Hypertension mediated organ damage (HMOD) refers to structural or functional changes in arteries or target organs that can be present in long-standing hypertension, but it can be also found in naïve never treated patients. Traditionally, cardiovascular risk is stratified with charts or calculators that tend to underestimate the real cardiovascular risk. The diagnosis of HMOD automatically reclassifies patients to the highest level of cardiovascular risk. Subclinical HMOD can be present already at the diagnosis of hypertension and more than 25% of hypertensives are misclassified with the routine tests recommended by hypertension guidelines. Whether HMOD regression improves cardiovascular outcomes has never been investigated in randomized clinical trials and remains controversial. However, different drugs have been probed with promising results in high cardiovascular risk patients, such as the new antidiabetic or the novel non-steroid mineralocorticoid antagonists. Accordingly, trials have shown that lowering blood pressure reduces cardiovascular events. In this narrative review, we will discuss the role of HMOD in cardiovascular risk stratification, the different types of organ damage, and the evidence available to define whether HMOD can be used as a therapeutic target.
El daño orgánico mediado por hipertensión (HMOD) se refiere a cambios estructurales o funcionales de larga duración en las arterias u órganos diana de la hipertensión, pero también se puede encontrar en pacientes que nunca han recibido tratamiento antihipertensivo previo. Tradicionalmente, el riesgo cardiovascular se ha estratificado utilizando tablas, calculadoras o algoritmos que tienden a subestimar el riesgo cardiovascular real. El diagnóstico del HMOD reclasifica automáticamente a los pacientes al nivel más alto de riesgo cardiovascular. El HMOD subclínico ya puede estar presente en el momento del diagnóstico de hipertensión y más del 25% de los hipertensos están mal clasificados con las pruebas de rutina recomendadas por las guías de hipertensión. Sin embargo, si la regresión del HMOD mejora los resultados cardiovasculares no suele ser un objeto de investigación en ensayos clínicos aleatorizados y sigue siendo un aspecto controvertido. A pesar de ello, se han probado diferentes fármacos con resultados prometedores en pacientes de alto riesgo cardiovascular, como los nuevos antidiabéticos o los nuevos antagonistas de mineralocorticoides no esteroides. De hecho, diferentes estudios han demostrado que bajar la presión arterial reduce los eventos cardiovasculares. En esta revisión narrativa, se discutirá el papel del HMOD en la estratificación del riesgo cardiovascular, los diferentes tipos de daño orgánico y la evidencia disponible para definir si HMOD puede usarse como un objetivo terapéutico.
Hypertension is the main cause of mortality and disability in the world. The correct diagnosis and management of this population are mandatory to reduce cardiovascular events. In the past years, different cardiovascular risk estimators have been developed to allow physicians to predict the risk of having a cardiovascular event in the next 10 years. These tools can improve adherence and make physicians intensify treatment in high-risk patients. There are different calculators available for clinicians, the traditional Framingham cardiovascular risk score,1 the American ASCVD (AtheroSclerotic CardioVascular Disease) estimator included in ACC/AHA clinical Guidelines,2,3 the English QRisk included in the NICE Guidelines,4,5 and the European SCORE (Systematic COronary Risk Evaluation) that is recommended by the ESC/ESH clinical Guidelines.6,7 However, the cumulative cardiovascular risk during the lifespan of young subjects and women tends to be underestimated independently of their cardiovascular risk factor burden because of the strong power of sex and age in the short-term. This cumulative cardiovascular risk is known as lifetime cardiovascular risk, and tends to be misconceived in the general population despite the fact that the majority of the population has low short-term cardiovascular risk and high lifetime cardiovascular risk.8 Accordingly, lifetime cardiovascular risk estimators have been developed in the US,9 UK10 and Spain,11 and are recommended by clinical Guidelines as a way to increase awareness of their cardiovascular risk in populations with low 10-year cardiovascular risk.12,13
Hypertension produces vascular and other organ damage. The organ damage generated by hypertension is called hypertension mediated organ damage (HMOD)14 and can be found not only in patients with inadequate blood pressure (BP) control, but also in naïve patients. Hypertensive vascular damage can be found in the brain, kidneys, heart, retinal, and arteries. The detection of subclinical organ damage can improve the classical cardiovascular risk estimation relocating patients from low-moderate to high cardiovascular risk, especially in middle-aged patients who tend to be asymptomatic. Moreover, detecting HMOD in younger and in naïve patients allows physicians to treat them as soon as possible. Some authors consider HMOD as a surrogate marker of inadequate BP control.14 Adequate BP control is needed, but whether HMOD is reversible with antihypertensive treatment is still controversial.
