANCA-negative PING has not been clarified yet.9 More severe complement and neutrophil activation and thus greater renal damage were found in ANCA-negative PING cases than in ANCA-positive ones.9 The main pathogenetic mechanism of AS is impaired regulation of interleukin-17 production and genetic mutations involving antigen presentation.1 As knowledge is lacking about the common pathogenic mechanism that leads to both PING and AS, this coexistence may be coincidental. In spite of the worse renal prognosis of patients with ANCA-negative PING, our patient recovered after 2 months of dialysis-dependent renal failure. Renal involvement was reported to be found in 21.7% of cases with AS.10 The renal pathologies (tubulointerstitial nephritis, IgA nephropathy, nonsteroidal anti-inflammatory drug-induced nephropathy, and amyloidosis) associated with AS were not found in our case. The renal pathology of the present case was PING. This case is important because of the rarity of cases with PING without ANCA, and the present case is the first to show the association of PING with AS.
FundingNo funding was obtained for this article
Inform consentWritten informed consent for publication of this case report was obtained from the patient
Conflict of interestThe authors declare no conflict of interest regarding the publication of this article.