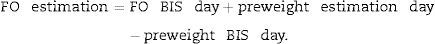Chronic fluid overload is frequent in hemodialysis patients (P) and it associates with hypertension, left ventricular hypertrophy (LVH) and higher mortality. Moreover, echocardiographic data assessing fluid overload is limited. Our aim was to evaluate the relationship between fluid overload measured by bioimpedance spectroscopy (BIS) and different echocardiographic parameters.
MethodsCross-sectional observational study including 76 stable patients. Dry weight was clinically assessed. BIS and echocardiography were performed. Weekly time-averaged fluid overload (TAFO) and relative fluid overload (FO/ECW) were calculated using BIS measurements.
ResultsBased on TAFO three groups were defined: A- dehydrated, TAFO <-0.25 L 32 P (42%); B- normohydrated, TAFO between -0.25 and 1.5 l: 26 (34%); C- overhydrated, TAFO>1.5 l: 18 (24%). We found significant correlation between TAFO and left atrial volume index (LAVI) (r: 0.29; p=0.013) but not with FO/ECW (r 0.06; p=0.61). TAFO, but not FO/ECW kept a significant relationship with LAVI (p=0.03) using One-Way ANOVA test and linear regression methods. LVH was present in 73.7% (concentric 63.2%, eccentric in 10.5%). No differences between groups in the presence of LVH or left ventricular mass index were found.
ConclusionsWe found that left atrial volume index determined by echocardiographic Area-length method, but not left ventricle hypertrophy or dimensions of cavities, are related on hydration status based on bioimpedance measured time-averaged fluid overload (TAFO), and not with FO/ECW.
La sobrehidratación es frecuente en pacientes en hemodiálisis (P) y se asocia con hipertensión, hipertrofia ventricular izquierda (LVH) y mayor mortalidad. Los datos ecocardiográficos evaluando sobrecarga hídrica son escasos. Nuestro objetivo fue evaluar la relación entre sobrehidratación medida por Bioimpedancia multifrecuencia (BIS) y parámetros ecocardiográficos.
MétodosEstudio transversal observacional, con 76 P estables; El peso seco fue determinado clínicamente; se realizaron ecocardiograma, BIS y analítica sanguínea. Se calcularon la sobrehidratación promedio semanal (TAFO) y sobrehidratación relativa (FO/ECW).
Resultados3 grupos: A- deshidratados, TAFO <-0.25 L: 32 P (42,1%); B- normohidratado, TAFO -0.25 - 1.5 L: 26 P (34,2%); C- sobrehidratados TAFO > 1.5 L: 18 P (23,7%). Encontramos correlación significativa entre TAFO e índice de volumen auricular izquierdo (LVAI) (r: 0.29; p=0.013) y no con FO/ECW (rho 0,06; p = 0,61). TAFO, pero no FO/ ECW, mantuvo una relación significativa con LVAI (p = 0,03) utilizando test de ANOVA y regresión lineal. LVH estuvo presente en 73,7% de P (concéntrica 63,2%, excéntrica 10,5%). No encontramos diferencias entre grupos en cuanto a la presencia de LVH, ni del índice de masa ventricular izquierda.
ConclusionesNosotros observamos que el índice de volumen auricular izquierdo determinado por longitud de área medida por ecocardiograma y no la hipertrofia ventricular izquierda o dimensión de cavidades se relaciona con el estado de hidratación medido por sobrehidatación semanal y no con FO/ECW.
Chronic fluid overload is a frequent problem in patients treated by hemodialysis; and it is known to be associated with different clinical conditions like hypertension, increased arterial stiffness, left ventricular hypertrophy, heart failure and consequently higher morbidity and mortality.1 In this regard, maintaining a normal extracellular volume status2 is one of the major targets of the therapy; yet establishing the hydration state of dialysis patients is one of the most challenging that nephrologists face in their daily practice.
Hydration state can be measured by different methods. In clinical routine, fluid management is largely based on subjective clinical assessment and the probing for dry weight procedure.3 New noninvasive bedside tools, such as bioimpedance spectroscopy (BIS), facilitate objective assessment of fluid status.
