Recent studies have demonstrated the effectiveness, safety, and tolerability of deferasirox in patients in peritoneal dialysis, however, its effect has not been studied in patients undergoing hemodialysis.
ObjectiveTo investigate the impact of iron chelation on telomere length, oxidative stress, and ferritin levels in patients undergoing hemodialysis.
MethodsThis is an open-label study, with a control group of patients undergoing hemodialysis, who will receive treatment with deferasirox 15mg/kg/day for 6 months for iron chelation. Telomere length was measured using real-time PCR. Serum ferritin levels and oxidation markers were evaluated. To evaluate the pharmacokinetics and safety of deferasirox, plasma concentrations were analyzed by HPLC.
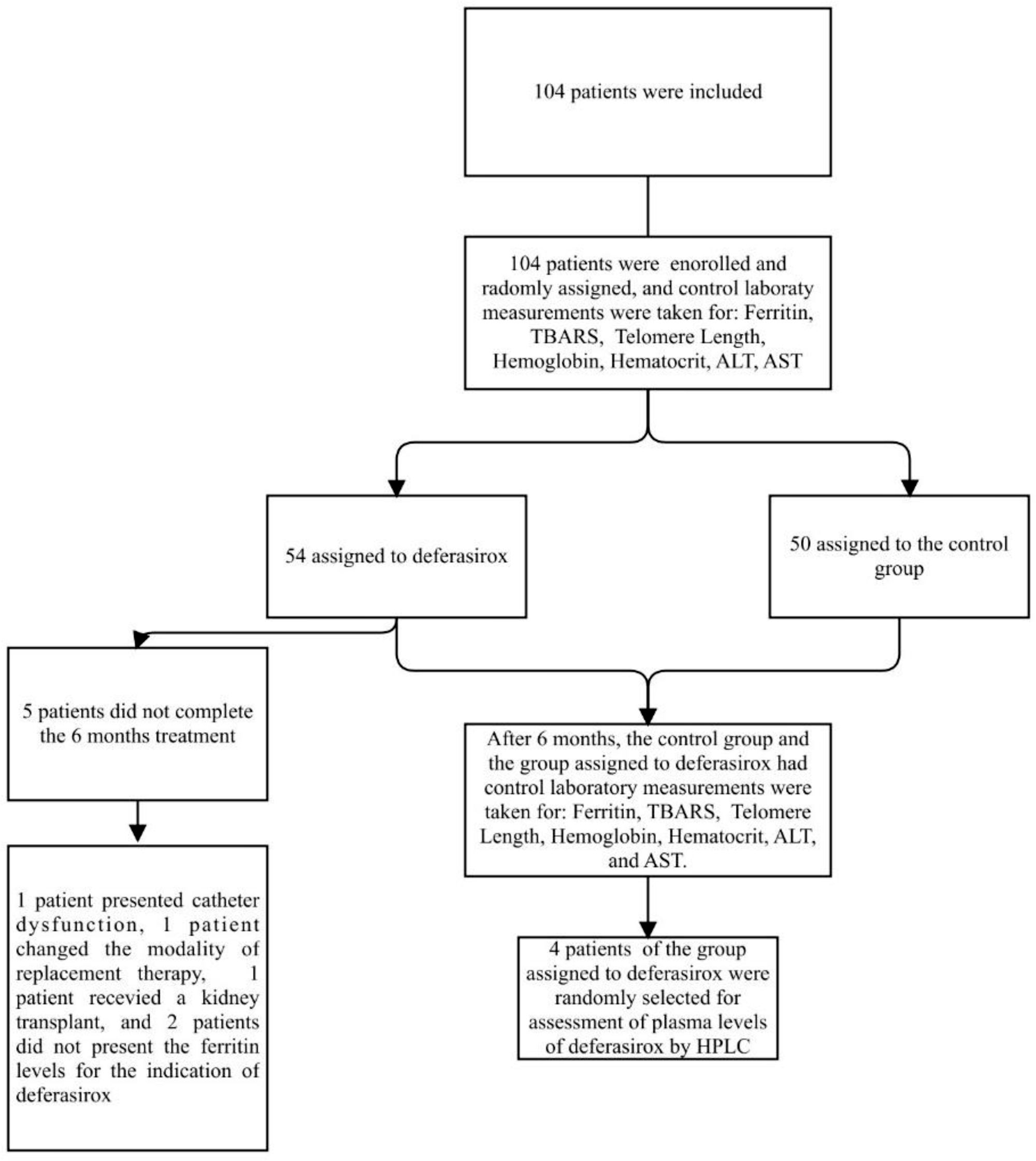
ResultsFifty-four patients were included to receive deferasirox, and a control group of 50 patients. Significant differences were observed in serum ferritin levels (p<0.0001), TBARS (thiobarbituric acid reactive substances) (p<0.01). Telomere length had a significant increase after chelation (p<0.001). The serum deferasirox concentration at zero time at 48h was maintained within a range of 2.67–23.78mmol/L.
