Chronic kidney disease morbimortality drives the development of new drugs. This work summarizes the evidence on the use of non-steroidal mineralocorticoid receptor antagonists on chronic kidney disease.
Materials and methodsA search was performed on PubMed and ClinicalTrials.gov using relevant keywords for clinical trials and observational studies, finished or ongoing, from 2019 to 2023.
ResultsFinerenone is currently approved for diabetic kidney disease with albuminuria, with results from two phase three trials, presenting satisfactory results on renal and cardiovascular outcomes and an appropriate safety profile, namely regarding potassium balance. Regarding nondiabetic kidney disease, combining sodium glucose transport protein-2 inhibitors and finerenone points toward reliable results, although these came from non-placebo-controlled trials. An ongoing phase three trial is assessing finerenone efficacy and safety on nondiabetic chronic kidney disease. Esaxerenone is approved for diabetic nephropathies and hypertension in Japan, based on two phase three clinical trials. These trials’ external validity is compromised, as they were only developed in Japan. Ocedurenone presents results from a phase two trial on managing refractory hypertension on chronic kidney disease. Apararenone is being investigated for diabetic kidney disease, with results from a phase two study, limited for only including Japanese patients.
ConclusionsWith this review we provide a global perspective on the available clinical results for these drugs to improve its evidence-based use.
La morbimortalidad de la enfermedad renal crónica impulsa el desarrollo de nuevos fármacos. Este trabajo resume la evidencia sobre el uso de antagonistas no esteroideos de receptores de mineralocorticoides en la enfermedad renal crónica.
Materiales y métodosSe realizó una búsqueda en PubMed y ClinicalTrials.gov utilizando palabras clave relevantes para ensayos clínicos y estudios observacionales, finalizados o en curso, de 2019 a 2023.
ResultadosLa finerenona está aprobada para la enfermedad renal diabética con albuminuria. En 2 ensayos de fase iii se presentan resultados renales, cardiovasculares y de seguridad (en concreto, en lo que respecta al equilibrio de potasio) satisfactorios. En cuanto a la enfermedad renal no diabética, la combinación de inhibidores de la proteína 2 transportadora de glucosa y sodio y finerenona apunta a resultados confiables, aunque estos provienen de ensayos no controlados. Un ensayo de fase iii en curso está evaluando la eficacia y seguridad de la finerenona en la enfermedad renal crónica no diabética. La esaxerenona está aprobada para las nefropatías diabéticas y la hipertensión en Japón, basada en 2 ensayos clínicos de fase iii. La ocedurenona cuenta con resultados favorables (ensayo de fase ii) en el manejo de la hipertensión refractaria en la enfermedad renal crónica. Por último, se está investigando la aplicación de la apararenona en la enfermedad renal diabética (estudio de fase ii en pacientes japoneses).
ConclusiónCon esta revisión proporcionamos una perspectiva global sobre los resultados clínicos disponibles de estos fármacos para mejorar su uso basada en la evidencia.
Chronic kidney disease (CKD) is defined as the presence of abnormalities on kidney structure or function for more than three months, having implications for health. Risk factors include hypertension and diabetes. CKD prognosis is based on estimated glomerular filtration rate (eGFR) and albuminuria.1 CKD affects approximately 13% of the global population, with important morbimortality by cardiovascular causes or end-stage kidney disease progression.2,3
Following primary renal lesion there is an inflammatory response mediated by vasoactive hormones, growth factors and cytokines, leading to hyperfiltration and hypertrophy of undamaged nephrons. This increase in function can compensate the loss of function but, on the long term, it becomes maladaptive.4
One important molecular mechanism involved is mineralocorticoid receptor (MR) overactivation.5 MR is a transcription factor from the steroid receptor family, expressed in the epithelium of distal nephrons, podocytes, mesangial cells, endothelial cells, smooth muscle cells, fibroblasts, macrophages, and T cells.6 Its agonists are the steroidal hormones aldosterone and cortisol. Cortisol-dependent activation is the main mechanism of MR activation on cardiomyocytes, podocytes, and macrophages. The MR functions include tissue repair and fluid, electrolyte and hemodynamic homeostasis.7 On the epithelium of distal nephrons its activation regulates blood pressure (BP), sodium retention and potassium secretion.6,8 The non-epithelial role of MR is different, as it is thought to promote inflammation, collagen formation, fibrosis and necrosis.5 Furthermore, diabetes, hypertension and heavy proteinuria are thought to promote MR expression.6 Patients treated with RAAS inhibitor may present with a serum aldosterone increase, known as the aldosterone breakthrough phenomenon, which may further contribute to the overactivation of MR.7,8 Promoting inflammation and fibrosis, MR could be an important target on CKD.
The mineralocorticoid receptor antagonists (MRA) can be categorized according to its chemical properties in steroidal (sMRA) (spironolactone and eplerenone) and nonsteroidal (nsMRA) (finerenone, esaxerenone, apararenone, ocedurenone).
When comparing finerenone with sMRA, finerenone does not have active metabolites, has a short half-life (2–3h) in patients with kidney compromise, and strongly inhibits inflammation and fibrosis (compared to an equi-natriuretic dose of eplerenone). Besides, it is a potent and selective agent that works as an inverse passive antagonist, which means it inhibits cofactor binding even in the absence of aldosterone, whilst sMRA act as partial agonists.7
Finerenone has an equipoised distribution between heart and kidney, and it does not cross the blood-brain barrier. Globally, there is a more marked inhibition of pro-fibrotic and pro-inflammatory markers on renal and cardiac environment when compared to sMRA.7,5,9
The KDIGO 2022 Clinical Practice Guidelines for Diabetes Management in Chronic Kidney Disease advocates reducing the risk of kidney disease progression and CV disease through the recommendation of pharmacological and non-pharmacological interventions. The nsMRA, namely finerenone, is clinically approved on patients with T2D and persistent albuminuria (UACR>30mg/g and eGFR≥25mL/min/1.73m2), notwithstanding treatment with maximum tolerated dose of RAAS inhibitor, insofar as the serum potassium levels are <4.8mg/dL.10 Finerenone therapy is discouraged on patients with basal eGFR<25mL/min/1.73m2 and should be restrained in patients who develop end stage kidney disease (ESKD) – defined as an eGFR<15mL/min/1.73m2 – during treatment.
Regarding practical management issues, patients should initiate finerenone on different doses depending on basal kidney function – 10mg/od for eGFR≥25 to <60mL/min/1.73m2 or 20mg/od for eGFR≥60mL/min/1.73m2. Finerenone is not advised when basal serum potassium is >5mg/dL. If serum potassium is between 4.8 and 5mg/dL, clinicians can begin finerenone, but additional potassium screening is mandatory, through the first month of treatment.