Traditional CVR stratification: the role of HMODOne important issue in the management of hypertensive patients is the stratification of cardiovascular risk, which refers to the probability of having a cardiovascular event in a certain timeframe. A significant proportion of asymptomatic essential hypertensive patients could have a better assessment of cardiovascular risk based on cardiovascular risk estimation. The SCOREc, the QRisk and the ASCVD estimators include age, cholesterol levels, smoking status, systolic BP, and whether patients are receiving antihypertensive drugs in their algorithms,15 but consider different primary end-points and accordingly set different thresholds to consider a patient with high cardiovascular risk. SCOREc estimates the risk of a first fatal cardiovascular event with a threshold of 5%, QRisk-2 estimates the risk of a first cardiovascular event (fatal or not) with a threshold of 10%, and ASCVD estimates the risk of developing atherosclerotic cardiovascular disease with a threshold of 7.5%.2,5,7 As an example, a 44 years old non-smoking hypertensive male from a low cardiovascular risk region treated with combination therapy of losartan/hydrochlorothiazide 100/25mg/day, with systolic BP of 120mmHg, a total cholesterol level of 180mg/dL and an HDL-C of 42mg/dL has a 10-year cardiovascular risk of 1% calculated with SCORE, 4.2% calculated with QRisk-2, and 2% calculated with ASCVD estimator. As we can see, this middle-age hypertensive male is considered at a low risk of cardiovascular events in all cases. However, screeing for subclinical organ damage reveals that this patient has low-grade albuminuria (220mg/day) and left ventricular hypertrophy (LVH). With this additional information, our patient would be considered at high risk of cardiovascular events in the next 10 years. Even if this subject had resistant hypertension, which is the presence of uncontrolled hypertension in subjects receiving at least three antihypertensive drugs of different classes, estimators would retrieve the same value of cardiovascular risk. Therefore, it is important to note that cardiovascular risk estimators underestimate the real cardiovascular risk of subjects with sub-clinical organ damage because markers of organ damage are not considered for routine cardiovascular risk assessment.
Sustained exposure to cardiovascular risk factors induces a progressive development or aggravation of organ damage, so primary prevention strategies to reverse it in subclinical phases are crucial to stop the progression of cardiovascular disease. Guidelines define routine and non-routine tests that many times are expensive and depend on the health policy of each country. Subclinical HMOD is frequent in patients with a long history of hypertension such as resistant hypertensives, but sometimes are present in naïve-never treated middle age hypertensives, that would be misclassified with traditional cardiovascular risk stratification as low/moderate.16–18 In fact, the presence of HMOD automatically reclassifies patients from low or moderate to high or very high cardiovascular risk. In this sense, Viazzi et al. studied the impact of assessing organ damage in the evaluation of 380 never-treated hypertensive patients by adding albuminuria, echocardiogram, and carotid ultrasound to the routine studies.16 They found that the combined use of all of these tests improved the detection of organ damage, leading to the identification of a higher percentage of patients who were at high/very high cardiovascular risk, as compared with those who were detected by routine clinical workup (73% instead of 42%; P<0.0001). The prevalence of microalbuminuria, carotid thickening or plaque, and LVH at echocardiogram was 13, 32, and 49%.16 Using non-routine tests, Gómez-Marcos et al. reclassified 25.4% of hypertensive patients from low or moderate to high cardiovascular risk.19 Moreover, 34% of asymptomatic patients receiving primary prevention therapy have silent asymptomatic cardiac abnormalities, especially LVH, and 6.3% of them had silent myocardial ischemia.20 The presence of subclinical organ damage at renal or artery level significantly improved cardiovascular risk assessment with SCORE, which could significantly strengthen the prevention of cardiovascular disease by implementing aggressive treatment strategies to prevent organ damage progression.21 Regression of HMOD might be possible with BP control, but whether the regression of HMOD improves cardiovascular outcomes is still a matter of debate.