ConclusionsOur results demonstrate that iron chelation in hemodialysis patients significantly reduces ferritin and TBARS, resulting in an increase in telomere length. Deferasirox proves to be beneficial for patients with iron overload undergoing hemodialysis.
Estudios recientes han demostrado la eficacia, seguridad y tolerabilidad del deferasirox en pacientes en diálisis peritoneal, sin embargo, su efecto no ha sido estudiado en pacientes sometidos a hemodiálisis.
ObjetivoInvestigar el impacto de la quelación del hierro sobre la longitud de los telómeros, el estrés oxidativo y los niveles de ferritina en pacientes sometidos a hemodiálisis.
MétodoSe trata de un estudio abierto, con un grupo control de pacientes en hemodiálisis, que recibieron tratamiento con deferasirox 15mg/kg/día durante 6 meses para la quelación del hierro. La longitud de los telómeros se midió mediante PCR en tiempo real. Se evaluaron los niveles séricos de ferritina y los marcadores de oxidación. Para evaluar la farmacocinética y la seguridad del deferasirox, se analizaron las concentraciones plasmáticas mediante HPLC.
ResultadosSe incluyeron 54 pacientes para recibir deferasirox, y un grupo control de 50 pacientes. Se observaron diferencias significativas en los niveles séricos de ferritina (p<0,0001), TBARS (sustancias reactivas al ácido tiobarbitúrico) (p<0,01). La longitud de los telómeros aumentó significativamente tras la quelación (p<0,001). La concentración sérica de deferasirox a tiempo cero a las 48h se mantuvo dentro de un rango de 2,67 a 23,78mmol/L.
ConclusionesNuestros resultados demuestran que la quelación del hierro en pacientes en hemodiálisis reduce significativamente la ferritina y el TBARS, lo que se traduce en un aumento de la longitud de los telómeros. El deferasirox demuestra ser beneficioso para los pacientes con sobrecarga de hierro sometidos a hemodiálisis.
Anemia in chronic kidney disease shares characteristics with anemia in other chronic diseases, although the decrease in erythropoietin production mediated by renal failure and the antiproliferative effects of accumulated uremic toxins contribute significantly.1
Ferritin is the iron storage protein. While large amounts of ferritin are present in iron-storing tissues such as the liver and bone marrow, only tiny amounts are present in the serum. This makes serum ferritin concentration a valuable indicator of the stored iron status. However, ferritin acts as an acute-phase reactant, leading to increased serum concentrations during acute inflammatory processes.2 Iron homeostasis dependents on regulatory feedback mechanisms.3
Stored iron levels in healthy subjects range from approximately 800–1200mg. Iron overload is a common complication in patients with chronic renal failure undergoing dialysis. The frequent need for red cell transfusions to treat symptomatic anemia. The repetitive use of parenteral iron with or without red cell transfusions, also contributes to iron overload.4 However, excess iron can generate highly toxic free radicals that cause oxidative damage to almost all cellular components, including DNA, membranes, and proteins.5,6
Currently, iron overload and the use of iron therapy are not well established in patients with chronic kidney disease. In the latest KDIGO update, a serum ferritin ≥500ng/mL and transferrin saturation (TSAT) ≥30 are recommended as benchmarks for stopping iron therapy.7 However, some guidelines even suggest serum ferritin values of 500–800ng/mL. MRI is now the gold standard for estimation and monitoring of iron stores, although it is not yet widely accepted due to its limited availability in resource-limited countries.8 Furthermore, it is difficult to determine whether iron detected in the liver is deposited within parenchymal hepatocytes or stored safely within reticuloendothelial cells. Therefore, serum ferritin and TSAT remain the values considered for iron overload, as noted in the study by Ghoti et al. where hemodialysis patients with ferritin >1000ng/dL, who had increased iron deposition in the liver and spleen, were studied.9 Therefore, in our study, a cut-off point of ferritin >1000ng/dL was considered as a reference.
Postmortem studies have detected iron in atherosclerotic lesions compared to arteries of healthy patients.10,11 Additionally, the Bruneck study indicates that serum ferritin and LDL cholesterol have synergistic effects associated with the progression of carotid atherosclerosis, suggesting that iron promotes lipid peroxidation.12
Patients with end stage renal disease have a markedly increased risk of presenting cardiovascular complications compared to the general population.13 In the general population, it has been associated with basal concentrations of ferritin and transferrin with multiple abnormalities of metabolic syndrome, primarily with hyperinsulinemia and a high insulin resistance index (HOMA-IR).14
Interestingly, the recently published Pivotal trial,15 non-inferiority was demonstrated whit a slight superiority related to fewer cardiovascular events fatal and no fatal myocardial infarction or hospitalizations for cardiac failure. Also confirmed that maintenance iron therapy is better than an iron loading strategy for sparing recombinant erythropoiesis stimulating agents.
A published study found that elevated levels of ferritin, even those much lower than those that are not normally regarded as high, are associated with a decreased probability of deterioration of cardiovascular fitness, primarily in young adults.16 Although there is evidence that the total iron in the body is related to the development of various disease, however, there is limited scientific evidence regarding what occurs at the DNA level.