Monitoring eGFR and potassium parameters is essential as it influences the clinical decision to either escalate, maintain or de-escalate the dose or withhold the treatment. Patients with serum potassium≤4.8mg/dL whom eGFR has not decreased>30%, either keep or begin 20mg/od, the latter in the case they had started with 10mg/od. Patients with serum potassium of 4.8–5.5mg/dL should not change dose. Patients with hyperkalemia (>5.5mg/dL) should withhold treatment and be treated for this electrolyte imbalance accordingly. When serum potassium reverts to <5mg/dL, finerenone 10mg daily can be reintroduced.11
Materials & methodsA comprehensive literature search was performed on December 2023 using the Pubmed database with the following key concepts: ((“Mineralocorticoid Receptor Antagonists”[Mesh] AND (“nonsteroidal” OR “non steroidal” OR “non-steroidal”)) OR “non steroidal mineralocorticoid receptor antagonist” OR “nonsteroidal mineralocorticoid receptor antagonist” OR “non-steroidal mineralocorticoid receptor antagonist” OR “finerenone” OR “kerendia” OR “BAY 94-8862” OR “esaxerenone” OR “apararenone” OR “ocedurenone”) AND (“Renal Insufficiency, Chronic”[Mesh] OR CKD OR “chronic kidney disease” OR “kidney disease” OR “kidney failure” OR CKF OR “chronic kidney failure” OR “renal failure” OR CRF OR CRD OR “chronic renal disease” OR “proteinuria” OR “albuminuria” OR “urine albumin” OR “urine protein” OR “microalbuminuria” OR “macroalbuminuria”). The search was restricted to the last five years (2019–2023) to clinical trials and observational studies.
To summarize the latest ongoing clinical trials regarding nsMRA, we searched for Finerenone, Esaxerenone, Apararenone and Ocedurenone, on chronic kidney disease, on ClinicalTrials.gov, in December 2023.
Some of the articles from the Pubmed search regarding pharmacokinetics, pharmacodynamics and mineralocorticoid receptor (MR) pathophysiology were considered, as well as some publications cited on relevant articles.
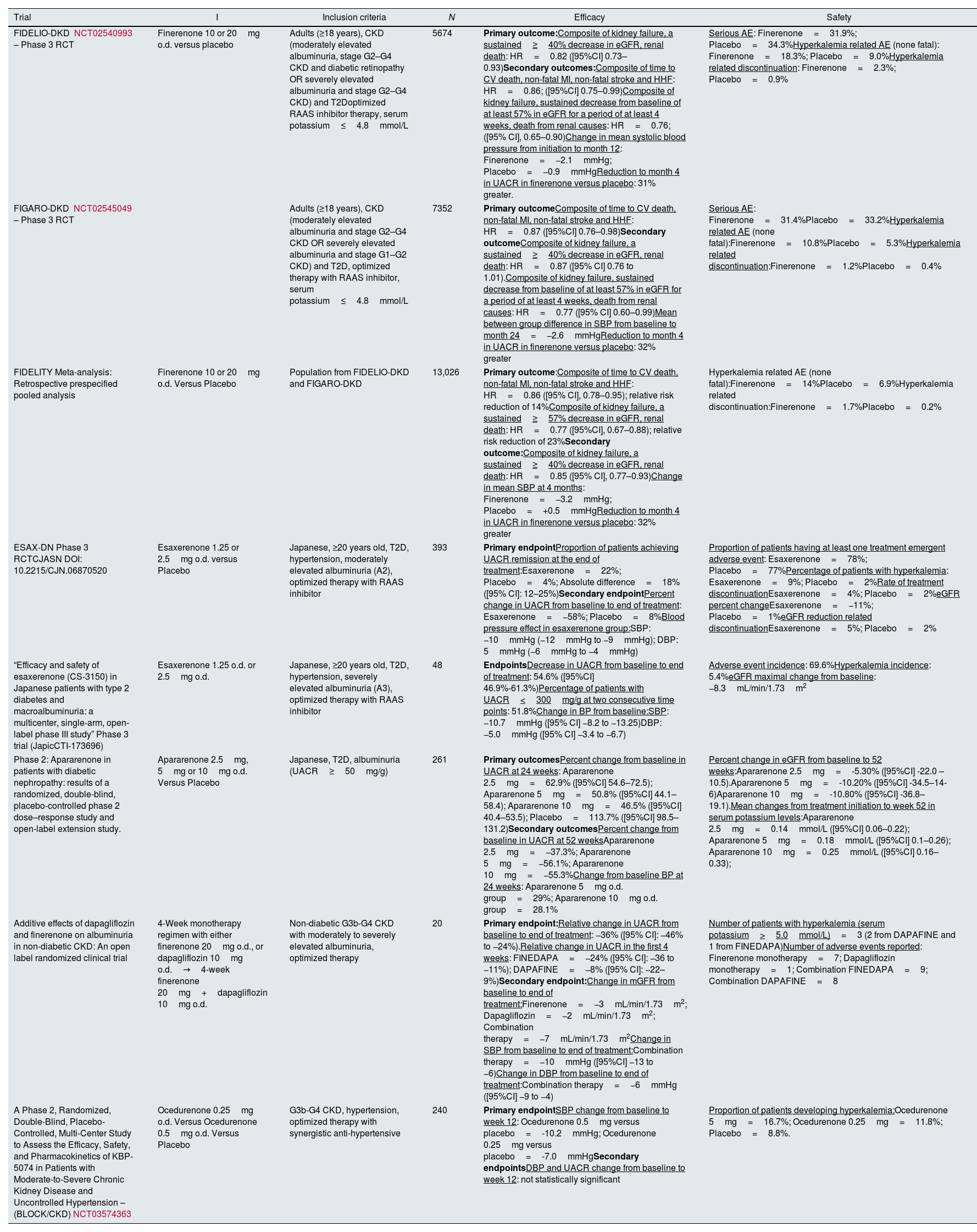
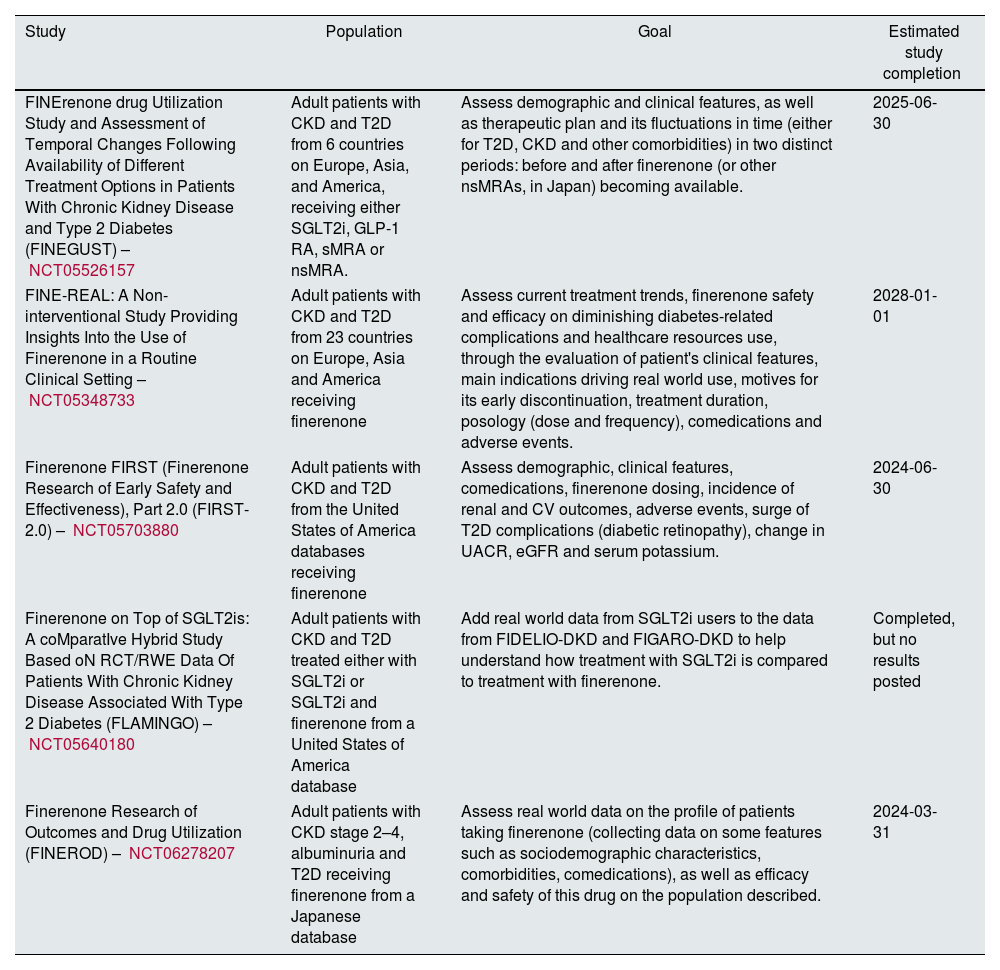
Results and discussionThe results from either ongoing or finished clinical trials on nonsteroidal mineralocorticoid receptor antagonists are summarized in Tables 1 and 2, respectively. The observational studies gathered are presented in Table 3.