Assessment of HMODHypertension mediated cardiac damageLVH is highly prevalent among hypertensive patients and is associated with poor outcomes.22 Different methods can be used to study cardiac alterations. Despite the low sensitivity of ECG for LVH assessment, the prevalence of LVH assessed by ECG increases with the severity and duration of hypertension.23 The Losartan Intervention for End-point Reduction in Hypertension Study (LIFE) trial including 9193 hypertensive patients with LVH described that the composite endpoint of cardiovascular mortality, myocardial infarction, and stroke was reduced with regression of ECG voltage parameters.24 Another sub-analysis of the same study showed that for every one standard deviation of regression of the ECG parameters, there was a 19% lower adjusted risk of sudden cardiac death,24 and also a lower risk of new-onset heart failure and mortality.25 Then, the majority of the cardiovascular benefit in the LIFE study derived from the reduction in left ventricular mass.26 A normal ECG does not exclude the presence of LVH, so echocardiography is recommended for precise information on cardiac structure and function.27 De Simone et al. showed in 8848 free of prevalent cardiovascular disease hypertensive patients that all the different forms of left ventricular changes as eccentric dilated LVH, concentric non-dilated LVH, and concentric dilated LVH were associated with higher cardiovascular risk.28 The presence of increased left ventricular mass was also associated with incident atrial fibrillation.29 Moreover, Verdecchia et al. found in 880 patients with untreated hypertension that the risk for a future cerebrovascular event was 2.8 times higher in those without regression of LVH or with new development of LVH than in those who exhibited LVH regression during a follow-up of 3.5 years.30 A reduction in office systolic BP of approximately 9mmHg, leads to a reduction in left ventricular mass,31 but whether LVH reduction improved cardiovascular mortality is not conclusive. Many studies showed that the regression of LVH reduced the risk of cardiovascular events, especially when patients were treated earlier and aggressively but others showed conflicting results. A possible reason for discrepancies is the definition of LVH, as it may be defined as the reduction in left ventricular mass to normal levels or a certain percentage or net reduction in left ventricular mass. A significant reduction in left ventricular mass in subjects with severe LVH has been suggested to be more clinically relevant than a slight reduction to normal levels in subjects with mild LVH because of the wide variations between measurements.32,33 Accordingly, while the percentage of total regression of LVH was 14% in the Campania Salute Network, this percentage raised to 23% when considering patients with a reduction of >5g/m.34
Hypertension mediated kidney damageElevated albumin excretion is considered a marker of subclinical organ damage35 whose prevalence in arterial hypertension varies between 8 and 15%.36,37 Moreover, albuminuria influences the levels of nighttime BP.38,39 The presence of high albuminuria correlates with cardiac damage, especially concentric hypertrophy.40,41 Accordingly, reducing high albuminuria is a favorable marker to improve LVH.42,43 Moreover, albuminuria is associated with several vascular structural and functional alterations44 including carotid intima media thickness,45 and atherosclerosis development and progression.46 In type 1 diabetes mellitus patients, moderately increased albuminuria is a powerful predictor for the development of proliferative diabetic retinopathy and blindness.47 Furthermore, increased albuminuria is a marker of coronariopathy in diabetic and non-diabetic individuals, as well as in the general population.48 Moreover, albuminuria was associated with silent target organ damage in resistant hypertension.49 As the worsening of albuminuria leads to chronic kidney disease (CKD) progression,50 its reduction with specific treatment leads to improving kidney function, so that albuminuria could be considered a therapeutic target in clinical practice and a surrogate endpoint for end-stage renal disease (ESRD).51,52 The European Guidelines for hypertension emphasize the importance of assessing the presence of albuminuria as a marker of organ damage for cardiovascular risk stratification.7 Therefore, physicians must determine the presence of albuminuria or subclinical renal involvement in hypertensive patients to better assess cardiovascular risk.
