The rapid accumulation of plastic waste poses a significant global threat, with micro/nanoplastics emerging as key pollutants. Common plastic polymers degrade into micro/nanoplastics, which contaminate oceans and enter the human body via ingestion, inhalation, or dermal absorption. Once inside, micro/nanoplastics can accumulate in organs such as the lungs, kidneys, and brain, triggering oxidative stress, inflammation, and endocrine disruption. Annual human intake is estimated at 39,000–121,000 particles.1 Recent studies suggest a growing link between MNP exposure and kidney dysfunction, making nephrotoxicity a rising concern2,3 (Table 1).
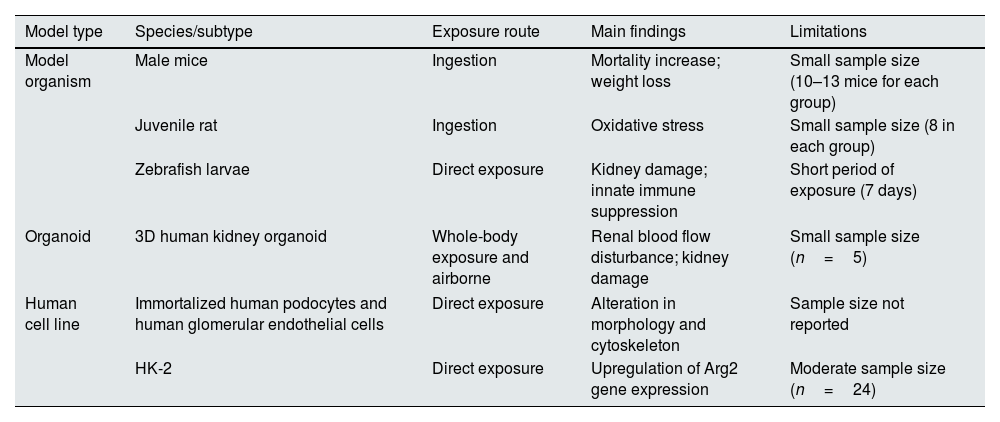
The research summary of micro/nanoplastics in kidney.
| Model type | Species/subtype | Exposure route | Main findings | Limitations |
|---|---|---|---|---|
| Model organism | Male mice | Ingestion | Mortality increase; weight loss | Small sample size (10–13 mice for each group) |
| Juvenile rat | Ingestion | Oxidative stress | Small sample size (8 in each group) | |
| Zebrafish larvae | Direct exposure | Kidney damage; innate immune suppression | Short period of exposure (7 days) | |
| Organoid | 3D human kidney organoid | Whole-body exposure and airborne | Renal blood flow disturbance; kidney damage | Small sample size (n=5) |
| Human cell line | Immortalized human podocytes and human glomerular endothelial cells | Direct exposure | Alteration in morphology and cytoskeleton | Sample size not reported |
| HK-2 | Direct exposure | Upregulation of Arg2 gene expression | Moderate sample size (n=24) | |
The effects of polystyrene micro/nanoplastics (50nm polystyrene nanoplastic as well as 4μm, 600nm and 300nm polystyrene microplastic) were examined on male mice kidney.4 As a result, histological impairment and inflammation of the kidney were observed beside the increase in mortality rate and significant weight loss observed across all treatments.4 Furthermore, they have identified a plethora of biomarkers such as SOD, IL-6, MCP, CAT, IL-10, MDA and GSH-Px that are altered across control and micro/nanoplastic treatments in mice.4 A long-term (28 days) polystyrene microplastic (1000nm) ingestion experiment was conducted on juvenile rats.5 The outcome showed that microplastics induced oxidative stress in both endoplasmic reticulum and kidney of treated rats.5 Significant increase in apoptosis associated gene expression (caspase-12, Bax, caspase-3, Bcl-2 and caspase-9) was also detected in microplastic treated rats.5
The polystyrene micro/nanoplastic effects was experimented on kidney of model fish species zebrafish (Danio rerio) larvae.6 Both the polystyrene microplastic (5μm) and polystyrene nanoplastic (100nm) induced severe kidney damage and innate immune function suppression in zebrafish larvae.6 These micro- and nanoplastic treated larvae were also discovered to be more susceptible to Edwardsiella piscicida bacterial infection as compared to the negative controls. Additionally, the transcriptomic analysis revealed that cytosolic DNA-sensing and C-type lectin receptor pathways were compromised in both micro- and nanoplastics treated zebrafish groups.6 They concluded that nanoplastics introduced stress within the endoplasmic reticulum whereas microplastics can lead to lipid accumulation in zebrafish larvae.6