Ongoing trials on nonsteroidal mineralocorticoid receptor antagonists.
| Drug | Trial | Phase | Population | Goal | Estimated trial completion |
|---|---|---|---|---|---|
| Finerenone | A Prospective, Interventional, Multicenter, Phase IV, Open-label, Single Arm Study to Assess the Safety and Effectiveness of Finerenone in Participants From India With Chronic Kidney Disease Associated With Type 2 DiabetesNCT05705271 | 4 | Adult patients with CKD and T2D from India. Excludes patients with HbA1c>12%, pregnant or nursing women, and patients co medicated with certain medications, such as MRA and medications interfering with serum potassium. | Assess the safety and efficacy of finerenone treatment through the analysis of adverse events and its impact; time to first occurrence of a composite kidney and CV outcome and the change in UACR. | 2025-07-24 |
| The Feasibility of Remote Clinical Trial Conduct in Patients With Diabetes and Elevated Albuminuria, Individual Responses to Empagliflozin, and Whether Suboptimal Treatment Responses Can be Overcome by the Addition or Switch With Finerenone (Optimize@Home) NCT06094920 | 4 | Adult participants with T2D, UACR 40mg/g and ≤2655mg/g, eGFR≥25mL/min/1.73m2 on optimized RAAS inhibitor treatment from primary or secondary healthcare settings. Excludes pregnant and breastfeeding women. | Assess the possibility of conducting a remote clinical trial in this population.Figure the individual response to empagliflozin in UACR, SBP, body weight, eGFR and plasma glucose. Understand the impact of adding or replacing empagliflozin with finerenone in overcoming patients not optimally controlled. | 2024-10-01 | |
| COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with CKD and type 2 diabetes using a UACR Endpoint study (CONFIDENCE) NCT05254002 | 2 | Adult participants with T2D, UACR 300-5000mg/g (A3), eGFR 30–89mL/min/1.73m2 (G2-G3b). | Assess the superiority of combination therapy with finerenone and empagliflozin on reducing UACR, when compared to monotherapy with either one of them, based on change in UACR. | 2025-02-28 | |
| The Effects of Finerenone on Vascular Stiffness and Cardiorenal Biomarkers in Type 2 Diabetes and Chronic Kidney Disease (FIVE-STAR)NCT05887817 | 4 | Patients from FIDELIO-DKD and FIGARO-DKD with UACR up to 3500mg/g. | Further understand finerenone's mechanisms, through the evaluation of CAVI, and assessing long-term effects, through cardiorenal biomarkers (UACR and BP). | 2026-07-31 | |
| A Trial to Learn How Well Finerenone Works and How Safe it is in Adult Participants With Non-diabetic Chronic Kidney Disease (FIND-CKD)NCT05047263 | 3 | Adult participants with non-diabetic G2-G4 CKD and moderately to severely elevated albuminuria, optimized RAAS inhibitor therapy, serum potassium≤4.8mmol/L. | Assess the efficacy and safety of finerenone on top of standard of care for non-DKD on adults, through the evaluation of mean rate of change on eGFR, time to either kidney failure, sustained eGFR decline≥57%, HHF or CV death, and number of participants with adverse events. | 2026-02-09 | |
| A Parallel-group, Randomized, Prospective, Interventional, Double-blind, Multicenter Global Phase 3 Study to Investigate the Efficacy and Safety of Finerenone Versus Placebo, in Addition to Standard of Care, in Participants With Chronic Kidney Disease and Type 1 Diabetes” FINE-ONE NCT05901831 | 3 | Adult participants with G2-G4 CKD, T1D and moderately to severely elevated albuminuria on optimized RAAS inhibitor therapy with serum potassium≤4.8mmol/L. | Assess the efficacy and safety of finerenone on top of standard of care for CKD and T1D, mainly through measuring UACR change. | 2025-10-02 | |
| A 6-month Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy, Safety and PK/PD of an age-and Body Weight-adjusted Oral Finerenone Regimen, in Addition to an ACEI or ARB, for the Treatment of Children, 6 Months to <18 Years of Age, With Chronic Kidney Disease and ProteinuriaFIONANCT05196035 | 3 | Children aged from 6 months to 17 years with G1-G3b CKD and proteinuria, serum potassium<5.0mmol/L if ≥2 years of age, or 5.3mmol/L if <2 years of age. | Assess the efficacy and safety of finerenone on a pediatric population with CKD, through the evaluation of UPCR reduction and reported adverse events. | 2027-03-11 | |
| An 18-month, Open-label, Single-arm Safety Extension Study of an age-and Bodyweight-adjusted Oral Finerenone Regimen, in Addition to an ACEI or ARB, for the Treatment of Children and Young Adults From 1 to 18 Years of Age With Chronic Kidney Disease and ProteinuriaFIONA-OLENCT05457283 | 3 safety extension study | Patients from the FIONA trial aged between 1 and 19 years old. | Assess the safety of finerenone use on a pediatric population with CKD, through the evaluation of number of patients with treatment emergent adverse events, changes in serum potassium and SBP. Secondly, to also validate its efficacy further, it will evaluate changes in UACR, UPCR and eGFR. | 2028-09-10 | |
| The EFfect of FinErenone in Kidney TransplantiOn Recipients: The EFFEKTOR Study EFFEKTORNCT06059664 | 2 | Adult participants who are KTR, in the 1–10 years after the transplant, stable kidney allograft function and albuminuria. | Assess the safety, efficacy, and feasibility of finerenone on KTR through the evaluation of total number of patients eligible, time on the intervention, percentage of discontinuation, percentage of adverse events, UACR reduction and relative risk of CHF. | 2025-12 | |
| Ocedurenone | A Phase 3 Randomized Double-Blind Placebo-Controlled Multicenter Study to Assess the Efficacy and Safety of KBP-5074 Mineralocorticoid Receptor Antagonist in Subjects With Uncontrolled Hypertension and Moderate or Severe (Stage 3b/4) CKD CLARION-CKD NCT04968184 | 3 | Adult participants with stage 3/4b CKD and uncontrolled hypertension (SBP≥140 and <180mm Hg) on therapy with ≥2 antihypertensive drugs and BMI between 19 and 45kg/m2. Excludes patients with hyperkalemia and MRA treatment in the 4 weeks prior to screening. | Assess the efficacy, safety, and durability of ocedurenone on diminishing blood pressure and UACR. | 2025-01-15 |
Note. ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; BP, blood pressure; CAVI, cardio ankle vascular index; CHF, congestive heart failure; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; HHF, hospitalization for heart failure; KTR, kidney transplant recipient; MRA, mineralocorticoid receptor antagonist; non-DKD, non-diabetic kidney disease; nsMRA, nonsteroidal mineralocorticoid receptor antagonist; PD, pharmacodynamics; PK, pharmacokinetics; RAAS, renin–angiotensin–aldosterone system; SBP, systolic blood pressure; SGLT2i, sodium glucose transport protein 2 inhibitor; sMRA, steroidal mineralocorticoid receptor antagonist; T1D, type 1 diabetes; T2D, type 2 diabetes; UACR, urinary albumin to creatinine ratio; UPCR, urinary protein to creatinine ratio.
