Guselkumab, one of the several monoclonal antibodies targeting Interleukin-23 (IL-23), was approved in 2017 for the treatment of psoriasis. Guselkumab is generally regarded as a safe agent with mostly mild adverse effects. While the well-known adverse events include infections, headaches, and diarrhea, a recent case publication reported a focal segmental glomerulosclerosis (FSGS) associated with guselkumab in a patient with psoriasis.1,2
Drug-induced nephrotoxicity is most commonly associated with tubular injury, but glomerular injury is also not uncommon.3 Commonly known medications causing nephrotic syndrome are nonsteroidal anti-inflammatory drugs (NSAIDs), but there are also some data with regard to pamidronate, lithium, sirolimus, and monoclonal antibodies including denosumab and bevacizumab.4,5
We present a 57-year-old female with severe psoriasis. Her medical history includes diabetes mellitus and hypertension for 10 years. Previous treatments included conventional systemic agents and multiple biologics, but with inadequate outcomes, guselkumab was initiated.
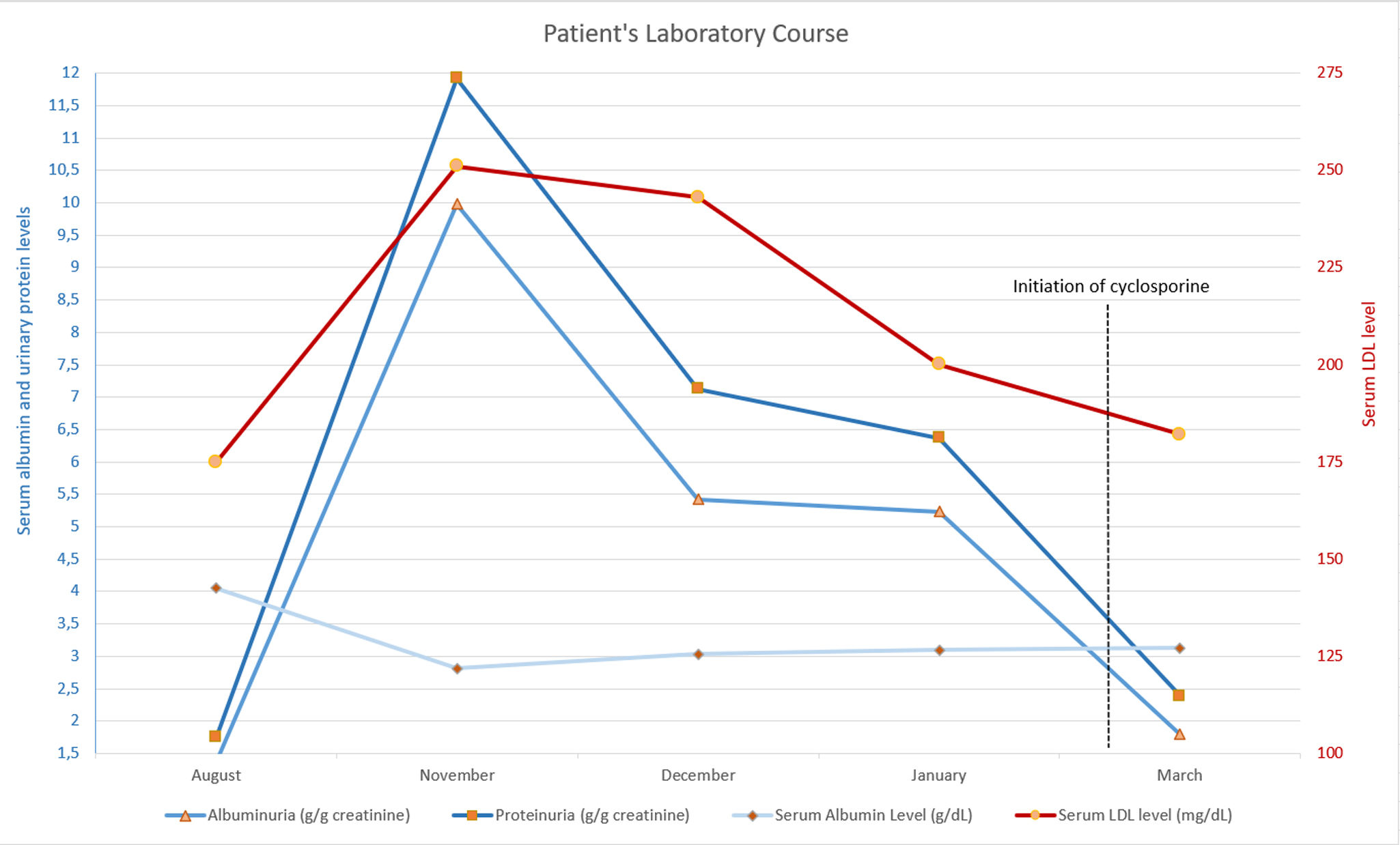
Routine pre-biological therapy recommendations were followed. Baseline urinalysis showed no proteinuria. However, after two maintenance doses (∼3 months), urinalysis revealed ‘++’ dipstick proteinuria. Spot urine protein/creatinine ratio was 1755mg/g. She was already on empagliflozin 25mg for her diabetes and 40mg olmesartan for her antihypertensive therapy. One month later, proteinuria rose to 11,917mg/g, with 9980mg as albuminuria. Serum albumin level decreased to 2.81g/dL, and low-density lipoprotein (LDL) was 251mg/dL. Despite normal blood pressure and no peripheral edema, the patient was diagnosed with nephrotic syndrome.