Telomeres are considered indicators of biological age and are specialized structures at the ends of human chromosomes. Early studies showed the essential role of telomeres in the integrity of chromosomes. These nucleoprotein hoods or caps are conserved by the telomerase enzyme.17
Human studies have correlated the shortening of telomeres in peripheral blood leukocytes with high mortality rates.18 A study of centenarians and their descendants found a positive relationship between telomere length and longevity.19,20
Telomere shortening increases with the progressive exposure to different factors of inflammation and oxidative stress that have a direct effect on the progressive loss of telomere length.21 Chronic oxidative stress accelerates cellular aging, renal dysfunction is associated with shorter telomere length in heart failure.22 Moreover, telomere shortening has been associated with hypertension, endothelial dysfunction, atherosclerosis, and cardiovascular mortality.23 Recent evidence shows that there is a strong association between telomere shortening and moderate chronic kidney disease and increased risk of death.24
In patients with type 2 diabetes mellitus telomeric length is shorter than healthy subjects of the same age.25 An association with microalbuminuria and albumin excretion has been reported. The evolution time increases oxidative stress, inflammation and loss of telomere length.26
During the aging process, renal function decreases, leading to a noticeable reduction in the glomerular flow rate, along with an increase in vascular resistance and loss of 20–25% of the renal mass. Research has shown that telomere shortening initially occurs in cells of the cortex rather than in the renal medulla.27 The increased oxidative stress caused by iron oversaturation may contribute to this telomere shortening, potentially leading to renal diseases such as glomerulosclerosis and preventing renal regeneration. Measuring telomere length will provide us with evidence of aging that occurs as a result of oxidative stress due to the high iron content stored within the body.28
Deferasirox (Exjade, ICL670) is a potent and specific iron chelator administered orally, approved as a first-line therapy in patients with chronic iron overload in transfusion-dependent anemias. The recommended starting dose is 20mg/kg/day, with a maximum recommended dose of 40mg/kg/day.29,30
Pharmacodynamic effects tested in the metabolic balance of iron have shown that deferasirox at doses of 10, 20, and 40mg/kg/day is capable of inducing a net iron excretion of 0.119, 0.329 and 0.445mg/Fe/kg/day with clinical relevance in the range of 0.1–0.5mg/kg/day. In addition to achieving a reduction in plasma iron levels, deferasirox has been shown to reduce liver iron concentration.31–33
The background outlined above suggests that, due to iron overload, oxidative stress, which affects all cells and causes accelerated telomere shortening increases. In patients with chronic renal failure at the bone marrow level, replicative power is affected in erythroid progenitor cells due to telomere length shortening. Therefore, the use of iron chelation therapy is important for this patient population, as elevated ferritin levels will continue to increase with the constant use of intravenous or oral iron supplement, as well as multiple red blood cells transfusions.
The general objective of this study was to identify the effect of iron chelation with deferasirox on telomere length and oxidative stress levels in patients undergoing hemodialysis.
MethodsThis is a randomized, single-arm, open-label simple arm and controls study to determine the effect of iron chelation with deferasirox on telomere length and oxidative stress markers in patients undergoing renal replacement therapy with hemodialysis and a glomerular filtration rate <15mL/min/1.73m2. The study includes patients ≥18 years, with a history of being multitransfused and having received oral and/or intravenous iron replacement therapy, with ferritin levels greater than 1000ng/mL that require iron chelation therapy, with leukocytes >5000/mL and platelets >150,000/mL, with liver enzymes <2.0 times normal levels, and total bilirubin <1.5, coming from the Unidad Médica de Alta Especialidad No. 1 Bajío.
Exclusion criteriaPatients with moderate to severe smoking, with active alcoholism at the time of the study, who have already received a kidney transplant, who present uncontrolled systemic arterial hypertension, systemic cardiovascular disease, hepatic impairment (ALT>300U/L). Patients with a history of autoimmune diseases (lupus erythematosus, focal segmental glomerulosclerosis), with any surgical or medical condition that prevents the correct absorption of Exjade. Female patients in pregnancy and/or lactation, were not included.
All the patients were invited to participate, and informed consent was obtained; they signed written informed consent to understand the effects of treatment with deferasirox (an iron chelator). The study complies with the Consolidated Standards of Reporting Trials,34 and the Helsinki Declaration and was approved by the Institutional Ethical Committee of the Mexican Institute of Social Security (IMSS R-2015-785-125).
Patients of both genders were included. Two groups of patients undergoing renal replacement therapy with hemodialysis, who had a history of iron overload and were not currently undergoing intravenous iron therapy, were randomly selected to receive deferasirox or to be part of the control group. They were randomly assigned using a computer-generated list of random numbers, centrally. Patients were evaluated monthly for 6 months of treatment with deferasirox at a dose to 15mg/kg. Routine biochemical marker tests were performed to assess liver function, and blood counts were observed during all visits to determine if any adverse effects had occurred. Timely reports were made to the national pharmacovigilance center according to NOM-220-SSA1-2012 evaluating the possible need for dose adjustment or discontinuation of treatment.