Finished trials on nonsteroidal mineralocorticoid receptor antagonists.
| Trial | I | Inclusion criteria | N | Efficacy | Safety |
|---|---|---|---|---|---|
| FIDELIO-DKD NCT02540993 – Phase 3 RCT | Finerenone 10 or 20mg o.d. versus placebo | Adults (≥18 years), CKD (moderately elevated albuminuria, stage G2–G4 CKD and diabetic retinopathy OR severely elevated albuminuria and stage G2–G4 CKD) and T2Doptimized RAAS inhibitor therapy, serum potassium≤4.8mmol/L | 5674 | Primary outcome:Composite of kidney failure, a sustained≥40% decrease in eGFR, renal death: HR=0.82 ([95%CI] 0.73–0.93)Secondary outcomes:Composite of time to CV death, non-fatal MI, non-fatal stroke and HHF: HR=0.86; ([95%CI] 0.75–0.99)Composite of kidney failure, sustained decrease from baseline of at least 57% in eGFR for a period of at least 4 weeks, death from renal causes: HR=0.76; ([95% CI], 0.65–0.90)Change in mean systolic blood pressure from initiation to month 12: Finerenone=−2.1mmHg; Placebo=−0.9mmHgReduction to month 4 in UACR in finerenone versus placebo: 31% greater. | Serious AE: Finerenone=31.9%; Placebo=34.3%Hyperkalemia related AE (none fatal): Finerenone=18.3%; Placebo=9.0%Hyperkalemia related discontinuation: Finerenone=2.3%; Placebo=0.9% |
| FIGARO-DKD NCT02545049 – Phase 3 RCT | Adults (≥18 years), CKD (moderately elevated albuminuria and stage G2–G4 CKD OR severely elevated albuminuria and stage G1–G2 CKD) and T2D, optimized therapy with RAAS inhibitor, serum potassium≤4.8mmol/L | 7352 | Primary outcomeComposite of time to CV death, non-fatal MI, non-fatal stroke and HHF: HR=0.87 ([95%CI] 0.76–0.98)Secondary outcomeComposite of kidney failure, a sustained≥40% decrease in eGFR, renal death: HR=0.87 ([95% CI] 0.76 to 1.01).Composite of kidney failure, sustained decrease from baseline of at least 57% in eGFR for a period of at least 4 weeks, death from renal causes: HR=0.77 ([95% CI] 0.60–0.99)Mean between group difference in SBP from baseline to month 24=−2.6mmHgReduction to month 4 in UACR in finerenone versus placebo: 32% greater | Serious AE: Finerenone=31.4%Placebo=33.2%Hyperkalemia related AE (none fatal):Finerenone=10.8%Placebo=5.3%Hyperkalemia related discontinuation:Finerenone=1.2%Placebo=0.4% | |
| FIDELITY Meta-analysis: Retrospective prespecified pooled analysis | Finerenone 10 or 20mg o.d. Versus Placebo | Population from FIDELIO-DKD and FIGARO-DKD | 13,026 | Primary outcome:Composite of time to CV death, non-fatal MI, non-fatal stroke and HHF: HR=0.86 ([95% CI], 0.78–0.95); relative risk reduction of 14%Composite of kidney failure, a sustained≥57% decrease in eGFR, renal death: HR=0.77 ([95%CI], 0.67–0.88); relative risk reduction of 23%Secondary outcome:Composite of kidney failure, a sustained≥40% decrease in eGFR, renal death: HR=0.85 ([95% CI], 0.77–0.93)Change in mean SBP at 4 months: Finerenone=−3.2mmHg; Placebo=+0.5mmHgReduction to month 4 in UACR in finerenone versus placebo: 32% greater | Hyperkalemia related AE (none fatal):Finerenone=14%Placebo=6.9%Hyperkalemia related discontinuation:Finerenone=1.7%Placebo=0.2% |
| ESAX-DN Phase 3 RCTCJASN DOI: 10.2215/CJN.06870520 | Esaxerenone 1.25 or 2.5mg o.d. versus Placebo | Japanese, ≥20 years old, T2D, hypertension, moderately elevated albuminuria (A2), optimized therapy with RAAS inhibitor | 393 | Primary endpointProportion of patients achieving UACR remission at the end of treatment:Esaxerenone=22%; Placebo=4%; Absolute difference=18% ([95% CI]: 12–25%)Secondary endpointPercent change in UACR from baseline to end of treatment: Esaxerenone=−58%; Placebo=8%Blood pressure effect in esaxerenone group:SBP: −10mmHg (−12mmHg to −9mmHg); DBP: 5mmHg (−6mmHg to −4mmHg) | Proportion of patients having at least one treatment emergent adverse event: Esaxerenone=78%; Placebo=77%Percentage of patients with hyperkalemia: Esaxerenone=9%; Placebo=2%Rate of treatment discontinuationEsaxerenone=4%; Placebo=2%eGFR percent changeEsaxerenone=−11%; Placebo=1%eGFR reduction related discontinuationEsaxerenone=5%; Placebo=2% |
| “Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study” Phase 3 trial (JapicCTI-173696) | Esaxerenone 1.25 o.d. or 2.5mg o.d. | Japanese, ≥20 years old, T2D, hypertension, severely elevated albuminuria (A3), optimized therapy with RAAS inhibitor | 48 | EndpointsDecrease in UACR from baseline to end of treatment: 54.6% ([95%CI] 46.9%-61.3%)Percentage of patients with UACR<300mg/g at two consecutive time points: 51.8%Change in BP from baseline:SBP: −10.7mmHg ([95% CI] −8.2 to −13.25)DBP: −5.0mmHg ([95% CI] −3.4 to −6.7) | Adverse event incidence: 69.6%Hyperkalemia incidence: 5.4%eGFR maximal change from baseline: −8.3mL/min/1.73m2 |
| Phase 2: Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose–response study and open-label extension study. | Apararenone 2.5mg, 5mg or 10mg o.d. Versus Placebo | Japanese, T2D, albuminuria (UACR≥50mg/g) | 261 | Primary outcomesPercent change from baseline in UACR at 24 weeks: Apararenone 2.5mg=62.9% ([95%CI] 54.6–72.5); Apararenone 5mg=50.8% ([95%CI] 44.1–58.4); Apararenone 10mg=46.5% ([95%CI] 40.4–53.5); Placebo=113.7% ([95%CI] 98.5–131.2)Secondary outcomesPercent change from baseline in UACR at 52 weeksApararenone 2.5mg=−37.3%; Apararenone 5mg=−56.1%; Apararenone 10mg=−55.3%Change from baseline BP at 24 weeks: Apararenone 5mg o.d. group=29%; Apararenone 10mg o.d. group=28.1% | Percent change in eGFR from baseline to 52 weeks:Apararenone 2.5mg=-5.30% ([95%CI] -22.0 – 10.5).Apararenone 5mg=-10.20% ([95%CI] -34.5–14-6)Apararenone 10mg=-10.80% ([95%CI] -36.8–19.1).Mean changes from treatment initiation to week 52 in serum potassium levels:Apararenone 2.5mg=0.14mmol/L ([95%CI] 0.06–0.22); Apararenone 5mg=0.18mmol/L ([95%CI] 0.1–0.26); Apararenone 10mg=0.25mmol/L ([95%CI] 0.16–0.33); |
| Additive effects of dapagliflozin and finerenone on albuminuria in non-diabetic CKD: An open label randomized clinical trial | 4-Week monotherapy regimen with either finerenone 20mg o.d., or dapagliflozin 10mg o.d.→4-week finerenone 20mg+dapagliflozin 10mg o.d. | Non-diabetic G3b-G4 CKD with moderately to severely elevated albuminuria, optimized therapy | 20 | Primary endpoint:Relative change in UACR from baseline to end of treatment: −36% ([95% CI]: −46% to −24%).Relative change in UACR in the first 4 weeks: FINEDAPA=−24% ([95% CI]: −36 to −11%); DAPAFINE=−8% ([95% CI]: −22–9%)Secondary endpoint:Change in mGFR from baseline to end of treatment:Finerenone=−3mL/min/1.73m2; Dapagliflozin=−2mL/min/1.73m2; Combination therapy=−7mL/min/1.73m2Change in SBP from baseline to end of treatment:Combination therapy=−10mmHg ([95%CI] −13 to −6)Change in DBP from baseline to end of treatment:Combination therapy=−6mmHg ([95%CI] −9 to −4) | Number of patients with hyperkalemia (serum potassium>5.0mmol/L)=3 (2 from DAPAFINE and 1 from FINEDAPA)Number of adverse events reported: Finerenone monotherapy=7; Dapagliflozin monotherapy=1; Combination FINEDAPA=9; Combination DAPAFINE=8 |