The etiology for nephrotic syndrome was investigated. She had an HbA1c level of 9.9% which was similar (10.3%) after 2 months of guselkumab. Multiple myeloma was ruled out via serum protein electrophoresis. Negative anti-nuclear antibody (ANA) and anti-neutrophilic-cytoplasmic antibody (ANCA) tests excluded vasculitis and systemic lupus erythematosus. Based on the data showing guselkumab-associated FSGS in recent literature, guselkumab therapy was discontinued. One month later, 24-h proteinuria was 7127mg/day, with albuminuria at 5425mg/day. Persisting proteinuria led to a kidney biopsy.
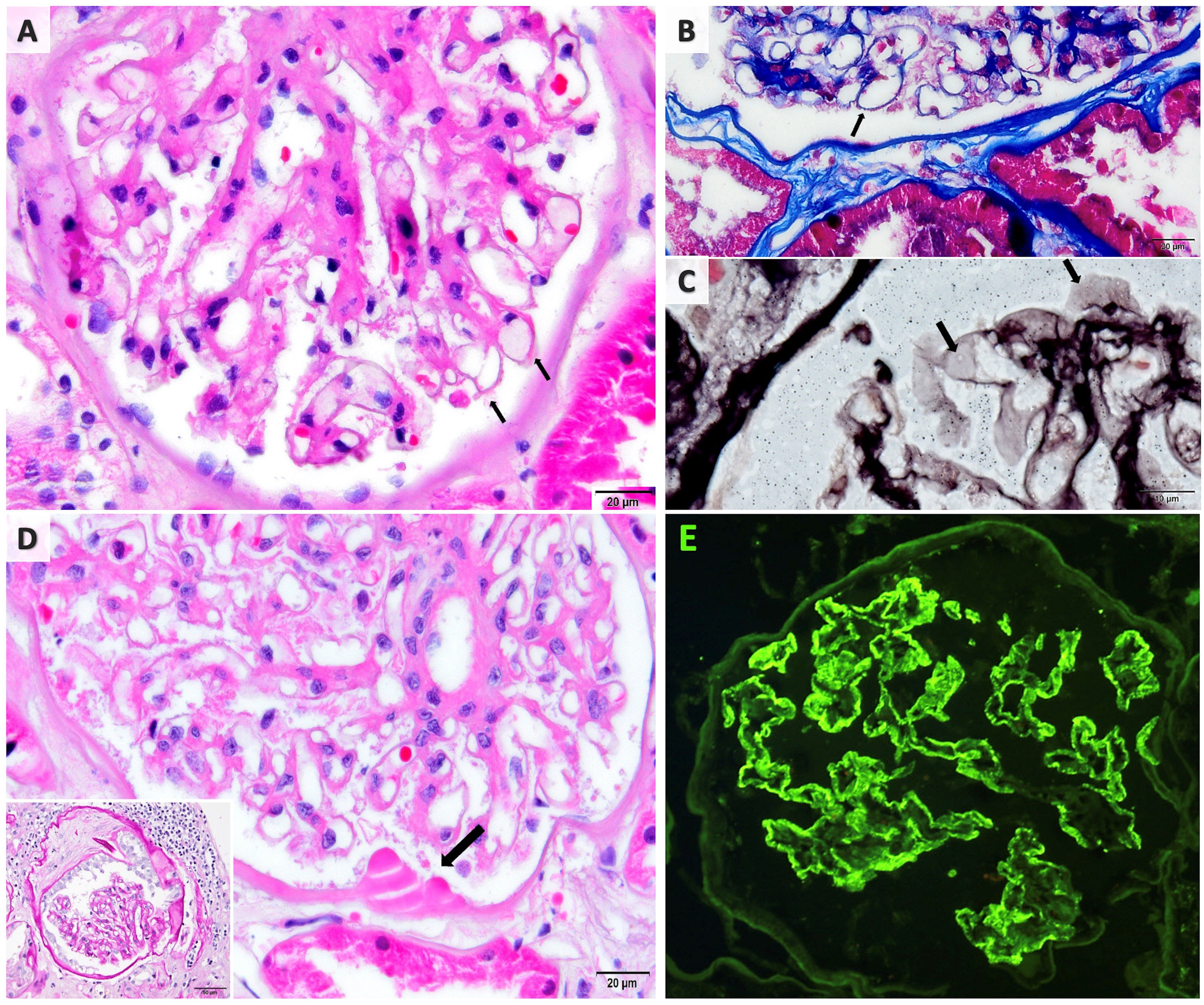
Renal biopsy revealed membranous nephropathy with diffuse IgG deposits and no proliferative or vasculitic changes. Since IgG subclass analysis was not available, biopsy couldn’t determine the predominant IgG type. Only mild diabetic and hypertensive alterations were noted including Bowman capsule duplication and hyaline droplets, as well as mild arteriolar hyalinosis (Fig. 1).