Multifrequency bioimpedance spectroscopy is a validated technique that objectively defines the individual overhydration status, taking into account the individual's body composition.4 The hydration status, evaluated by bioimpedance spectroscopy, is an important and independent predictor of mortality in chronic hemodialysis patients.5,6 Bioimpedance can be useful in guiding fluid management with a favorable impact in cardiovascular parameters.7 Fluid status can be expressed as pre- or postdialytic fluid overload, but to assess the cardiovascular condition of a patient, the time averaged fluid overload (TAFO) seems to better reflect the long-term cardiovascular load.8
Cardiovascular disease is the major cause of death in patients with advanced chronic disease9 and echocardiography is an established technique to estimate the risk for cardiovascular complications in patients with end-stage renal; been a radiation free technique that provides noninvasive assessment of cardiac structures and function. It is widely available and recommended in patients with end-stage renal disease for diagnosis, guidance of treatment, and pretransplantation evaluation.10 However, there is limited data on echocardiographic parameters evaluating hydration status in patients undergoing dialysis.
Our aim was to assess the relationship between hydration status and echocardiographic parameters in hemodialysis patients.
MethodsPatientsWe conducted a cross-sectional study including 76 patients (68.4% male) from the dialysis unit of a secondary hospital with 103 hemodialysis patients. Patients that were more than two months in the technique in stable condition and without hospital admissions during the previous two months were included. Patients with contraindication for BIS (implanted electronic device, metallic prostheses of any type, amputated patients, pregnant or lactating women) were excluded. Six patients (7.3%) with a previously known severe valvulopathy or bad acoustic window also were excluded. Patients were 18 years old or above and had signed an informed consent approved by the Institutional Ethics Committee of Severo Ochoa Hospital.
Blood samples for standard laboratory parameters and BIS were obtained in the same day, before the second dialysis treatment of the week and after 20min in semi-recumbent position. Patients underwent echocardiography on the day following midweek dialysis.
Demographic and clinical characteristics were recorded. Dry weight was determined by the patient's attending physician in each hemodialysis session without knowledge of the bioimpedance results. Under these conditions bioimpedance was performed and weekly TAFO measured. Pre- and postdialysis body weight and blood pressure were collected. Intradialytic symptoms were noted and classified as hypotension, cramps, or other. Hypertension was defined as blood pressure greater than 140–90 and or antihypertensive therapy. Interdialytic weight gain and ultrafiltration rates in mL/h/kg were recorded.
Bioimpedance assessmentBioimpedance was assessed using a Body Composition Monitor (Fresenius Medical Care, Deutschland GmbH). Patients were measured before dialysis in a semirecumbent position in their dialysis chair. Following measures were taken: Fluid overload (FO), extracellular water (ECW), intracellular water (ICW) in liters, ECW/ICW ratio, lean tissue mass in percentage (LTM %), lean tissue index (LTI) defined as the quotient of LTM/height2 (kg/m2), fat mass in percentage (FAT %), fat tissue index (FTI) defined as the quotient of FAT/height2 (kg/m2) and phase angle (PA) in grades. Phase angle is calculated as the arctangent of reactance over resistance at frequency 50kHz and is related to body cell mass and to the distribution of fluid between intracellular and extracellular compartments.11
Relative fluid overload (FO/ECW) and TAFO were calculated. For calculating TAFO, FO was additionally estimated for each of the other two treatment days of the week by using differences in predialysis body weight (preweight) according to next equation:
FO BIS day indicates real measurements. This approach assumes that treatment-to-treatment changes in preweight over a few days reflect mainly changes in FO and that body composition remains stable within this short time period. Postdialysis FO was calculated by subtracting ultrafiltration volume from the predialysis measurement.
Definition of time averaged fluid overload (TAFO)Fluid status in patients on intermittent ultrafiltration therapy is time-dependent, being lowest just after dialysis and reaching a maximum before the next dialysis session. In addition, fluid status is usually higher after the long interdialytic interval. To make different measurements of fluid status during the week comparable, the concept of weekly TAFO was introduced. It represents the average cardiovascular fluid load over one complete week, assuming linear fluid accumulation in the interdialytic period. It is defined as the average between all three FO predialysis and postdialysis FO (FOpost) values of the week.