| A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Assess the Efficacy, Safety, and Pharmacokinetics of KBP-5074 in Patients with Moderate-to-Severe Chronic Kidney Disease and Uncontrolled Hypertension – (BLOCK/CKD)NCT03574363 | Ocedurenone 0.25mg o.d. Versus Ocedurenone 0.5mg o.d. Versus Placebo | G3b-G4 CKD, hypertension, optimized therapy with synergistic anti-hypertensive | 240 | Primary endpointSBP change from baseline to week 12: Ocedurenone 0.5mg versus placebo=-10.2mmHg; Ocedurenone 0.25mg versus placebo=-7.0mmHgSecondary endpointsDBP and UACR change from baseline to week 12: not statistically significant | Proportion of patients developing hyperkalemia:Ocedurenone 5mg=16.7%; Ocedurenone 0.25mg=11.8%; Placebo=8.8%. |
Note. I, intervention; N, number of randomized participants; AE, adverse events; CKD, chronic kidney disease; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HHF, hospitalization for heart failure; HR, hazard ratio; MI, myocardial infarction; RAAS, renin–angiotensin–aldosterone system; RCT, randomized controlled trial; SBP, systolic blood pressure; T2D, type 2 diabetes; UACR, urinary albumin to creatinine ratio.
Observational studies on nonsteroidal mineralocorticoid receptor antagonists.
| Study | Population | Goal | Estimated study completion |
|---|---|---|---|
| FINErenone drug Utilization Study and Assessment of Temporal Changes Following Availability of Different Treatment Options in Patients With Chronic Kidney Disease and Type 2 Diabetes (FINEGUST) – NCT05526157 | Adult patients with CKD and T2D from 6 countries on Europe, Asia, and America, receiving either SGLT2i, GLP-1 RA, sMRA or nsMRA. | Assess demographic and clinical features, as well as therapeutic plan and its fluctuations in time (either for T2D, CKD and other comorbidities) in two distinct periods: before and after finerenone (or other nsMRAs, in Japan) becoming available. | 2025-06-30 |
| FINE-REAL: A Non-interventional Study Providing Insights Into the Use of Finerenone in a Routine Clinical Setting – NCT05348733 | Adult patients with CKD and T2D from 23 countries on Europe, Asia and America receiving finerenone | Assess current treatment trends, finerenone safety and efficacy on diminishing diabetes-related complications and healthcare resources use, through the evaluation of patient's clinical features, main indications driving real world use, motives for its early discontinuation, treatment duration, posology (dose and frequency), comedications and adverse events. | 2028-01-01 |
| Finerenone FIRST (Finerenone Research of Early Safety and Effectiveness), Part 2.0 (FIRST-2.0) – NCT05703880 | Adult patients with CKD and T2D from the United States of America databases receiving finerenone | Assess demographic, clinical features, comedications, finerenone dosing, incidence of renal and CV outcomes, adverse events, surge of T2D complications (diabetic retinopathy), change in UACR, eGFR and serum potassium. | 2024-06-30 |
| Finerenone on Top of SGLT2is: A coMparatIve Hybrid Study Based oN RCT/RWE Data Of Patients With Chronic Kidney Disease Associated With Type 2 Diabetes (FLAMINGO) – NCT05640180 | Adult patients with CKD and T2D treated either with SGLT2i or SGLT2i and finerenone from a United States of America database | Add real world data from SGLT2i users to the data from FIDELIO-DKD and FIGARO-DKD to help understand how treatment with SGLT2i is compared to treatment with finerenone. | Completed, but no results posted |
| Finerenone Research of Outcomes and Drug Utilization (FINEROD) – NCT06278207 | Adult patients with CKD stage 2–4, albuminuria and T2D receiving finerenone from a Japanese database | Assess real world data on the profile of patients taking finerenone (collecting data on some features such as sociodemographic characteristics, comorbidities, comedications), as well as efficacy and safety of this drug on the population described. | 2024-03-31 |
Note. CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon like peptide-1 receptor agonist; nsMRA, nonsteroidal mineralocorticoid receptor antagonist; SGLT2i, sodium glucose transport protein 2 inhibitor; sMRA, steroidal mineralocorticoid receptor antagonist; T2D, type 2 diabetes; UACR, urinary albumin to creatinine ratio;
Finerenone usage on DKD was evaluated on two large randomized, double-blind, multicenter (48 countries), phase 3 placebo-controlled trials – Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD). These trials were designed similarly to combine their results on FInerenone in CKD and type 2 diabetes: combined FIDELIO-DKD and FIGARO-DKD Trial program analysis (FIDELITY) – a retrospective prespecified pooled analysis, endorsing their individual results and achieving statistical power to conclude on the efficacy of finerenone on the joined population.12–14
The two aimed at determining the efficacy and safety of finerenone compared to placebo on patients with T2D and CKD on top of an optimized therapeutic regimen, but on different outcomes: FIDELIO-DKD on renal outcomes and FIGARO-DKD on CV outcomes. Patients included were adults (≥18 years old), on a maximum tolerated dose of RAAS inhibitor, had T2D and a serum potassium ≤4.8mmol/L. It excluded patients with non-DKD; uncontrolled cardiovascular risk factors; pregnant and breastfeeding women; concomitant therapy that could interact with nsMRA and other comorbidities. A key difference on the pair was CKD definition: FIDELIO-DKD included patients with overall worse kidney function and more renal damage than FIGARO-DKD, with an overlapping, nonetheless. Patients on the intervention group received finerenone on an initial dose dependent on basal kidney function – 10mg/od to eGFR 25 to <60mL/min/1.73m2 or 20mg/od to eGFR≥60mL/min/1.73m2 – titrated to 20mg/od depending on serum potassium and eGFR. Patients on the control group received a placebo pill/od. Total study duration was longer on FIGARO-DKD, to be able to detect CV outcomes – 3.4 years vs 2.6 years. Statistical analysis included 5674 participants for FIDELIO-DKD and 7352 patients for FIGARO-DKD. In terms of efficacy, on FIDELIO-DKD, the primary outcome, a renal outcome, was a composite of kidney failure, a sustained ≥40% decrease in eGFR and renal death. The results showed a hazard ratio (HR) of 0.82 ([95%CI] 0.73–0.93) on the finerenone group vs the placebo group, consistent across pre-stated subgroups