Serum levels of glucose were determined using the glucose oxidase-peroxidase method (Biosystems, Spain). Creatinine, urea, cholesterol, and triglycerides were estimated using enzymatic methods (STANBIO Laboratory, Boerne, TX, USA). Ferritin levels in plasma were determined with an automated analyzer using dry chemistry technique and reported in units of the IS (ng/mL). Serum TBARS levels were quantified to assess oxidative damage to lipids. To evaluate oxidative damage to oxidized proteins, serum carbonyls were quantified.
DNA samples were extracted from white blood cells. The ratio of telomere repeat copy number to a single gene copy number (T/S) was determined using a modified version of the quantitative real-time PCR telomere assay, as previously described.20
Deferasirox was administered orally every day at a dose of 15mg/kg/day weight, for 3 months. Ferritin levels were evaluated on this date to recalculate the dose. If ferritin levels were below 500mg, the dose was adjusted to 10mg/kg for an additional for three more months. Patient monitoring took place during each visit to assess treatment adherence and the presence of adverse events. Biochemical and anthropometric parameters (such as body weight) were monitored to determine whether the dose needed adjustment or if treatment with deferasirox should be continued.
If a patient did not attend their scheduled hemodialysis therapy visit, the principal investigator was alerted to reschedule the visit. If a patient did not present on two consecutive occasions, an investigation was conducted to determine the reason for the absence. This assessment aimed to determine if it was necessary to temporarily discontinue treatment or if some event had occurred that excluded the patient from the study (for example, a fistula infection, or another complication that prevented continued treatment).
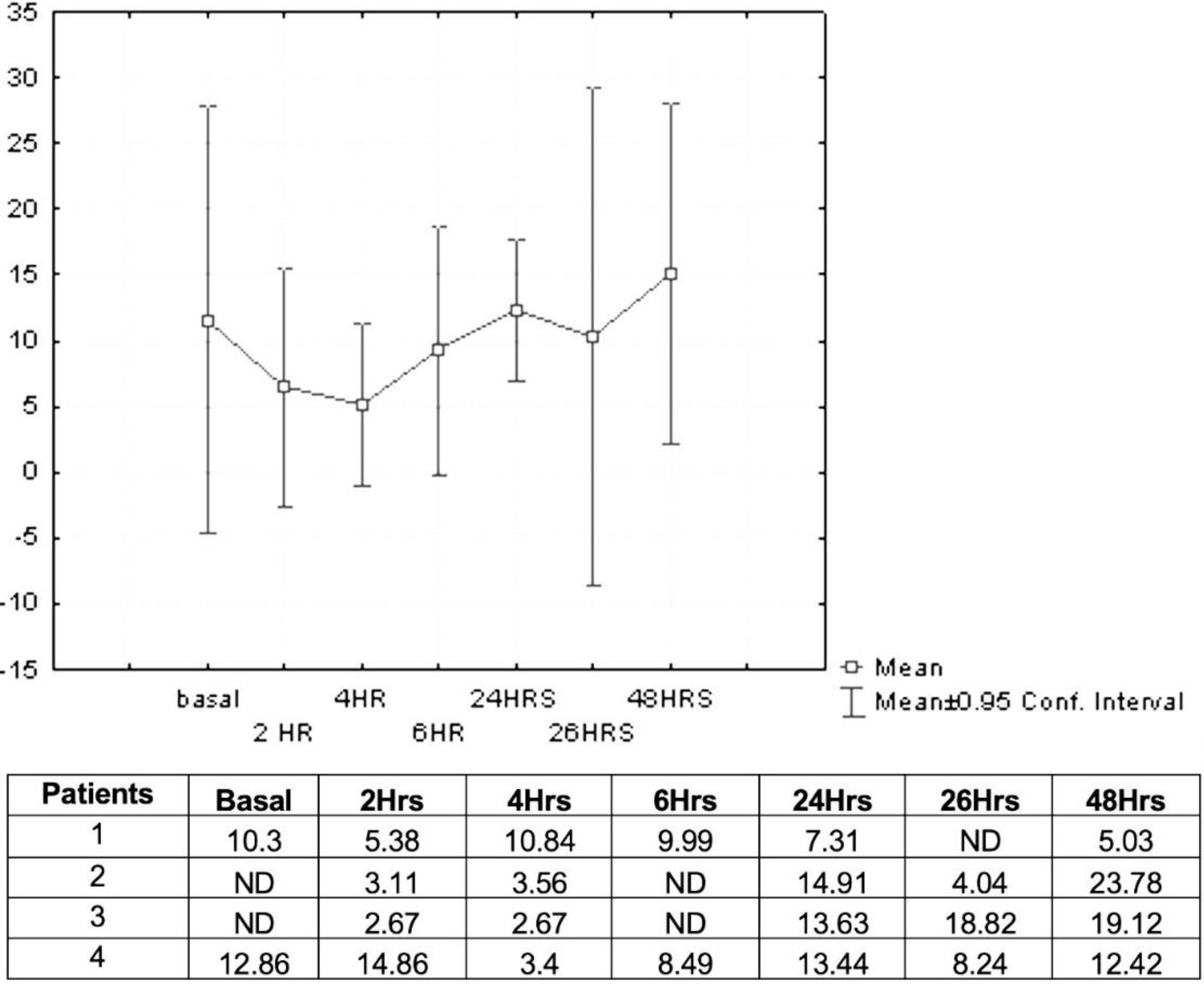
Pharmacokinetics and safety of deferasiroxBased on the results of Maker et al.,33 we proposed to evaluate the pharmacokinetics and safety of deferasirox by HPLC analyzing the plasma concentration in a representative pilot group of four patients. This group was chosen randomly. The dose to be administered is 15mg/kg per day, as reported in the pilot study. Samples were collected at 2, 4, 6h after the first dose of deferasirox. At 24h, just before the second dose of deferasirox, a new sample was taken, and 2h later corresponding to the serum evaluation at 26h. At 48h, the last sample was taken before the administration of oral deferasirox and after hemodialysis.