Average weekly TAFO: (FO pre1+FO pre2+FO pre3+FOpost1+FOpost2+FOpost3)/6.12
In other studies, different TAFO values have been used to determined hydration status among patients.8,12 In this study, patients were divided into 3 groups based on time-averaged fluid overload: Group A (dehydrated), with TAFO <−0.25L; Group B (normohydrated), with TAFO between −0.25 and 1.5L; Group C (overhydrated), with TAFO >1.5L.
Echocardiography2D, M-mode and Doppler echocardiography was performed on patients lying in the left decubitus position at baseline, on the day after dialysis, by an experienced group of sonographers from the echocardiography laboratory, using a General Electric Vivid7 echocardiographic system with a 5S transthoracic probe. Registries were reviewed by a single sonographer, and all of them were blinded to the clinical details of patients. All echocardiographic data were measured according to the guidelines of the European and American Societies of Echocardiography.13,14 Left ventricle (LV) mass was calculated using Devereux formula and indexing by body surface area. Left atrial dilation was considered for a LAV >34ml/m2. Diastolic function measurements were assessed14 from the apical view using transmitral power-Doppler. Right ventricle diameter and function15 were determined from apical view measuring subvalvular diameter, tricuspid annular plane systolic excursion (TAPSE) and tricuspid annulus systolic velocity by tissue-Doppler.
Statistical analysisData were processed with IBM SPSS19 program. Quantitative data are expressed by mean (median for time variables) and a confidence interval of 95%, and qualitative variables by the number of subjects (n) and its relative percentage. Groups were defined from time-averaged fluid overload (TAFO) and the OH/ECW ratio, and were compared by one-way ANOVA and linear multivariate regression analysis (quantitave variables), and Chi-square for trend (qualitative variables). A variation >10% was settled for confounding factors. Correlations were compared by Spearman's rho. Statistically significance was considered when p<0.05.
ResultsStudy participantsAmong the 76 patients included the median age was 63.1 (CI 95% 60.0–66.2) years, men 52 P (68.4%) with a median dialysis vintage of 36 (13.5–84) months. 31 patients (40, 8%) were diabetics, 23 patients (30.3%) were hypertensive. The underlying renal disease was Diabetes 21 P; Vascular disease 17P; Cystic hereditary disease 3P; Glomerulopathy 14P; interstitial 7 P; Unknown 12 P; Others 2 P. Regarding dialysis technique, 55 P (72.4%) patients were in conventional hemodialysis and 21 (27.6%) patients were in on-line hemodiafiltration. The vascular access used was native arteriovenous fistula in 40 (52%) patients, Graft in 8 (10, 5%) patients and permanent central catheter in 28 (37%) patients.
