We read with great interest the manuscript titled “Osteoporosis Management in Patients with Chronic Kidney Disease (ERCOS Study): A Challenge in Nephrological Care” published in Nefrologia. The authors have provided important insights into the challenges of managing osteoporosis (OP) in patients with chronic kidney disease (CKD), highlighting gaps in treatment and opportunities for improvement.1 This study is a timely and significant contribution to nephrology practice, addressing a critical area of patient care that warrants further exploration. While commending the authors for their efforts, we would like to offer a few suggestions based on recent evidence to optimize the clinical and research implications of their findings.
Firstly, the authors have focused on CKD patients with either dual-energy X-ray absorptiometry (DXA)-confirmed OP or a history of fragility fractures. However, this approach may have inadvertently introduced a selection bias, likely overestimating the prevalence of OP and fractures in the broader CKD population. Recent studies suggest substantial variability in bone mineral density (BMD) and fracture risk across CKD stages.2,3 Including CKD patients without prior OP diagnoses could offer a more comprehensive understanding of bone health in this population and enable earlier interventions for those at risk.
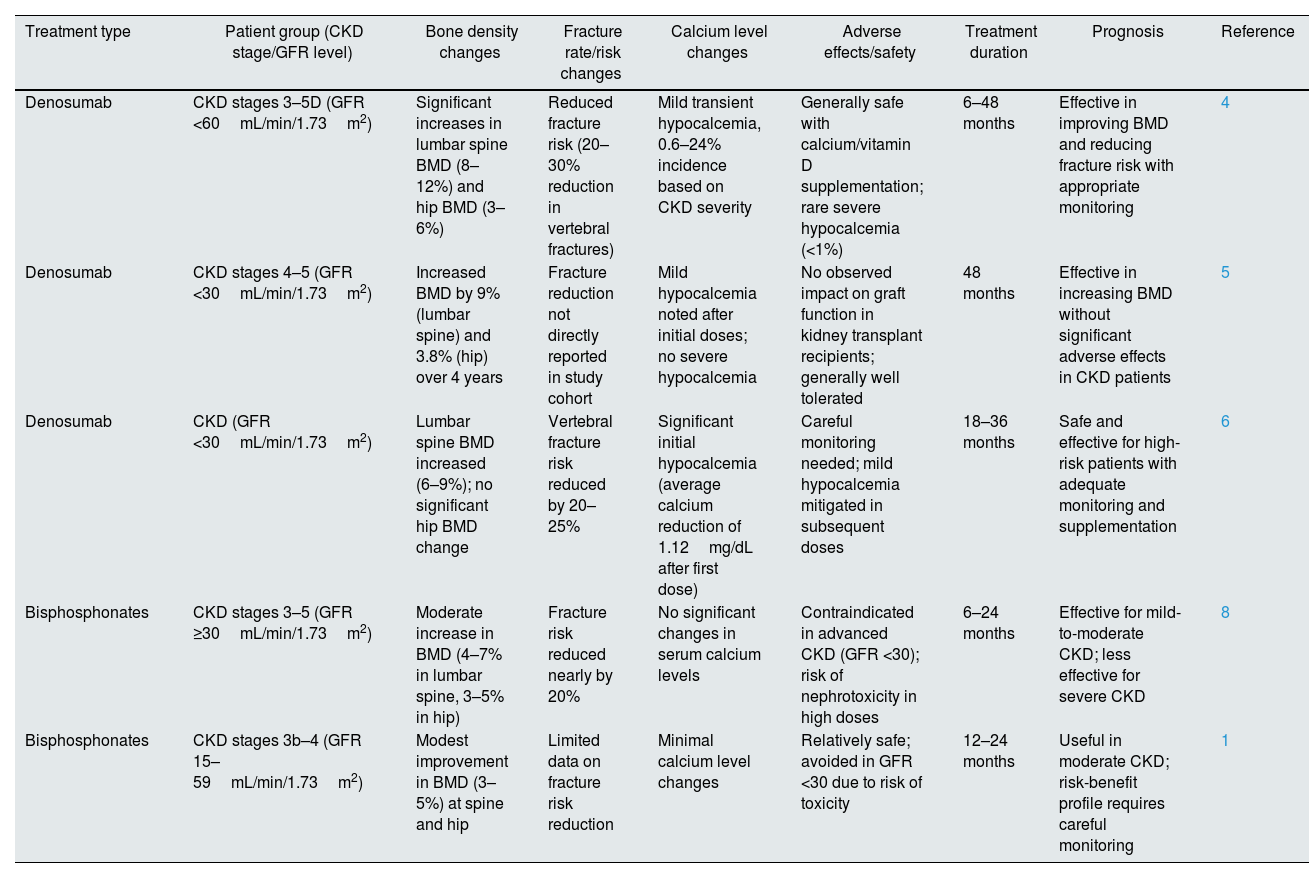
Secondly, the study highlighted the underutilization of antiresorptive therapies in CKD patients but data on treatment outcomes was limited. Evidence suggests that denosumab improves BMD and reduces fracture risk in advanced CKD stages, including transplant recipients, albeit with a higher risk of hypocalcemia.4–6 Bisphosphonates, while effective in early CKD stages, are contraindicated in advanced stages due to nephrotoxicity.7,8 We propose integrating data on treatment outcomes from recent studies. Table 1 summarizes the effectiveness and safety profiles of denosumab and bisphosphonates in CKD patients, stratified by treatment type, CKD stage, and clinical outcomes.