Statistical analysisStatistical analysis was performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were obtained for continuous variables, and comparisons were made using corresponding non-parametric approaches (Wilcoxon signed-rank test). To analyze the difference in serum concentrations of deferasirox over time, the Mann–Whitney U test was used, with p <0.05 considered significant.
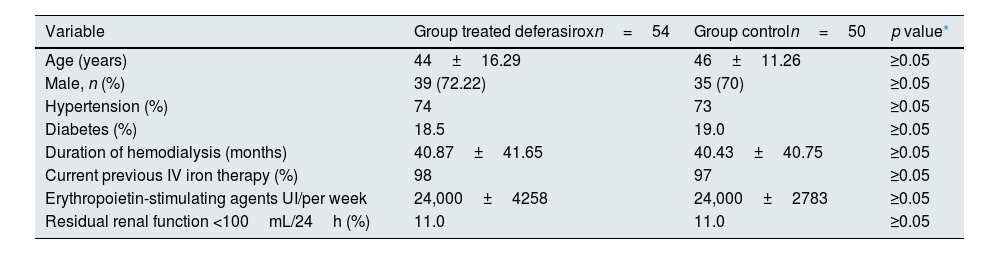
ResultsIn the present study, 54 patients were included to receive deferasirox, and a control group of 50 patients was established. The treated group had an average age of 44±16.29 years, 27. 77% were female and 72.22% male. Among them, 74% had systemic arterial hypertension, and 18.5% had type 2 diabetes mellitus. The patients had a history of therapy with intravenous iron dextran at a dose of 100mg in each hemodialysis session for 3 months, subsequently adjusted according to serum ferritin concentration and TSAT (Table 1).
Characteristics demographic, clinical and therapeutic.
| Variable | Group treated deferasiroxn=54 | Group controln=50 | p value* |
|---|---|---|---|
| Age (years) | 44±16.29 | 46±11.26 | ≥0.05 |
| Male, n (%) | 39 (72.22) | 35 (70) | ≥0.05 |
| Hypertension (%) | 74 | 73 | ≥0.05 |
| Diabetes (%) | 18.5 | 19.0 | ≥0.05 |
| Duration of hemodialysis (months) | 40.87±41.65 | 40.43±40.75 | ≥0.05 |
| Current previous IV iron therapy (%) | 98 | 97 | ≥0.05 |
| Erythropoietin-stimulating agents UI/per week | 24,000±4258 | 24,000±2783 | ≥0.05 |
| Residual renal function <100mL/24h (%) | 11.0 | 11.0 | ≥0.05 |
Five patients did not complete the 6 months of treatment, one male patient presented with catheter dysfunction, so he retired from the study; another male patient changed the modality of replacement therapy to peritoneal dialysis. One female patient received a kidney transplant, and two patients did not have ferritin levels available for the indication of deferasirox. No deaths occurred during the study, there were no serious adverse events (Fig. 1).