Hypertension mediated vascular damageHypertension generates vascular changes that are characterized by endothelial dysfunction and remodeling of the small and large arteries. This leads to a reduced dilation capability of the high resistance vasculature and stiffening of the arteries, which finally manifest in an accelerated aging of the vasculature.53 Carotid intima-media thickness (CIMT), pulse wave velocity (PWV), augmentation index, and endothelial dysfunction have been associated with the prediction of future cardiovascular events.54,55 Carotid ultrasound to determine the extent of carotid stenosis is currently not recommended as a routine test by Guidelines. However, carotid stenosis is commonly associated with subclinical organ damage in other places16 and has a strong predictive value for both stroke and myocardial infarction independently of traditional cardiovascular risk factors.56,57 In fact, the presence of advanced carotid plaque (≥50% stenosis) is considered as documented cardiovascular disease in the current European Guidelines for the Management of Arterial Hypertension, and consequently the presence of advanced carotid plaque automatically reclassifies patients from intermediate to high cardiovascular risk.7 Age, male sex, and systolic BP are significantly related to the presence of carotid atherosclerosis, which reclassified 25.1% of a study population including 3778 volunteers from low-intermediate cardiovascular risk to higher cardiovascular risk.58 Moreover, the prevalence of subclicical atherosclerosis is greater in subjects with low 10-year cardiovascular risk and high lifetime cardiovascular risk than in those with low 10-year cardiovascular risk and low lifetime cardiovascular risk.59 Vascular damage can also be studied by different non-invasive methods as the ankle-brachial index and PWV. It is important to note that a positive ankle brachial index is indicative of relevant stenosis.60 Carotid-femoral PWV is the gold standard for measuring arterial stiffness.61 Values above 10m/s are classified as pathological and are associated with an increased risk of cardiovascular mortality.62 Ben-Shlomo et al. published a meta-analysis of 17635 patients from 16 trials describing that PWV improved the estimation of risk for future cardiovascular events in models that include standard cardiovascular risk factors as the SCORE and the Framingham risk score.63 Another meta-analysis that included 14,673 Japanese participants described the role of brachial-ankle PWV (baPWV) as an independent predictor of the risk of development of cardiovascular diseases in Japanese subjects without preexisting cardiovascular disease.64 The use of PWV could enhance the efficacy of prediction of the risk of development of cardiovascular disease over that of the Framingham risk score. Moreover, reducing arterial stiffness is associated with a decrease in LVH, although this association may be counfounded by a simultaneous reduction in BP.43 The presence of arterial stiffness is suggested to preceed the development of hypertension in normotensive patients,65 contributing to the vicious cycle in which hypertension aggravates HMOD, and narrowing of blood vessels increases blood pressure. Therefore, more studies are needed to uncover the causative associations between arterial stiffness, high BP, and LVH. However, routine use of PWV measurement increases health cost and it is currently not recommended as a routine test by Guidelines.7
Is it possible to consider organ damage as a surrogate therapeutic target?BP reduction improves HMOD and some authors have proposed that organ damage regression can improve cardiovascular mortality.66,67 It is well known that organ damage increases the risk of cardiovascular events and improves traditional cardiovascular risk estimators. However, despite some positive publications, whether HMOD can be considered a therapeutic target is still an unresolved question. The majority of the available evidence is based on subanalyses of previous interventional trials.