and statistically significant. On FIGARO-DKD, the primary outcome, a CV outcome, was a composite of time to CV death, non-fatal myocardial infarction (MI), non-fatal stroke and hospitalization for heart failure (HHF). The results showed a statistically significant lower incidence on finerenone group, with a hazard ratio (HR) of 0.87 ([95%CI] 0.76–0.98). The single outcome in which the reduction was more significant was HHF (HR 0.71 [95%CI] 0.56–0.90). There was no significant heterogeneity across prespecified groups. FIDELIO-DKD assessed the FIGARO-DKD's CV primary outcome as a secondary outcome, and the results were consistent with a HR of 0.86 ([95% CI] 0.75–0.99) on the finerenone group vs the placebo group. Of note, the incidence of individual outcomes was smaller in finerenone, but nonfatal stroke had an akin incidence on both groups. FIGARO-DKD assessed the FIDELIO-DKD's renal primary outcome and shown a lower incidence in the finerenone group versus placebo, although not statistically significant. Furthermore, both trials considered another key secondary outcome, a composite kidney outcome, composed of three items: first onset of kidney failure, sustained decrease from baseline of at least 57% in eGFR for a period of at least 4 weeks or death from renal causes. The results from FIDELIO-DKD showed a lower incidence on the finerenone group, with a HR of 0.76 ([95% CI] 0.65–0.90). The results from FIGARO-DKD pointed to a HR of 0.77 ([95% CI] 0.60–0.99), providing evidence on the efficacy of finerenone in lowering this composite outcome. Lastly, other outcomes measured on both trials were change in mean SBP and change in the UACR. In both FIDELIO-DKD and FIGARO-DKD there was a reduction in SBP. The researchers claim that this lowering effect on BP is not known to have an impact on hard clinical outcomes, showing that the benefits of finerenone are not necessarily related to its hypotensive effect. It also does not seem to be an inconvenience to use this drug on patients with low BP. The change in the UACR from baseline to month four in FIDELIO-DKD was 31% greater with finerenone than with placebo, and on the end of the trial the UACR was sustained lower on the finerenone's group. This same outcome on FIGARO-DKD showed results coincident with FIDELIO-DKD. Finerenone's safety was determined based on the adverse events reported. The results were similar, with a ratio of serious adverse events of about 30% on the finerenone groups on both studies, mainly hyperkalemia related. FIDELIO-DKD had a slightly bigger proportion of hyperkalemia-related adverse events, probably due to including patients with overall worse kidney function. Despite that, the incidence of hyperkalemia-related discontinuation was not concerning neither for FIDELIO-DKD – finerenone 2.3% and placebo 0.9% – nor for FIGARO-DKD – finerenone 1.2% and placebo 0.4% – and only a small percent of patients from FIGARO-DKD required hospitalization – 0.6% on finerenone and 0.1% on placebo.12,13 The incidence of severe hyperkalemia (>6mmol/L) was only 4.5% with finerenone versus 1.4% with placebo. No fatal events were reported. The studies’ protocol allowed the patients to maintain a normal diet and supplementation, not advising on specific measures to restrain potassium intake. The extent to which the results from these studies can be applied on real world are influenced by some limitations, as it did not include patients with non-albuminuric CKD, non-diabetic CKD and only a small percent of patients reported identifying themselves as black. Also, the study excluded pregnant women, children and teenagers, and ESKD patients, limiting the application of these results. 12,13
FIDELITY, the analysis joining the results from FIDELIO-DKD and FIGARO-DKD assessed two primary outcomes: the composite CV outcome (time to CV death, non-fatal MI, non-fatal stroke and HHF) and the composite renal outcome (kidney failure, renal death and a sustained ≥57% decrease in eGFR from baseline over ≥4weeks). The CV primary outcome was statistically significant, with a HR of 0.86 ([95% CI] 0.78–0.95), with a relative risk reduction on the primary CV outcome of 14%. The great reduction in single outcome was observed in the HHF, with a HR of 0.78 ([95%CI] 0.66–0.92) on finerenone versus placebo, meaning a relative risk reduction of 22%. The renal primary outcome was also statistically significant, with a 5.5% rate of events happening with finerenone versus 7.1% with placebo, proving a HR of 0.77 ([95%CI] 0.67–0.88) and a relative risk reduction of 23% with finerenone. Particularly, the relative risk of ESKD was reduced by 20%, the risk of kidney failure by 16%, and sustained decrease in eGFR to <15mL/min/1.73m2 by 19%, shedding light on the beneficial effects of finerenone on dreadful complications associated with CKD. Concerning other important results, the change in mean SBP at 4 months was −3.2mmHg on finerenone versus +0.5mmHg on placebo. The change in UACR from baseline to month four was 32% lower in finerenone than with placebo. Regarding safety outcomes, FIDELITY concluded that finerenone is safe. Despite a greater incidence of hyperkalemia related adverse events (14% on Finerenone vs. 6.9% on placebo), there was only a small incidence of permanent discontinuation (1.7% on finerenone vs. 0.6% on placebo), a modest incidence of hyperkalemia related hospitalization (0.9% on finerenone vs. 0.2% on placebo), and no fatal events. Concluding, this meta-analysis proved the clinical benefit of using finerenone on a vast spectrum of CKD in patients with T2D.14
One FIDELITY sub-analysis also assessed the same cardiovascular composite and kidney composite outcome on patients who were under concomitant therapy with SGLT2 inhibitors and finerenone. This analysis showed benefit for both composite outcomes, kidney and cardiovascular, irrespective of the SGL2i use.15 Furthermore, a network meta-analysis pooled data from 8 randomized controlled trials using SGLT2 inhibitors and finerenone and showed potential CV benefits with combination therapy.16 For better understanding of these positive outcomes the “COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with CKD and type 2 diabetes using a UACR Endpoint study” (CONFIDENCE – NCT05254002) is an ongoing phase 2 study aiming to demonstrate the superiority of combination therapy with finerenone and empagliflozin on reducing UACR when compared to monotherapy with either one of them on patients with T2D and CKD (KDIGO stage G2–G3 and UACR 300–5000mg/g). The primary outcomes assessed will be the relative change in UACR and mean ratio of change in UACR. Results are due February 2025.