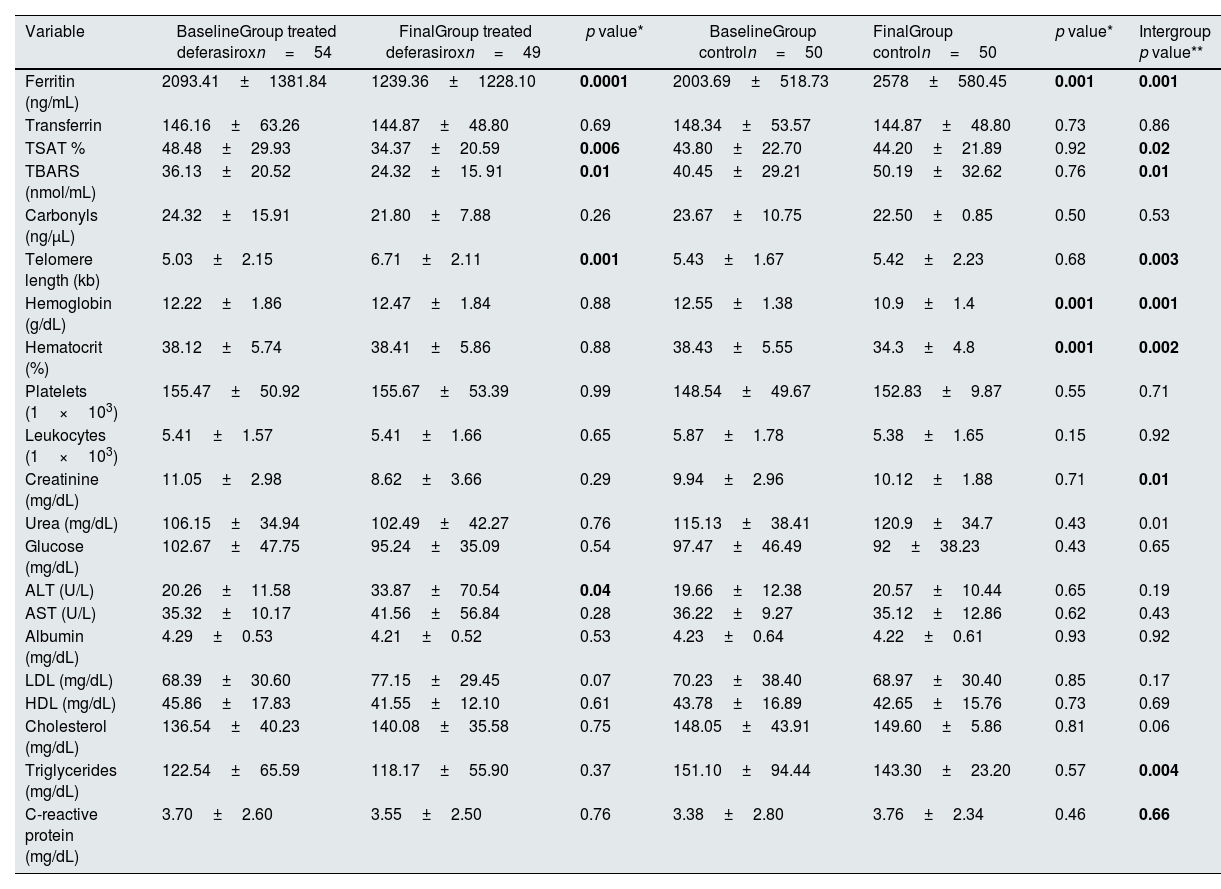
At the end of the treatment, significant differences were observed when comparing serum ferritin levels (1239.3±1228.1ng/mL vs 2093.4±1381.8ng/mL, p<0.0001), TBARS (24.32±15.91nmol/mL vs 36.13±20.52nmol/mL, p<0.01), but not in carbonyls (21.80±7.88ng/μL vs 24.32±15.91ng/μL, p=0.28).
The response in hemoglobin (12.47±1.84 vs 10.9±1.4, p=0.001) and hematocrit (38.41±5.86 vs 34.3±4.8, p<0.002) was significantly higher in the group treated with deferasirox when comparing the two groups. However, no significant response was response in leukocytes and platelets (p>0.05). The C-reactive protein showed no changes at the end of the treatment (3.70±2.60 vs 3.55±2.50, p=0.76). Telomere length increased significantly at the end of chelation (5.03±2.15kb vs. 6.71±2.11kb, p=0.001), while in the control group no changes were observed (5.43±1.67kb vs 5.42±2.23kb, p=0.68). The study variables were compared with the control group as seen in Table 2. Eleven percent of patients in both groups had residual renal function <100mL/24h. Creatinine levels decreased considerably in the treated group (8.62±3.66 vs 10.12±1.88, p=0.01). All patients received on average IU of erythropoietin, with a range of (4000–24,000 IU per week).
Comparison of biochemical markers at baseline and end of treatment.