It is well known that LVH is associated with poor cardiovascular outcomes such as heart failure, atrial fibrillation, acute myocardial infarction, or cardiovascular deaths.68,69 Therefore, it is logical to speculate that LVH regression with optimal BP control would improve cardiovascular events. In this sense, many authors found benefits from LVH regression on mortality.25,28,29,34,70 However, results from the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANS-CEND) showed that despite LVH reduction after 2 and 5 years in the intervention group, the beneficial effect on regression of LVH was not translated into benefits in cardiovascular events.71 In a large meta-analysis that included 14 studies and 12,809 participants, Costanzo et al. found that LVH reduction did not show a significant reduction for acute myocardial infarction and heart failure.72 Moreover, in the Systolic Blood Pressure Intervention Trial (SPRINT), intensive (vs. standard) BP lowering was 66% more likely to regress LVH (HR=1.66; 95% CI=1.31–2.11) in participants with baseline LVH.73 Nonetheless, adjustment for LVH as a time-varying covariate did not substantially attenuate the effect of intensive BP therapy on cardiovascular events. The authors concluded that this favorable effect on LVH did not explain most of the reduction in cardiovascular events associated with intensive BP lowering in the SPRINT trial.73 However, other authors did not find positive results of LVH regression on cardiovascular outcomes. Brooks et al. recently published an article to answer the question of whether LVH could be considered a therapeutic target in patients with hypertension.74 They reviewed studies that used ECG, echocardiogram, and cardiac magnetic resonance on LVH and found that higher BP is a risk factor for LVH and poor cardiovascular outcomes, and that regression of LVH is possible by successful BP lowering. Nevertheless, LVH improvement cannot yet be considered a reliable surrogate outcome measure to use in the context of hypertensive heart disease. They concluded that LVH may not be the “holy grail” regarding therapeutic targets in hypertensive heart disease, but it could be considered one of the markers in the successful management of hypertension.74
In CKD patients, the presence of albuminuria has largely been associated with poor cardiovascular and renal outcomes.75 Many authors showed the deleterious effect of albuminuria on ESRD and cardiovascular mortality and proposed it as a therapeutic target.51,76 Focusing on albuminuria, different trials were done to show the renoprotective effects of lowering BP, especially by blocking the renin–angiotensin–aldosterone system (RAAS).77,78 Results from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial79 showed that losartan reduced the incidence of a doubling of the serum creatinine concentration by 25% (P=0.006) and ESRD by 28% (P=0.002) but did not affect the rate of death.79 Similar findings were published in the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trial, where olmesartan delayed the onset of microalbuminuria but was associated with a higher rate of fatal cardiovascular events among patients with preexisting coronary heart disease.80 Moreover, various trials focused on dual RAAS blockade, despite reducing albuminuria, failed to reduce cardiovascular outcomes and induced acute kidney injury and hyperkalemia.81,82 Interestingly, in the African-American Study of Kidney Disease and Hypertension trial (AASK), patients in the intensive treatment group (BP<130/80mmHg) with a protein-to-creatinine ratio of more than 0.22 improved secondary endpoints of ESRD or death (HR 0.67; 95% CI=0.52–0.87; P=0.002).83 In the SPRINT trial 28% of the study population had CKD.84 In those patients, intensive BP management (BP<120/80mmHg) seemed to provide the same benefits in cardiovascular composite primary outcome and all-cause mortality as was seen in the full study cohort.85 In contrast, data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study indicated that strict BP control (systolic BP of less than 120mmHg) did not improve renal outcomes in patients with type 2 diabetes mellitus (T2DM).86 Given that most patients with CKD die from cardiovascular complications, SPRINT evidence supports a target of less than 130/80mmHg for non-diabetic CKD patients.84,85 Since the publication of SPRINT, different Guidelines have reviewed the BP target in the CKD population and support the BP target of less than 130/80mmHg for CKD patients independently of the presence of albuminuria.87 Moreover, KDIGO Guideline for the Management of Blood Pressure in CKD suggest that adults with high BP and CKD be treated with a target systolic blood pressure of <120mmHg, when tolerated, using standardized office BP measurement.88 On the other hand, BP reduction can improve arterial stiffness, but there is a lack of evidence showing positive results on cardiovascular mortality.89,90 Guerin et al. analyzed the incidence of arterial stiffness by PWV in dialyzed patients, indicating that the lack of response of PWV to decreased BP is predictor of mortality independently of BP change.91 More recently, results from the Strategy for Preventing cardiovascular and renal events based on ARTErial stiffness (SPARTE) study have demonstrated that targeting normalization of PWV (<10m/s) to guide antihypertensive therapy significantly reduces SBP and PWV compared to targeting SBP following clinical management Guidelines.92 However, cardiovascular outcomes were not affected.