The kidney biopsy findings. (A) Glomerulus demonstrating thickened capillary walls (H&E). (B) Fucsinophilic deposits barely seen on capillary walls (MTC stain). (C) Scarce glomerular wall vacuolization (JMS stain). (D) Diabetic changes; hyaline droplets on Bowman capsule (arrow; H&E) and capsule duplication (PAS stain; inset). (E) Immunoflorescence microscopy showing diffuse fine granular IgG deposition along the glomerular capillary wall.
Secondary cause screening showed no malignancy. Serum phospholipase A2 receptor antibodies were negative. The discontinuation of guselkumab treatment led to a significant exacerbation of psoriatic lesions. Due to the worsening of psoriasis lesions, departments of dermatology and nephrology jointly initiated cyclosporine 100mg twice daily and methylprednisolone 8mg. After 2 weeks of cyclosporine treatment, proteinuria decreased to non-nephrotic levels (2385mg/g creatinine).
This is an another case of guselkumab-associated nephrotic syndrome. The first case was an FSGS.2 In that case, the patient had no disease apart from psoriasis and was managed with a low-dose angiotensin-converting enzyme inhibitor (ramipril 5mg). However, our case was more complex. In our case, there was a clear temporal relationship between guselkumab therapy and the onset of nephrotic syndrome. Moreover, proteinuria and LDL levels decreased, and serum albumin increased before cyclosporine therapy was initiated. Unfortunately, we couldn’t perform neural epidermal growth factor-like 1(NELL1) antigen testing and IgG subclass analysis due to technical limitations which could show an association with secondary conditions.6 Taken together, and especially considering that no other alternative explanation was identified, we consider guselkumab to be the most likely cause (Fig. 2).
Laboratory findings of nephrotic syndrome clinical course. Notice that before cyclosporine and methylprednisolone therapy, the proteinuria and serum LDL levels started to decrease and serum albumin levels started to increase significantly. Due to patient's psoriasis lesions, patient received a brief cylosporine therapy in March 2024.
Since IL-17 and IL-23 secreted by Th17 are pivotal in nephrotic syndrome and podocyte injury, it is unexpected for a drug suppressing this pathway to cause nephrotic syndrome.7 IL-17A overproduction drives the inflammation in psoriasis. Studies show that IL-23, a cytokine composed of p19 and p40 subunits, triggers IL-17A production and induces pro-inflammatory cytokines by activating Th17 cells.8 Guselkumab is an entirely human IgG1 lambda monoclonal antibody that binds the p19 subunit of IL-23, blocking its pathways. Studies also suggest that IL-23 does not regulate all cellular mechanisms of IL-17 production, such as its role in cardiometabolic syndrome and pathogen defense.9 Therefore, considering that IL-23 has a partial blocking effect on IL-17, it is reasonable that the role of IL-17 in the pathogenesis of nephrotic syndrome persists. In fact, this partial blockade may potentiate the effect of IL-17. Additionally, the patient's improvement with cyclosporine, another potent Th17 inhibitor, supports this hypothesis. However, it should be noted that cyclosporine also directly suppresses B cells alongside T cells.10
To validate this information, the long-term use of guselkumab in a large number of psoriasis patients is necessary. Although current data are insufficient to establish a causal link, we suggest that monitoring dipstick proteinuria may be beneficial in patients receiving guselkumab therapy.
Ethics statementThe patient gave informed consent for publishing this case.
Conflict of interestAuthors declare no conflict of interests for this article.
We must express our special thanks to Dr. Meral Uner for her contributions to this article, providing the view of pathology, and preparing the figure.