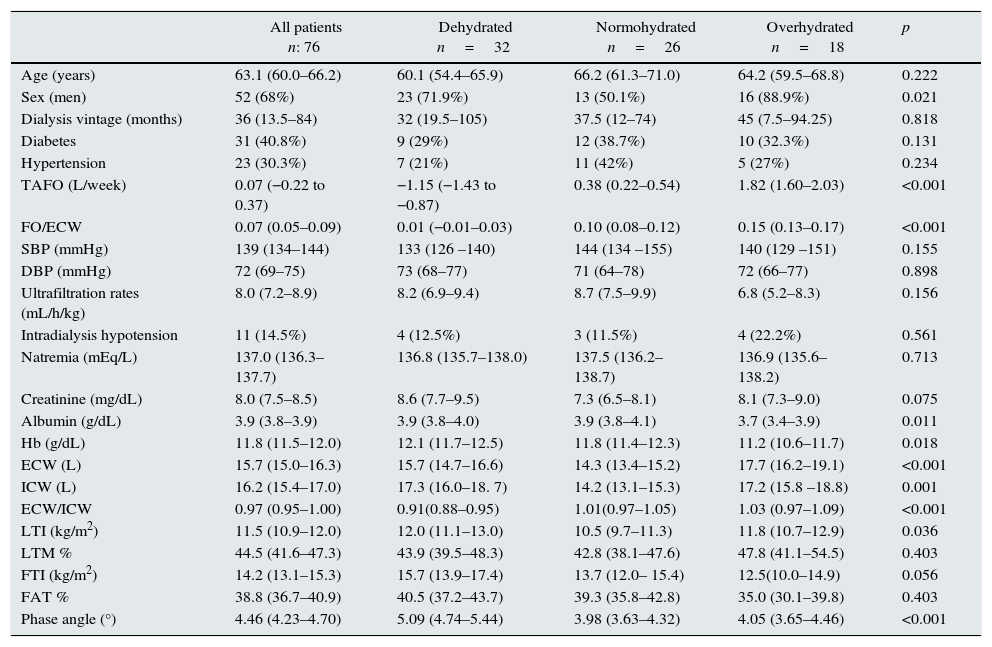
Overhydration status according TAFO measurementsBased on time-averaged fluid overload 32 (42%) patients were considered dehydrated; 26 (34%) patients were considered normohydrated and 18 (24%) patients were considered overhydrated. Table 1 summarizes general characteristics according TAFO groups. We found no differences between groups regarding age, systolic and diastolic blood pressure, diabetic history and dialysis vintage; regarding sex, men more often out of range (dehydrated or overhydrated). Additionally, no differences were found with the dialysis technique, the frequency of intradialysis hypotension and ultrafiltration rates. Interestingly, we founded that interdialytic weight gain was higher in dehydrated and overhydrated patients compared to normohydrated patients. As expected, in dehydrated group, ECW/ICW index was lower and phase angle was higher. While there was no difference of lean mass and fat mass percentage, LTI was higher in dehydrated patients. In regard of biochemical parameters, there was no difference between groups in serum sodium and creatinine; however, as usual, albumin and hemoglobin levels were lower in overhydrated patients.
Comparison of the whole population and the subgroups stratified according TAFO level.
| All patients n: 76 | Dehydrated n=32 | Normohydrated n=26 | Overhydrated n=18 | p | |
|---|---|---|---|---|---|
| Age (years) | 63.1 (60.0–66.2) | 60.1 (54.4–65.9) | 66.2 (61.3–71.0) | 64.2 (59.5–68.8) | 0.222 |
| Sex (men) | 52 (68%) | 23 (71.9%) | 13 (50.1%) | 16 (88.9%) | 0.021 |
| Dialysis vintage (months) | 36 (13.5–84) | 32 (19.5–105) | 37.5 (12–74) | 45 (7.5–94.25) | 0.818 |
| Diabetes | 31 (40.8%) | 9 (29%) | 12 (38.7%) | 10 (32.3%) | 0.131 |
| Hypertension | 23 (30.3%) | 7 (21%) | 11 (42%) | 5 (27%) | 0.234 |
| TAFO (L/week) | 0.07 (−0.22 to 0.37) | −1.15 (−1.43 to −0.87) | 0.38 (0.22–0.54) | 1.82 (1.60–2.03) | <0.001 |
| FO/ECW | 0.07 (0.05–0.09) | 0.01 (−0.01–0.03) | 0.10 (0.08–0.12) | 0.15 (0.13–0.17) | <0.001 |
| SBP (mmHg) | 139 (134–144) | 133 (126 –140) | 144 (134 –155) | 140 (129 –151) | 0.155 |
| DBP (mmHg) | 72 (69–75) | 73 (68–77) | 71 (64–78) | 72 (66–77) | 0.898 |