New evidence in the management of patients with HMOD: much more than antihypertensivesMineralocorticoid receptor antagonists (MRAs)The addition of a MRA is an interesting option for managing patients with albuminuria and high cardiovascular risk but has the inconvenience of frequent hyperkalemia.93 However, given the good results of spironolactone in resistant hypertension, spironolactone is recommended as four-line therapy to control BP.7,94 Finerenone is a new non-steroidal MRA (BAY948862) with better MRA selectivity than spironolactone that reduces albuminuria and end organ damage more effectively than eplerenone.95 In the MinerAlocorticoid Receptor Antagonist Tolerability Study (ARTS), finerenone reduced albuminuria and had a lower incidence of hyperkalemia when compared with spironolactone in patients with chronic heart failure.96 Furthermore, in the MinerAlocorticoid Receptor Antagonist Tolerability Study Diabetic Nephropathy (ARTS-DN) study, different oral doses of finerenone in patients with T2DM reduced albuminuria.97 Recently, the results of FIDELIO-DKD study (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) showed that finerenone is associated with a significant reduction compared to placebo group (HR=0.82 [95% CI=0.73–0.93], P=0.001) in the primary composite outcome of kidney failure, a sustained decrease of at least 40% in the eGFR from bseline, or death from renal causes, and a larger reduction (HR=0.76 [95% CI=0.65–0.90]) in the main secondary renal outcome, a composite of kidney failure, sustained eGFR decrease of ≥57% or renal death.98 Moreover, the FIDELIO-DKD trial has also demonstrated that finerenone benefits patients with and without cardiovascular disease.99 However, results from the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) demonstrated that the reduction in the kidney composite outcome is only significant in the case of a sustained decrease in the eGFR ≥57% 100. FIGARO-DKD and FIDELIO-DKD included patients with T2DM, but FIGARO-DKD included patients with moderately increased albuminuria and decreased renal function or patients with severely increased albuminuria and preserved renal function, while FIDELIO-DKD included patients with decreased renal function and moderately or severely increased renal function. Therefore, patients with decreased renal function might benefit more from finerenone treatment than patients with preserved renal function despite presenting with severey increased albuminuria.
Glucose-lowering agentsIn recent years, new anti-diabetic agents appeared not only to show benefits in glycemic control, but also to reduce cardiovascular mortality and improving kidney function. The sodium-glucose co-transporter-2 (SGLT2) inhibitor acts as a glycosuric agent. Two large randomized trials, the Empagliflozin Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME),101 and the CANagliflozin cardioVascular Assessment Study (CANVAS)102 reduced worsening nephropathy, doubling of serum creatinine and progression to very high albuminuria. Moreover, in the Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy study (CREDENCE),103 patients with T2DM and CKD assigned to receive canagliflozin reduced the relative risk of the primary outcome by 30% (HR=0.70; 95%, CI=0.59–0.82; P=0.00001) and also the relative risk of ESRD by 32% (HR=0.68, 95%, CI=0.54–0.86; P=0.002). The canagliflozin group had lower risks of hospitalization for heart failure (HR=0.61, 95% CI=0.47–0.80; P<0.001) and cardiovascular death, myocardial infarction, or stroke (HR=0.80, 95% CI=0.67–0.95; P=0.01). The DAPA-CKD (Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease), aimed to evaluate the effect of dapagliflozin 10mg once daily versus placebo in patients with CKD with or without diabetes, shows major reductions in the primary outcome of ≥50% decline in eGFR, ESRD, or cardiovascular or renal death (HR=0.61 [95% CI=0.51–0.72]), the composite of ≥50% decline in eGFR, ESRD, and renal death (HR=0.56 [95% CI=0.45–0.68]) and all its individual outcomes.104 Furthermore, the EMPA-KIDNEY (Study of Heart and Kidney Protection With Empagliflozin) is another renal outcome study evaluating the effects of empagliflozin on top of ACE or ARB treatment in patients with CKD. The trial started at November 2018 and is to be completed in June 2022. The primary outcome is kidney disease progression or cardiovascular death. The study is expected to add information on the cardiovascular effects of SGLT-2 inhibitors in patients with CKD.105 GLP-1 agonists liraglutide106 and semaglutide107 have also shown a significant improvement in cardiovascular outcomes of patients with T2DM and were accompanied by a more significant decrease in body weight and a less important drop in BP in the absence of natriuretic effects. This last point could explain the absence of effects on heart failure.108 Liraglutide also exhibited a renal protective capacity through a significant decrease in albuminuria.108
Endothelin receptor antagonistsEndothelin receptor antagonists reduce albuminuria and BP but can also cause sodium retention. The previous trial with a non-selective endothelin receptor antagonist avosentan in patients with diabetes and CKD was stopped prematurely because of an increased incidence of heart failure.109 By contrast, short-term treatment with low doses of the more selective endothelin A receptor antagonist atrasentan reduced albuminuria without causing significant fluid retention.110 More recently, the Study of Diabetic Nephropathy with Atrasentan (SONAR) was published and showed a reduction in the risk of renal events with no significant differences for hospitalization for heart failure and also mortality compared with placebo.111
Statins and fibratesDifferent meta-analyses showed reductions of albuminuria, proteinuria, and clinical deaths with statins.112,113 Statins showed positive results in cardiovascular events even in low-moderate cardiovascular risk populations, independently of the presence of hyperlipidemia.114 Furthermore, treatment with rosuvastatin at a dose of 10mg per day resulted in a significantly lower risk of cardiovascular events than placebo in an intermediate-risk, ethnically diverse population without cardiovascular disease.115 Fibrates showed also a reduction in albuminuria.116
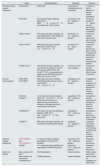
A summary of the new therapeutic strategies to manage the HMOD are shown in Table 1.