| Variable | BaselineGroup treated deferasiroxn=54 | FinalGroup treated deferasiroxn=49 | p value* | BaselineGroup controln=50 | FinalGroup controln=50 | p value* | Intergroup p value** |
|---|---|---|---|---|---|---|---|
| Ferritin (ng/mL) | 2093.41±1381.84 | 1239.36±1228.10 | 0.0001 | 2003.69±518.73 | 2578±580.45 | 0.001 | 0.001 |
| Transferrin | 146.16±63.26 | 144.87±48.80 | 0.69 | 148.34±53.57 | 144.87±48.80 | 0.73 | 0.86 |
| TSAT % | 48.48±29.93 | 34.37±20.59 | 0.006 | 43.80±22.70 | 44.20±21.89 | 0.92 | 0.02 |
| TBARS (nmol/mL) | 36.13±20.52 | 24.32±15. 91 | 0.01 | 40.45±29.21 | 50.19±32.62 | 0.76 | 0.01 |
| Carbonyls (ng/μL) | 24.32±15.91 | 21.80±7.88 | 0.26 | 23.67±10.75 | 22.50±0.85 | 0.50 | 0.53 |
| Telomere length (kb) | 5.03±2.15 | 6.71±2.11 | 0.001 | 5.43±1.67 | 5.42±2.23 | 0.68 | 0.003 |
| Hemoglobin (g/dL) | 12.22±1.86 | 12.47±1.84 | 0.88 | 12.55±1.38 | 10.9±1.4 | 0.001 | 0.001 |
| Hematocrit (%) | 38.12±5.74 | 38.41±5.86 | 0.88 | 38.43±5.55 | 34.3±4.8 | 0.001 | 0.002 |
| Platelets (1×103) | 155.47±50.92 | 155.67±53.39 | 0.99 | 148.54±49.67 | 152.83±9.87 | 0.55 | 0.71 |
| Leukocytes (1×103) | 5.41±1.57 | 5.41±1.66 | 0.65 | 5.87±1.78 | 5.38±1.65 | 0.15 | 0.92 |
| Creatinine (mg/dL) | 11.05±2.98 | 8.62±3.66 | 0.29 | 9.94±2.96 | 10.12±1.88 | 0.71 | 0.01 |
| Urea (mg/dL) | 106.15±34.94 | 102.49±42.27 | 0.76 | 115.13±38.41 | 120.9±34.7 | 0.43 | 0.01 |
| Glucose (mg/dL) | 102.67±47.75 | 95.24±35.09 | 0.54 | 97.47±46.49 | 92±38.23 | 0.43 | 0.65 |
| ALT (U/L) | 20.26±11.58 | 33.87±70.54 | 0.04 | 19.66±12.38 | 20.57±10.44 | 0.65 | 0.19 |
| AST (U/L) | 35.32±10.17 | 41.56±56.84 | 0.28 | 36.22±9.27 | 35.12±12.86 | 0.62 | 0.43 |
| Albumin (mg/dL) | 4.29±0.53 | 4.21±0.52 | 0.53 | 4.23±0.64 | 4.22±0.61 | 0.93 | 0.92 |
| LDL (mg/dL) | 68.39±30.60 | 77.15±29.45 | 0.07 | 70.23±38.40 | 68.97±30.40 | 0.85 | 0.17 |
| HDL (mg/dL) | 45.86±17.83 | 41.55±12.10 | 0.61 | 43.78±16.89 | 42.65±15.76 | 0.73 | 0.69 |
| Cholesterol (mg/dL) | 136.54±40.23 | 140.08±35.58 | 0.75 | 148.05±43.91 | 149.60±5.86 | 0.81 | 0.06 |
| Triglycerides (mg/dL) | 122.54±65.59 | 118.17±55.90 | 0.37 | 151.10±94.44 | 143.30±23.20 | 0.57 | 0.004 |
| C-reactive protein (mg/dL) | 3.70±2.60 | 3.55±2.50 | 0.76 | 3.38±2.80 | 3.76±2.34 | 0.46 | 0.66 |
TSAT: transferrin saturation; TBARS: thiobarbituric acid reactive substances; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
The significance of bold values is p < 0.05.
Wilcoxon signed-rank test. *p value. **Intergroup p value.
Plasma levels of deferasirox (mmol/L) were analyzed in four patients. Prior to hemodialysis treatment (zero day), blood samples were taken at time zero (before oral intake of deferasirox). Subsequently deferasirox was orally administered at a dose of 15mg/kg. Samples were collected, and the average concentration was measured at 2h (6.50mmol/L), 4h (5.11mmol/L), 6h (9.24mmol/L), after this first dose of deferasirox. At 24h (12.32mmol/L), just before the second dose of deferasirox, a new sample was taken, and 2h later corresponding to the serum evaluation at 26h (10.36mmol/L). At 48h (15.08mmol/L), the last sample was taken before the administration of oral deferasirox and after hemodialysis. Fig. 2 shows the concentrations (mmol/L) determined by HPLC at different times.