| Ultrafiltration rates (mL/h/kg) | 8.0 (7.2–8.9) | 8.2 (6.9–9.4) | 8.7 (7.5–9.9) | 6.8 (5.2–8.3) | 0.156 |
| Intradialysis hypotension | 11 (14.5%) | 4 (12.5%) | 3 (11.5%) | 4 (22.2%) | 0.561 |
| Natremia (mEq/L) | 137.0 (136.3–137.7) | 136.8 (135.7–138.0) | 137.5 (136.2–138.7) | 136.9 (135.6–138.2) | 0.713 |
| Creatinine (mg/dL) | 8.0 (7.5–8.5) | 8.6 (7.7–9.5) | 7.3 (6.5–8.1) | 8.1 (7.3–9.0) | 0.075 |
| Albumin (g/dL) | 3.9 (3.8–3.9) | 3.9 (3.8–4.0) | 3.9 (3.8–4.1) | 3.7 (3.4–3.9) | 0.011 |
| Hb (g/dL) | 11.8 (11.5–12.0) | 12.1 (11.7–12.5) | 11.8 (11.4–12.3) | 11.2 (10.6–11.7) | 0.018 |
| ECW (L) | 15.7 (15.0–16.3) | 15.7 (14.7–16.6) | 14.3 (13.4–15.2) | 17.7 (16.2–19.1) | <0.001 |
| ICW (L) | 16.2 (15.4–17.0) | 17.3 (16.0–18. 7) | 14.2 (13.1–15.3) | 17.2 (15.8 –18.8) | 0.001 |
| ECW/ICW | 0.97 (0.95–1.00) | 0.91(0.88–0.95) | 1.01(0.97–1.05) | 1.03 (0.97–1.09) | <0.001 |
| LTI (kg/m2) | 11.5 (10.9–12.0) | 12.0 (11.1–13.0) | 10.5 (9.7–11.3) | 11.8 (10.7–12.9) | 0.036 |
| LTM % | 44.5 (41.6–47.3) | 43.9 (39.5–48.3) | 42.8 (38.1–47.6) | 47.8 (41.1–54.5) | 0.403 |
| FTI (kg/m2) | 14.2 (13.1–15.3) | 15.7 (13.9–17.4) | 13.7 (12.0– 15.4) | 12.5(10.0–14.9) | 0.056 |
| FAT % | 38.8 (36.7–40.9) | 40.5 (37.2–43.7) | 39.3 (35.8–42.8) | 35.0 (30.1–39.8) | 0.403 |
| Phase angle (°) | 4.46 (4.23–4.70) | 5.09 (4.74–5.44) | 3.98 (3.63–4.32) | 4.05 (3.65–4.46) | <0.001 |
Mean and CI 95% are expressed for quantitative variables. N and (%) is given for qualitative variables. TAFO, time average fluid overload; FO, fluid overload; SBP, systolic blood pressure; DBP, dyastolic blood pressure; HB, hemoglobin; ECW, extracelular water; ICW, intracelular water; LTI, lean tissue index; LTM %, percentage lean tissue mass; FTI, fat tissue index; FAT %, percentage fat mass.
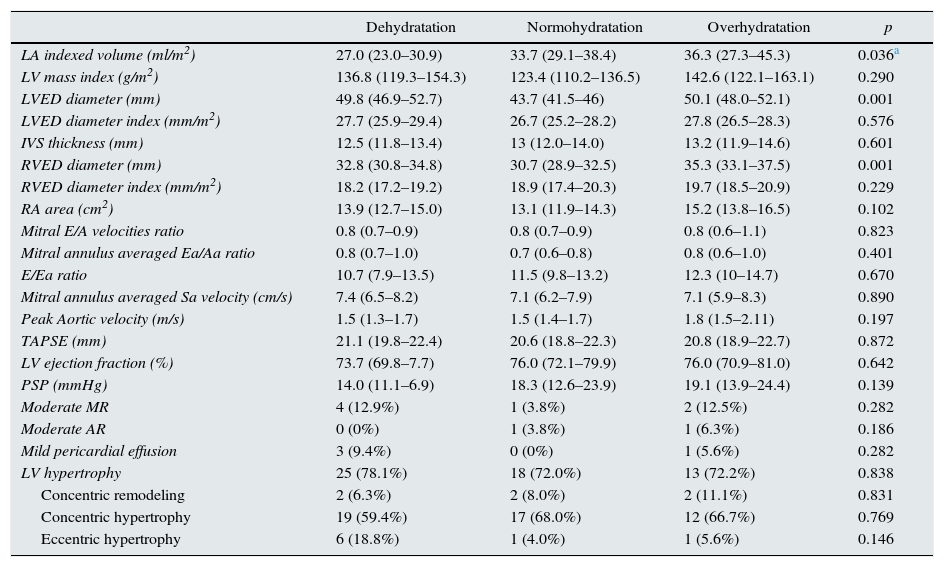
Table 2 shows data about echocardiographic parameters. Left atrial dilatation (LAVI >34ml/m2) was present in 24 (33.3%) patients without atrial fibrillation, being moderate in 3 (4.2%) and severe in 8 (18.1%). We found a significant correlation between the hydration status measured by TAFO and left atrial volume index (LAVI) (Spearman's rho 0.29; p=0.013). Interestingly, this correlation was not found when the hydration status was defined by FO/ECW (Spearman's rho 0.06; p=0.61), in spite of the significant internal correlation between both measurements (TAFO and FO/ECW) (Spearman's rho 0, 46; p<0.0001). Three patients with atrial fibrillation (3.9%, one from Group A and 2 from Group C) were excluded of the analysis.