A summary of the new therapeutic strategies to manage the HMOD.
| Study | Model/population | Treatment | Outcome | |
|---|---|---|---|---|
| Mineralocorticoid receptor antagonists | Experimental | DOCA rats95 | Finerenone vs placeboEplerenone vs placebo | Finerenone, but not eplerenone, reduces cardiac hypertrophy |
| ARTS-DN97 | 812 adults with type 2 diabetes, albuminuria, eGFR>30mL/min/1.73m2, and treated with a RAS blocker | Finerenone 1.25–20mg/day vs placebo | Finerenone dosage of >5mg/day reduces albuminuria after 90 days of treatment | |
| FIDELIO-DKD98 | 5674 adults with type 2 diabetes and CKD treated with an ACE inhibitor or ARB at the maximum dose | Finerenone 10–20mg/day vs placebo | Finerenone reduces albuminuria after 4 months of treatment | |
| FIDELIO-DKD99 | 5658 adults with type 2 diabetes, albuminuria, eGFR ≥25 to <75mL/min/1.73m2 | Finerenone 10–20mg vs placebo | Finerenone reduces albuminuria after 4 months of treatment in patients with and without history of cardiovascular disease | |
| FIGARO-DKD100 | 7352 adults with type 2 diabetes, and urine albumin-to-creatinine ratio 30-300 mg/g and eGFR ≥25 to ≤90 mL/min/1.73 m2, or urine albumin-to-creatinine ratio 300-5000 mg/g and eGFR ≥60 mL/min/1.73 m2 | Finerenone 10-20 mg vs placebo | Finerenone reduces albuminuria after 4 months of treatment | |
| Glucose-lowering agents | EMPA-REG OUTCOME101 | 7020 adults with type 2 diabetes, established cardiovascular disease, and an eGFR ≥30mL/min/1.73m2 | Empagliflozin 10–25mg/day vs placebo | Empagliflozin reduces progression or incidence of albuminuria after 48 months of treatment |
| CANVAS Program102 | 10,142 adults with type 2 diabetes, eGFR ≥30mL/min/1.73m2, and ≥30 years old and history of atherosclerotic cardiovascular disease, or ≥50 years old and ≥2 cardiovascular risk factors | Canagliflozin 100–300mg/day vs placebo | Canagliflozin reduces progression of albuminuria after 338 weeks of treatment | |
| CREDENCE103 | 4401 adults ≥30 years old with type 2 diabetes, eGFR ≥30 to <90mL/min/1.73m2, and albuminuria | Canagliflozin 100mg/day vs placebo | Canagliflozin reduces albuminuria after 6 months of treatment | |
| LEADER108 | 9340 adults with type 2 diabetes and a high risk of cardiovascular disease | Liraglutide 1.8mg/day vs placebo | Liraglutide reduces the progression and incidence of albuminuria after 12 months of treatment | |
| Edothelin receptor antagonists | NCT01356849 and NCT01424319110 | 211 adults with type 2 diabetes, nephropathy, and under RAS blockade | Atrasentan 0.75–1.25mg/day vs placebo | Atrasentan reduces albuminuria |
| SONAR111 | 4711 adults with type 2 diabetes and nephropathy, under RAS blockade | Atrasentan 0.75mg/day vs placebo | Atrasentan reduces albuminuria | |
| Statins and fibrates | Meta-analysis of 23 trials113 | 39,419 participants | 8 types of statins | Statins reduce albuminuria |
| Meta-analysis of 8 randomized controlled trials116 | 16,869 participants | 3 types of fibrates | Statins reduce the progression of albuminuria | |
DOCA: deoxycorticosterone acetate; ARTS-DN: MinerAlocorticoid Receptor Antagonist Tolerability Study Diabetic Nephropathy; eGFR: estimated glomerular filtration rate; RAS: renin angiotensin system; FIDELIO-DKD: Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease; CKD: chronic kidney disease; EMPA-REG OUTCOME: Empagliflozin Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial; CANVAS: CANagliflozin cardioVascular Assessment Study; CREDENCE: Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy study; LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; SONAR: Study of Diabetic Nephropathy with Atrasentan.