“The Effects of Finerenone on Vascular Stiffness and Cardiorenal Biomarkers in Type 2 Diabetes and Chronic Kidney Disease” (FIVE-STAR – NCT05887817) trial aspires to deepen the knowledge on the mechanisms underlying the benefits of finerenone observed on both FIDELIO-DKD and FIGARO-DKD. Patients with T2D and CKD will be randomized to either finerenone or placebo for 24 weeks. The primary endpoint will be the change from baseline to 24 weeks after treatment initiation on cardio ankle vascular index (CAVI), which will be the parameter to assess vascular stiffness. Results are due to July 2026.
“The A Prospective, Interventional, Multicenter, Phase IV, Open-label, Single Arm Study to Assess the Safety and Effectiveness of Finerenone in Participants From India With Chronic Kidney Disease Associated With Type 2 Diabetes” (NCT05705271) is a phase 4 trial evaluating the long-term effects, mainly safety wise, of finerenone use on a population with CKD and T2D from India, estimated to be completed by July 2025.
“The Feasibility of Remote Clinical Trial Conduct in Patients With Diabetes and Elevated Albuminuria, Individual Responses to Empagliflozin, and Whether Suboptimal Treatment Responses Can be Overcome by the Addition or Switch With Finerenone” (Optimize@Home, NCT06094920) is a phase 4 trial with multiple goals. Firstly, it will assess the feasibility of conducting a remote clinical trial on a small population of adults with T2D and albuminuria. Secondly, it is going to assess the individual response to empagliflozin, part of the intervention treatment, in UACR, SBP, eGFR and plasma glucose. Lastly, it will evaluate the impact of either adding or replacing empagliflozin with finerenone on patients with refractory albuminuria. Results are due to October 2024.
Non-DKDA recent prospective, single center, open label randomized clinical trial gave insight on benefits of combination therapy with finerenone and dapagliflozin in reducing albuminuria in a population with non-DKD. A population of twenty non-DKD patients aged between 18–80 years old, UACR of 150–2000mg/g and an eGFR in-between 25 and 45mL/min/1.73m2, on maximum tolerated dose of RAAS inhibitor for at least four weeks, was gathered and studied. Patients were excluded based on presence of comorbidities, such as diabetes, autosomal dominant polycystic kidney disease, HFrEF (left ventricular ejection fraction<40%), poorly controlled hypertension (SBP>160mmHg and DBP>100mmHg), hyperkalemia (>4.5mmol/L) or hyponatremia, previous renal transplantation, conditions requiring immunosuppressants use, and patients on other SGLT2 inhibitors. They were randomized on a 1:1 manner to two arms: the FINEDAPA arm and the DAPAFINE arm. The differences between these groups laid in which medicine was started first: FINEDAPA had a 4-week monotherapy regimen with finerenone 20mg od, while DAPAFINE had the same period with dapagliflozin 10mg od. Following week 4, patients were treated with the combination therapy (finerenone 20mg od+dapagliflozin 10mg od), for a period of 4 weeks, completing a total trial duration of 8 weeks. There was no lost to follow up and there were no significant differences in baseline characteristics among participants. The primary endpoint was the relative change in UACR from baseline to end of treatment and it showed a notable change of −36% [95%CI: −46% to −24%]. When data from the first 4 weeks of treatment is analyzed, it depicts a greater reduction in UACR with finerenone compared to dapagliflozin [−24% ([95% CI] −36 to −11%) vs −8% ([95% CI] −22 to 9%)]. Secondary endpoints showed a significant difference on mean eGFR (mGFR) from baseline to end of treatment, of −3mL/min/1.73m2 with finerenone, −2mL/min/1.73m2 with dapagliflozin and −7mL/min/1.73m2 [95% CI −8 to −5] with combination therapy. These results are indicative that both SGLT2 inhibitors and nsMRAs can cause a reduction in mGFR, at least on the short term. Regarding safety, only three patients developed hyperkalemia (>5.0mmol/L). The raise in serum potassium was more noticeable when finerenone was added to dapagliflozin (DAPAFINE arm), which is incongruent with a theory that states that SGLT2 inhibitors may counteract the hyperkalemia related to nsMRA use. Both events were self-limiting. There were no reports of acute kidney injury. Only one adverse event, from the DAPAFINE arm, was considered serious. These results could be limited, considering the small sample size, short follow up period, absence of a placebo group, and the continuity between single and combination therapy.17
Larger studies to validate finerenone on a population with non-DKD are being developed. The “A Trial to Learn How Well Finerenone Works and How Safe it is in Adult Participants With Non-diabetic Chronic Kidney Disease (FIND-CKD)” (NCT05047263) is a randomized double-blind, placebo-controlled, multicenter (24 countries) phase 3 trial researching the efficacy and safety of finerenone on top of an optimized therapeutic regimen on the evolution of non-DKD. The investigators estimate to gather a population of about 1580 adults (≥18 years old) with eGFR 25–90mL/min/1.73m2 and UACR 200–3500mg/g on an optimized treatment regimen with RAAS inhibitor for at least 4 months and a serum potassium≤4.8mmol/L. Patients are excluded based on the presence of T1D, T2D, or HbA1c≥6.5%; other nephropathies (polycystic kidney disease and any kidney disease requiring immunosuppressants in the 6 months before screening); and the presence of symptomatic HFrEF. Patients will be randomly attributed to two arms receiving either finerenone (10 or 20mg) or placebo daily, for an estimated period of 32 months, comprising 50 weeks total of study duration. The primary outcome is the mean rate of change as measured by total slope of eGFR, from baseline to month thirty-two. Secondary outcomes include the time to a composite kidney and CV outcome (a compound of kidney failure, sustained eGFR decline of ≥57%, HHF or CV death); and the time to both the kidney composite outcome (kidney failure, sustained eGFR decline of ≥57%) and the CV composite outcome (heart failure hospitalization or CV death), on their own. The trial is estimated to end in January 2026.
Finerenone is also currently being investigated for CKD and T1D on a phase 3 trial (NCT05901831), for CKD on pediatric age on two phase 3 trials (NCT05196035 and NCT05457283), and on kidney transplant recipients (NCT06059664).
Observational studiesThe widespread use of nsMRAs allows some observational studies to be developed, although there are no results yet. These studies mainly aim to assess the real-world data on the use of nsMRA, namely finerenone. The parameters studied include demographic data, patient characteristics and drug efficacy and safety. Table 3 summarizes the 5 observational studies we found, including the criteria used on choosing the population, the study's goal and the current status of the work.