Echocardiographic measurements.
| Dehydratation | Normohydratation | Overhydratation | p | |
|---|---|---|---|---|
| LA indexed volume (ml/m2) | 27.0 (23.0–30.9) | 33.7 (29.1–38.4) | 36.3 (27.3–45.3) | 0.036a |
| LV mass index (g/m2) | 136.8 (119.3–154.3) | 123.4 (110.2–136.5) | 142.6 (122.1–163.1) | 0.290 |
| LVED diameter (mm) | 49.8 (46.9–52.7) | 43.7 (41.5–46) | 50.1 (48.0–52.1) | 0.001 |
| LVED diameter index (mm/m2) | 27.7 (25.9–29.4) | 26.7 (25.2–28.2) | 27.8 (26.5–28.3) | 0.576 |
| IVS thickness (mm) | 12.5 (11.8–13.4) | 13 (12.0–14.0) | 13.2 (11.9–14.6) | 0.601 |
| RVED diameter (mm) | 32.8 (30.8–34.8) | 30.7 (28.9–32.5) | 35.3 (33.1–37.5) | 0.001 |
| RVED diameter index (mm/m2) | 18.2 (17.2–19.2) | 18.9 (17.4–20.3) | 19.7 (18.5–20.9) | 0.229 |
| RA area (cm2) | 13.9 (12.7–15.0) | 13.1 (11.9–14.3) | 15.2 (13.8–16.5) | 0.102 |
| Mitral E/A velocities ratio | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.8 (0.6–1.1) | 0.823 |
| Mitral annulus averaged Ea/Aa ratio | 0.8 (0.7–1.0) | 0.7 (0.6–0.8) | 0.8 (0.6–1.0) | 0.401 |
| E/Ea ratio | 10.7 (7.9–13.5) | 11.5 (9.8–13.2) | 12.3 (10–14.7) | 0.670 |
| Mitral annulus averaged Sa velocity (cm/s) | 7.4 (6.5–8.2) | 7.1 (6.2–7.9) | 7.1 (5.9–8.3) | 0.890 |
| Peak Aortic velocity (m/s) | 1.5 (1.3–1.7) | 1.5 (1.4–1.7) | 1.8 (1.5–2.11) | 0.197 |
| TAPSE (mm) | 21.1 (19.8–22.4) | 20.6 (18.8–22.3) | 20.8 (18.9–22.7) | 0.872 |
| LV ejection fraction (%) | 73.7 (69.8–7.7) | 76.0 (72.1–79.9) | 76.0 (70.9–81.0) | 0.642 |
| PSP (mmHg) | 14.0 (11.1–6.9) | 18.3 (12.6–23.9) | 19.1 (13.9–24.4) | 0.139 |
| Moderate MR | 4 (12.9%) | 1 (3.8%) | 2 (12.5%) | 0.282 |
| Moderate AR | 0 (0%) | 1 (3.8%) | 1 (6.3%) | 0.186 |
| Mild pericardial effusion | 3 (9.4%) | 0 (0%) | 1 (5.6%) | 0.282 |
| LV hypertrophy | 25 (78.1%) | 18 (72.0%) | 13 (72.2%) | 0.838 |
| Concentric remodeling | 2 (6.3%) | 2 (8.0%) | 2 (11.1%) | 0.831 |
| Concentric hypertrophy | 19 (59.4%) | 17 (68.0%) | 12 (66.7%) | 0.769 |
| Eccentric hypertrophy | 6 (18.8%) | 1 (4.0%) | 1 (5.6%) | 0.146 |
Mean and CI 95% are expressed for quantitative variables. N and (%) is given for qualitative variables.