HMOD progresses more rapidly and severely in high cardiovascular risk patients compared with patients at low/moderate cardiovascular risk. Cardiovascular risk assessment in clinical practice is mostly based on risk estimators, such as Framingham risk score and SCORE. However, cardiovascular risk estimators frequently underestimate the real cardiovascular risk because their algotrithms do not include the presence of organ damage, which reclassifies low/moderate risk patients to high risk. Guidelines support the use of different routine tests (see Fig. 1) and non-routine tests to assess organ damage,19 although the possibility of performing a non-routine test depends on the suspicion skills of physicians and on the coverage of health care systems. The main problem of this approach is that subclinical organ damage is asymptomatic and the majority of patients are middle aged subjects without a correct diagnosis of HMOD, who consequently do not receive adequate treatment and usually do not return to a medical office in many years. Therefore, we suggest adding an echocardiogram as a routine test to better specify cardiac structure and the presence of LVH in the initial approach and annually in patients with adequate BP control (Fig. 1).
Although there are no available studies designed to focus on organ damage regression and cardiovascular mortality as the main outcome, post hoc analyses of previous studies showed that lowering BP improved kidney function, and reduced LVH and CIMT. In fact, intensive strategies to prevent recurrent cardiovascular events result in levels of oxidative stress similar to those of naïve subjects without hypertension and with normal levels of cholesterol.117 However, the impact of organ damage regression on cardiovascular mortality is still controversial. Despite a similar BP reduction after one year, LVH (9.2% vs 41.7%; P=0.001), fundus oculi advanced damage (0.99% vs 14.3%; P=0.001), high urine albumin excretion rate (0.3% vs 5.1%; P=0.005) and amount of target organ damage (9.2% vs 44.0%; P=0.001) were better controlled in patiens with low/moderate cardiovascular risk than in those with high/very high cardiovascular risk,118 suggesting that high cardiovascular risk patients still have residual organ damage after achieving BP control.119
PerspectivesThen, how can we reduce cardiovascular mortality? Different trials showed benefit with BP control and strategies of treatment with dual, triple antihypertensive drug combinations. It is interesting that in the SPRINT trial, a BP target of <120/80 reduced mortality in high cardiovascular risk patients at all ages.84 Moreover, in the same trial, cardiovascular events were reduced in patients with LVH or CKD in the intensive BP control group, independently of the organ damage regression (LVH or albuminuria). The question now is how we can translate this evidence into real life patients. We can conclude that adding tests to diagnose subclinical HMOD will optimize the traditional cardiovascular risk assessment and improve our therapeutic strategies. For the moment HMOD is not considered as a surrogate therapeutic target, but its regression can be used as a marker of optimal BP control, and strategies need to be elucidated to improve the rates of BP control.
FundingThis study was supported by grants from the National Institute of Health Carlos III [PI17/01093, PI17/01193, PI20/00763, CP15/00129, CPII20/00022, FI18/00261] and co-funded by the European Regional Development Fund (Fondos FEDER).
Conflict of interestNone to be declared.
This work was mainly supported by projects from the Instituto de Salud Carlos III [PI17/01093, PI17/01193, PI20/00763, CP15/00129, CPII20/00022, FI18/00261] and co-funded by the European Regional Development Fund (Fondos FEDER).