DiscussionThere is currently strong evidence for the benefit of intravenous iron therapy in the treatment of anemia in patients with chronic kidney disease.35 However, on the other hand, we know that iron accumulation leads to adverse effects with increased ferritin levels, increased risk of infection, and increased mortality from cardiovascular events.36,37 In our study, iron chelation in patients with a glomerular filtration rate of less than 15mL/min and undergoing hemodialysis significantly reduces ferritin and TBARS levels, which increases the length of the telomeres. We found significant differences at the end of the treatment when comparing serum ferritin levels, and the response in hemoglobin and hematocrit between the two groups, observing significant differences and higher levels in the group treated with deferasirox. Similarly, creatinine levels were lower after 6 months of treatment, which may indicate an improvement in dialysis efficiency by reducing inflammation and oxidative stress factors. The treatment of chronic kidney disease includes the administration of parenteral iron; unfortunately, this treatment leads to iron overload. Randomized trials in hemodialysis patients have demonstrated significantly greater increases in hemoglobin levels with IV iron when compared to oral iron, and a low rate of treatment related adverse events during these short trials.38,39
However, little is known about the efficacy and safety of iron chelation in patients with renal replacement therapy. Deferasirox is an oral iron chelator that is hepatically metabolized and excreted by the intestine.29
Marker and colleagues performed a pilot study to evaluate the pharmacokinetics and safety of deferasirox in patients with chronic renal failure undergoing hemodialysis and presenting iron overload. Deferasirox was administered at two doses of 10mg/kg and 15mg/kg per day for two weeks. They observed that at a dose of 10mg/kg/day, insufficient concentrations were obtained in the blood (14.1–22.8μmol/L) while at a dose of 15mg/kg/day, the observed concentration was higher (40–50μmol/L) without showing clinically adverse events.33 In our study, the dose of 15mg/kg maintained the plasma concentration required without adverse events during treatment. The deferasirox concentration in serum was determined by HPLC, and much higher concentrations were observed (2.67–23.78mmol/L).
Tsai et al. reported the response of deferasirox to 15mg/kg in patients with chronic renal failure on dialysis; serum ferritin levels decreased significantly, and those who presented amounts of 3252ng/mL continued with a maintenance dose of 10mg/kg.39
Few studies have been conducted to determine the pharmacokinetics and safety of deferasirox in hemodialysis patients. Deferasirox may cause acute renal failure, and a creatinine clearance rate of <40mL/min and serum creatinine level of >2-fold the upper limit of normal are listed as contraindications by Novartis and the Food and Drug Administration.40 Although the pharmacokinetics of deferasirox in patients with chronic kidney disease suggests minimal risk of accumulation because its excretion by the renal route is minimal.9 In our study, it has been shown that there is a significant increase in plasma levels when the dose is elevated from 10mg/kg/day to 15mg/kg/day. It has been argued that uremia is one of the reasons why deferasirox levels are increased in the plasma level, because uremia can reduce fecal excretion and enhance reabsorption at the intestinal level. In uremic rats there is a decrease in intestinal membrane transporter protein (IMTP) and related protein (MRP2). The increased bioavailability of deferasirox can be explained by a reduction in excretion through MRP2.41
In this investigation, the concentration of deferasirox in serum from time zero to 48h was maintained in a range of 2.67–23.78mmol/L. We were able to determine the concentration of deferasirox at a dose of 15mg/kg/day from baseline pre hemodialysis, and the following concentrations during hemodialysis (at 2, 4, 6h), at 24h before the next deferasirox intake, and at 48h before entering hemodialysis therapy again. During this time there were no significant adverse events. Some authors have reported complications in hematology patients caused by iron chelation treatment such as kidney damage.42,43
In the present study, follow-up was conducted for six months, without significant elevations in creatinine levels, and the majority of patients had liver function tests remained within the normal limits.
Previous epidemiological studies have shown that elevated iron status is associated with an increased risk of chronic conditions such as type 2 diabetes, cardiovascular disease, and mortality.44 Furthermore, high ferritin levels have been found to be associated with shortened telomeres, a biomarker of biological aging, and chronic age-related diseases, among patients with iron overload due to disease. However, the association between body iron status and telomere length in the general population remains unknown.
In a nationally representative population of the USA, high body iron level was associated with shorter telomeres, especially in adults 65 years of age and older.28 Cell culture experiments indicate that pro-inflammatory conditioning and high glucose have an effect on telomere shortening, with former accelerating the process.45 Oxidative stress also induces telomere attrition,46 with the telomere GGG sequence particularly vulnerable to damage caused by reactive oxygen species.47 The increased oxidative stress resulting from iron overload may induce this telomere shortening. Elevated ferritin levels contribute to telomere loss in hemodialysis patients. Indeed, shorter telomere length has been associated with an increased risk of death in CKD.48,49
In our experience with deferasirox in patients with chronic kidney failure undergoing hemodialysis, we observed that iron chelation prevented telomere shortening, reduced ferritin levels and lipid peroxidation, and reduced oxidative stress, with an increase in telomere length at the end of iron chelation. Iron chelation therapy is an alternative that addresses new paradigms in the management of patients undergoing renal replacement therapy; this study demonstrated recovery of hemoglobin levels and improved response to erythropoietin. Deferasirox was generally well tolerated; common adverse events included nausea, vomiting, diarrhea, and abdominal pain. Further studies are needed to support iron chelation in the prevention of survival-limiting complications in patients with CKD.
ConclusionOur findings demonstrate that iron chelation in patients undergoing hemodialysis significantly reduces ferritin levels and oxidative damage to lipids, which results in an increase in telomere length. Treatment with deferasirox during renal replacement therapy provides an alternative approach that benefits patient by addressing complications with iron overload.
Conflict of interestThe authors have no relevant conflicts of interest to disclose.
This study was sponsored by Novartis Pharmaceuticals Corporation and Mexican Institute of Social SecurityFIS/IMSS/1631.