Adjusted for mitral E/A ratio, percentage lean tissue mass and fat mass percentage.
LA, left atrium; LV, left ventricle; LVED, left ventricle end-diastolic: RVED, right ventricle end-diastolic; IVS, interventricular septum; IVC, inferior vena cava; RA, right atrium: TAPSE, tricuspid annular plane systolic; PSP, pulmonary systolic pressure; MR, mitral regurgitation; AR, aortic regurgitation.
Using One-Way ANOVA test and linear regression methods, the TAFO defined hydration status, but not the FO/ECW, kept a significant relationship with LAVI (p=0.03). When including age, sex, ethiology, dialysis vintage, comorbidities, dialysis technique, hypertension, biochemical parameters (Hemoglobin, Na, K, Albumin, Creatinin), and all echocardiographic measurements including valvular regurgitation and left ventricle mass index, the only confounding factors identified were lean and fat mass percentages related to total weight, and diastolic function measured by transmitral velocities ratio, but the adjusted relationship kept significant (p=0.036).
Plasma sodium was not different between the 37 patients with (136.6mEq/l; CI 95% 135.8–137.4) and 35 patients without (137.3mEq/l; IC95% 136.1–138.6) atrial dilatation (p=0.314).
Left ventricle hypertrophy was present in 73.7% of patients and concentric remodeling in 7.9%. Concentric hypertrophy was present in 63.2% of patients and eccentric hypertrophy only in 10.5%. No statistical difference between TAFO defined groups (p=0.838) nor regarding left ventricle mass index (p=0.29) was found. Left ventricle ejection fraction (LVEF) was normal (in fact, all groups tended to hypercontractility), but in two cases from group A (LVEF 36.47 and 45.2%, moderate and mild systolic dysfunction respectively) and one from group C (LVEF 46.7%, mild systolic dysfunction). 48 patients (66.7%) had a pattern of diastolic dysfunction type I, defined by tissue-Doppler study of mitral annulus and power Doppler transmitral velocities, with no difference between groups (p=0.401). There was no significant difference in the prevalence of interdialysis hypertension (Group A 21.9%, Group B: 42.3%, Group C: 27.8% (p=0.241)).
No severe valvulopathy was found: Moderate mitral regurgitation was present in 9.2% of patients and moderate aortic regurgitation in 2.6%, without differences between groups. Mild pericardial effusion was present in only 5.3% with no significant difference between groups. No pulmonary hypertension was present, although pulmonary systolic pressure could only be determined in 40.8% of patients.
DiscussionIn our patients from a single haemodialysis center, we found that LAVI determined by echocardiographic Simpson's rule, but not left ventricle hypertrophy or dimensions of cavities, is related to hydration status based on TAFO, and does not on FO/ECW. Both parameters are determined by bioimpedance, the latter having shown to be a predictor for mortality.5,16,6 Several reports on bioimpedance-guided fluid management have shown improvement in blood pressure control and other cardiovascular parameters5,6 but still are under debate the most appropriate target for volume management. In this study we found a significant correlation between the hydration status measured by TAFO and left atrial volume index. It has been hypothesized that weekly TAFO could be an adequate measure of cardiovascular overload.7,12
Left atrial volume (LAV) is more accurate and is superior to diameter and area for predicting cardiovascular outcomes.17 In dialysis patients, left atrium enlarges in response to an increased preload (volume overload, arteriovenous fistula, mitral valve regurgitation, chronic anemia) or afterload (left ventricular systolic dysfunction, valve stenosis, hypertension, and left ventricle hypertrophy).10 In our study, there was no difference between groups regarding these factors with the exception of hydration status. Atrial fibrillation promotes and is maintained by atrial enlargement, and so we excluded the patients with atrial fibrillation from atrial volume data. Some authors18 have proposed to index LAV to height instead of body surface area (BSA) in order to avoid possible fluctuations in body fluid, muscle or fat affecting BSA in haemodialysis patients, but we did not find different results when doing so, perhaps because of using a more steady parameter as TAFO instead of FO/ECW, and a similar interdialysis weight gain (about 2.6kg) in dehydrated and overhydrated patients, so that we used the more stablished indexation to BSA. The same reflection applies to left ventricle mass index (LVMI) where indexing to height2,7 could detect more patients with left ventricle hypertrophy and have a more powerful prognostic impact.19
Left atrial volume predicts mortality and cardiovascular events in patients undergoing hemodialysis20–22 independently from left ventricle hypertrophy or function. Tripepi et al.18 demonstrated that an increase in LAV by 1ml/m2.7 per years independently predicts cardiovascular events. LAV is a strong nonspecific indicator of cardiovascular pathology and an ideal simple marker of diastolic dysfunction in everyday clinical practice, and two dimensional echocardiography is more readily accessible than 3D echocardiography or cardiac magnetic resonance.