OrganoidsThe toxicological effects of renal injury induced by inhalation of airborne polystyrene nanoplastics were investigated using a kidney organoid.7 First, they set up a whole-body exposure system to allow the simulation of airborne nanoplastic inhalation exposure. Then, they have also established a cost-effective, reliable, and high-performing protocol for culturing 3D human kidney organoids.7 Their results indicated that airborne nanoplastics deposition in kidney is possible via inhalation alone.7 Polystyrene nanoplastics showed a tendency to promote apoptosis through the NR4A1/CASP3 signaling pathway and simultaneously activated the TF/F12 pathway, initiating an external coagulation cascade.7 These polystyrene nanoplastics also contributed to dysfunction in the renin–angiotensin–aldosterone system (RAAS), impairing renal circulation and worsening kidney damage.7 They deduced that chromic exposure to these airborne nanoplastics can lead to severe embryotoxicity, renal blood flow disturbance and nephrotoxicity.7 However, due to limitations in experimental models, they mentioned that caution is advised when applying these findings to human health.
Human cell lineThe effects of 0.05μm polystyrene nanoplastics on the kidney was investigated via cultured immortalized human podocytes and human glomerular endothelial cells.8 The resultant treated podocytes and glomerular endothelial cells experienced alteration in both morphology and cytoskeleton, but the cell specific markers did not depict significant differential expressions across treated and non-treated cells.8 Micro/nanoplastics was subjected to normal human adult male kidney HK-2 cell line used commonly in toxicology researches.9 Interestingly, they unearthed that the Arg2 gene expression was increased significantly upon micro/nanoplastic exposure in HK-2 cells.9 The Arg2 gene encodes arginase 2, a mitochondrial enzyme involved in arginine metabolism. This enzyme plays indispensable roles in vascular function, metabolic processes as well as inflammation. Following lentivirus-induced knockdown of the Arg2 gene, HK-2 cells exposed to micro/nanoplastics during sepsis exhibited SOD, GSH, and MDA levels comparable to those in non-septic HK-2 cells.9 This indicates an enhanced resistance to oxidative stress adapted by the HK-2 cells treated with micro/nanoplastics. In the same study, they have also conducted similar treatment to mice and as a result the mortality rate of mice was elevated due to exacerbated kidney damage induced by micro/nanoplastic ingestion.9 Similarly, the Arg2 gene expression in micro/nanoplastic treated mice was also found to be significantly abundant than their non-treated counterparts.9
Future outlooksCurrently, no established method exists to remove nanoplastics from human kidneys, but emerging strategies include enhancing renal clearance through hydration and nephroprotective agents, using nanomedicine tools like plastic-binding nanoparticles, and improving dialysis techniques. Experimental approaches such as magnetic nanocarriers, AI-driven detox planning, and gut microbiota modulation are also being explored. Continued interdisciplinary research is crucial to develop safe, effective removal techniques for this growing environmental and health concern. Standardized exposure models, 3D organoids, and omics approaches10 are essential for deeper insight. Integrating AI3 can accelerate research and boost prediction accuracy. Interdisciplinary efforts are vital to understand chronic impacts and guide public health and regulatory responses.