Treatment effectiveness and safety of denosumab and bisphosphonates in CKD patients.
| Treatment type | Patient group (CKD stage/GFR level) | Bone density changes | Fracture rate/risk changes | Calcium level changes | Adverse effects/safety | Treatment duration | Prognosis | Reference |
|---|---|---|---|---|---|---|---|---|
| Denosumab | CKD stages 3–5D (GFR <60mL/min/1.73m2) | Significant increases in lumbar spine BMD (8–12%) and hip BMD (3–6%) | Reduced fracture risk (20–30% reduction in vertebral fractures) | Mild transient hypocalcemia, 0.6–24% incidence based on CKD severity | Generally safe with calcium/vitamin D supplementation; rare severe hypocalcemia (<1%) | 6–48 months | Effective in improving BMD and reducing fracture risk with appropriate monitoring | 4 |
| Denosumab | CKD stages 4–5 (GFR <30mL/min/1.73m2) | Increased BMD by 9% (lumbar spine) and 3.8% (hip) over 4 years | Fracture reduction not directly reported in study cohort | Mild hypocalcemia noted after initial doses; no severe hypocalcemia | No observed impact on graft function in kidney transplant recipients; generally well tolerated | 48 months | Effective in increasing BMD without significant adverse effects in CKD patients | 5 |
| Denosumab | CKD (GFR <30mL/min/1.73m2) | Lumbar spine BMD increased (6–9%); no significant hip BMD change | Vertebral fracture risk reduced by 20–25% | Significant initial hypocalcemia (average calcium reduction of 1.12mg/dL after first dose) | Careful monitoring needed; mild hypocalcemia mitigated in subsequent doses | 18–36 months | Safe and effective for high-risk patients with adequate monitoring and supplementation | 6 |
| Bisphosphonates | CKD stages 3–5 (GFR ≥30mL/min/1.73m2) | Moderate increase in BMD (4–7% in lumbar spine, 3–5% in hip) | Fracture risk reduced nearly by 20% | No significant changes in serum calcium levels | Contraindicated in advanced CKD (GFR <30); risk of nephrotoxicity in high doses | 6–24 months | Effective for mild-to-moderate CKD; less effective for severe CKD | 8 |
| Bisphosphonates | CKD stages 3b–4 (GFR 15–59mL/min/1.73m2) | Modest improvement in BMD (3–5%) at spine and hip | Limited data on fracture risk reduction | Minimal calcium level changes | Relatively safe; avoided in GFR <30 due to risk of toxicity | 12–24 months | Useful in moderate CKD; risk-benefit profile requires careful monitoring | 1 |
BMD: bone mineral density; CKD: chronic kidney disease; GFR: glomerular filtration rate.
Denosumab has consistently demonstrated superior efficacy in improving BMD and reducing fracture risk over longer durations (≥4 years), particularly in high-risk CKD patients when accompanied by appropriate calcium and vitamin D supplementation.4,5 However, careful monitoring is required to mitigate the risk of hypocalcemia, particularly after the initial dose.6,8,9 Such data could further enrich the manuscript by providing a clearer picture of treatment efficacy and risks.
While the authors focus on traditional therapies, incorporating emerging options like romosozumab could provide a broader overview of available treatments. Recent studies suggest that romosozumab may benefit CKD patients by improving BMD and reducing fractures without significant adverse effects.2 Furthermore, addressing barriers to guideline implementation in nephrology practice – such as concerns about medication safety, limited access to DXA, and lack of awareness of updated guidelines – would enhance the practical applicability of the findings. Proposing actionable solutions, such as interdisciplinary collaboration or educational initiatives, could bridge the gap between evidence and practice.4,10
ConclusionWe commend the authors for their valuable contribution to the understanding of OP management in CKD patients. By broadening the scope of study to include undiagnosed patients, incorporating treatment-specific efficacy and safety data, addressing confounding variables, and exploring newer therapies, the manuscript could provide even greater clinical relevance. We look forward to future research that advances the care of CKD patients at risk of OP and fractures.
Conflict of interestThe authors declare no conflicts of interest.