Other emerging clinical applicationsFIDELITY unveiled the positive effect of finerenone on diminishing HHF on a population that included patients with chronic symptomatic heart failure with preserved ejection fraction (HFpEF). With a view to obtain more data on the efficacy and safety of finerenone versus placebo on patients with HFpEF, there are multiple ongoing clinical trials. “The Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients with Heart Failure” (FINEARTS-HF), a double-blind trial including 6001 patients, showed that finerenone reduced both heart-failure worsening (rate ratio 0.82; [95%CI 0.71–0.94]) and cardiovascular mortality (HR 0.93 [95%CI 0.78–1.11]) in patients with HF (NYHA II–IV) and left ventricular ejection fraction≥40%. There was approximately 50% of patients with eGFR<60mL/min/1.73m2. There was no difference between groups (HR 1.33; [95% CI: 0.94–1.89]) in the prespecified secondary kidney composite outcome (a composite of a sustained decrease in the eGFR≥50%, a sustained decline in the eGFR to <15mL/min/1.73m2, or the initiation of long-term dialysis/kidney transplantation). There was no difference in the rate of serious adverse events.18
EsaxerenoneEsaxerenone is currently approved for the treatment of hypertension in Japan.19
“Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria” (ESAX-DN) is a multicenter, randomized, double-blind, placebo-controlled phase 3 trial aiming to obtain evidence on the efficacy and safety of esaxerenone on reducing and eventually leading to remission of microalbuminuria. It included 393 patients aged above 20 years old with T2D, hypertension, microalbuminuria (UACR 45–300mg/g) and eGFR≥30mL/min/1.73m2 on top of an optimized RAAS inhibitor treatment (for at least 12 weeks), conducted in Japan. UACR remission was defined as the concomitant presence of UACR<30mg/g and a ≥30% reduction in UACR from baseline at two consecutive time points. Exclusion criteria included the presence of T1D, secondary glucose intolerance, poorly controlled diabetes (HbA1c≥8%) and SBP<120mmHg or ≥160mmHg and DBP<60mmHg or ≥100mmHg; malignant hypertension or hypertension not DKD related; alterations in serum potassium concentration and some renal diseases. Patients were treated with either esaxerenone 1.25mg titrated to a target dose of 2.5mg od, based on serum potassium levels or placebo. The primary endpoint, the proportion of patients achieving UACR remission at the term of intervention, had a significant absolute difference of 18% ([95%CI]: 12–25%) with equivalent results in the various subgroups of patients. Further efficacy results showed a modest effect in reducing both SBP and DBP and a greater decrease in UACR with esaxerenone. Hyperkalemia, defined as a serum potassium of either ≥6.0mEq/L or ≥5.0mEq/L on two consecutive measurements, was more frequent in patients treated with esaxerenone (9% versus 2%). Nonetheless, it was not associated with complications, was controlled with dosage reduction, notwithstanding a higher discontinuation of treatment rate in the esaxerenone group (4% versus 2%). The greater risk of hyperkalemia happened on the first 2 weeks of treatment and was more frequent in patients with a higher baseline potassium (≥4.5mEq/L) and in those with worse prior kidney function (baseline eGFR<60mL/min/1.73m2). In terms of kidney function decline, eGFR decline was bigger with esaxerenone when compared to placebo. Indeed, 5% in esaxerenone group had to stop the treatment due to two consecutives ≥30% reductions in eGFR, versus 2% on the placebo group. There was a weak correlation between change in UACR and eGFR. Applying these results to the real world is limited: this study only included a Japanese population with well-controlled diabetes. Another constraint is the short duration of treatment (52 weeks) and short follow up period(4 weeks), as the rate of decrease in eGFR may not have been fully evaluated in all patients.20
Another phase 3 trial was developed, evaluating esaxerenone on a population with macroalbuminuria. The “Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study” trial enrolled a population of 56 patients using the same inclusion and exclusion criteria as ESAX-DN, apart from only including patients with UACR≥300mg/g and allowing for HbA1c values up to 8.6%. The results showed benefits on diminishing UACR from baseline, decreased by 54.6% ([95%CI] 46.9–61.3%), leading to two consecutive measures of UACR<300mg/g in 51.8% of the patients, which indicates it can improve the nephropathy. Furthermore, it also lowered SBP and DBP. Its safety profile was congruent with an adverse event incidence of 69.6%, and with 5.4% patients developing hyperkalemia. It diminished eGFR, with a maximal change from baseline of −8.3mL/min/1.73m2. Without a placebo group and including only Japanese patients, these results are limited.21
Apararenone and ocedurenoneThese drugs will be more briefly mentioned, as their evidence is scarcer.
Apararenone's safety and tolerability on reducing UACR on a Japanese population with stage 2 diabetic nephropathy (HbA1≤10.5%, eGFR≥30mL/min/1.73m2 and UACR≥50mg/g) was already investigated on a phase 2 dose–response study, including and extension study. Investigators analyzed the effect of apararenone on 3 different doses (2.5, 5 and 10mg/od) versus placebo on UACR, BP and eGFR change from baseline to various timepoints. Results showed a significant dose dependent UACR decrease in the three apararenone groups, albeit not on the placebo group. The effect was more marked on those already treated with RAAS inhibitor. There did not appear to be a significant effect on lowering BP, with slight reduction in patients with higher baseline BP and concomitant use of RAAS inhibitor. Regarding safety, there was a greater percent change from baseline in eGFR with the three apararenone groups versus the placebo group, but even a ≥30% decrease in eGFR was not associated with adverse events and there was an improvement on kidney function after treatment discontinuation, implying the absence of associated structural damage. There was also a dose dependent increase in serum potassium and a sizable proportion of patients included on the extension group developed adverse events, but no deaths were reported. These results point to an appropriate dosing of apararenone of either 5mg od or 10mg od. Limitations on this study are the small population (293 patients) and the fact that patients are all Japanese. Additionally, the results from the extension study lack the presence of a placebo comparator and were not blinded to patients.22
Ocedurenone is under investigation for uncontrolled hypertension in a population with moderate to severe CKD, with results already available, from “A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Assess the Efficacy, Safety, and Pharmacokinetics of KBP-5074 in Patients with Moderate-to-Severe Chronic Kidney Disease and Uncontrolled Hypertension – (BLOCK/CKD)” trial (NCT03574363). This study enrolled 240 adult patients with eGFR≥15mL/min/1.73m2 and ≤44mL/min/1.73m2 and SBP≥140mmHg on an optimized anti-hypertensive treatment with 2 or more synergistic agents. These patients were randomly assigned to daily treatment with either ocedurenone 0.25mg or 0.5mg or placebo. The primary endpoint was SBP change throughout the 12-weeks period, with a statistically significant reduction in both ocedurenone arms versus placebo. Secondary endpoints evaluated DBP and UACR change from baseline to week twelve, but these treatment differences did not reach significance. There were some adverse events reported, namely hyperkalemia, with a similar incidence in the three groups, albeit bigger on the intervention ones. Nonetheless, no severe hyperkalemia (≥6.0mmol/L) or hyperkalemia leading to hospitalization were recorded. AKI was also not reported and there was no significant decrease in eGFR. These results point to the need to further investigate these drugs, as it can be useful in treating resistant hypertension in a population with advanced CKD, with an apparently safe profile.23,24
ConclusionAs summarized in this work, there is a growing interest on nsMRAs and its benefits on CKD and hypertension. The present work does have limitations: the literature review was restricted to Pubmed and ClinicalTrials.gov, compromising the extent of the trials found and possible contributing to a lack of some trials. Nonetheless, we consider this a good picture on the main evidence and the state of the art for nsMRA, a new promising drug for CKD.
Finerenone is the nsMRA with the most robust evidence on either DKD or non DKD. Regarding DKD, The KDIGO guidelines for Diabetic Kidney Disease recommends its use on patients with T2D, CKD with albuminuria and appropriate serum potassium.
Esaxerenone is only currently available in Japan, explained by most of the evidence for the use of this drug coming from studies conducted on that country. The evidence gathered showed that esaxerenone efficacy on diminishing micro and macroalbuminuria and controlling hypertension on patients with DKD. Its safety profile was also appropriate.
Ocedurenone and apararenone are not yet available for clinical routine use but are being investigated on refractory hypertensive CKD and DKD on Japanese patients, respectively.
Conflict of interestEdgar Avito Fernandes de Almeida received grants as speaker from VIFOR, ASTRA ZENECA, BAYER and BIAL.
The other authors have no declarations of interest.
The authors thank Beatriz Rodrigues for the language revision in the Spanish version of the abstract.