Some data suggest a link between atrial enlargement and plasma sodium,23,24 but we did not find differences in plasma sodium between groups (p=0.713) or between patients with or without atrial dilatation (p=0.314).
Diastolic disfunction, measured by transmitral and tissue Doppler, was present in the three groups with no relevant difference between them, and considered for adjustment in the statistical analysis. Diastolic dysfunction is multifactorial and almost universal in these patients, and volume is not its only determinant.
Left ventricle hypertrophy was present in 73.7% of patients, similar to previously reported data.25,26 In patients undergoing dialysis, concentric hypertrophy, due to pressure overload (mainly hypertension) together with stimulating factors as mineral and bone disorder, renin-angiotensin system activation and endothelin,27,28 is more prevalent than eccentric hypertrophy. Many formulas exist for calculating left ventricular mass, been the Devereux formula commonly used and validated. Limitations due to endocardial borders delineation and the angle of probe during examination exist, and could have affected our results too. Cardiac magnetic resonance or 3D echocardiography are more accurate10,29,30 but less available for routine practice. LVH is predictive of mortality and cardiovascular events in dialysis patients.31,19
LVH is found early in end-stage chronic renal disease, and it is usually considered irreversible,32 though some recent studies suggest it can regress in the long term.33,34 Juan-García et al.35 find a relationship between hydration status and eccentric hypertrophy, but they do not consider sex in order to fix the threshold for hypertrophy. We did not demonstrate this relationship. As previously commented, volume overload may be decrease in our population, due to a relatively aggressive monitoring of volume status: right cavities were not dilated, pulmonary systolic pressure was very low (although its determination can be affected by dialysis), and pericardial effusion was scarcely present. As them, our prevalence of hypertension is less than data reported in literature (30.3% instead of about 60%) and there was no difference in distribution of interdialyisis hypertension. Inferior vena cava (IVC) diameter can be useful for estimating intravascular volume status36; we found no difference in IVC diameter between our groups. Our echocardiographic studies were performed in the day between dialysis sessions in order to keep homogeneity. It is also important to index diameter values to BSA or height in order to avoid inaccuracies, as shown by the change in signification when not doing so in our population. Time on dialysis and age affect prevalence of hypertrophy, and the relatively small population could affect to distribution of LVH between groups.
ConclusionsWe found that left atrial volume index determined by echocardiographic Area-length method, but not left ventricle hypertrophy or dimensions of cavities, are related on hydration status based on bioimpedance measured time-averaged fluid overload, and does not related on FO/ECW.
LimitationsAlthough relatively unselected, our population represents a single center and it is relatively small. People with previously known cardiopathy or bad acoustic window were excluded. No important valvulopathies were found, and systolic disfunction or previous cardiomiopathy was also rare, although this could favor homogeneity. Echocardiography measurements are subject to inaccuracies. Registries were reviewed by a single echocardiographer, and the studies performed by an experienced group into a hospital Echocardiography laboratory, but interobserver variability could affect results. Although values were adjusted for different variables in multivariate models, confounding factors may persist. Prognostic studies are ongoing. As in all observational studies, no conclusion regarding causality can be drawn.
Conflicts of interestThe authors declare no conflict of interest.