Patients with the dual burden of chronic kidney disease (CKD) and cardiovascular disease (CVD) experience unacceptably high rates of morbidity and mortality, which also entail unfavorable effects on healthcare systems. Currently, concerted efforts to identify, prevent and treat CVD in CKD patients are lacking at the institutional level, with emphasis still being placed on individual specialty views on this topic. The authors of this position paper endorse the need for a dedicated interdisciplinary team of subspecialists in cardio-nephrology that manages appropriate clinical interventions across the inpatient and outpatient settings. There is a critical need for training programs, guidelines and best clinical practice models, and research funding from nephrology, cardiology and other professional societies, to support the development of the subspecialty of cardio-nephrology. This position paper from the coordinating committee from the Working Group for Cardiorenal Medicine of the Spanish Society of Nephrology (S.E.N.) is intended to be the starting point to develop the subspecialty of cardio-nephrology within the S.E.N.. The implementation of the subspecialty in day-to-day nephrological practice will help to diagnose, treat, and prevent CVD in CKD patients in a precise, clinically effective, and health cost-favorable manner.
Los pacientes con enfermedad renal crónica (ERC) que presentan enfermedad cardiovascular (ECV) tienen índices de morbilidad y mortalidad inaceptablemente elevados, que impactan desfavorablemente sobre los sistemas de salud. En la actualidad, se requieren actuaciones multidisciplinares para identificar, prevenir y tratar la ECV en los pacientes con ERC, debiendo pues superarse la época de las actuaciones de las especialidades individuales. Los autores de este artículo respaldan la necesidad de un equipo interdisciplinar de subespecialistas en cardionefrología que gestione las intervenciones clínicas adecuadas en el entorno hospitalario y en el ambulatorio. Existe una gran necesidad de programas de formación, de guías y modelos de práctica clínica, y de fondos para la investigación en las sociedades de nefrología, cardiología y otras, para apoyar el desarrollo de la subespecialidad de cardio-nefrología. Este documento de opinión del comité coordinador del Grupo de Trabajo de Medicina Cardiorenal de la Sociedad Española de Nefrología (S.E.N.) pretende ser el inicio del desarrollo de la subespecialidad de Cardionefrología en el marco de la S.E.N. La implementación de la subespecialidad en la práctica nefrológica diaria contribuirá a diagnosticar, tratar y prevenir la ECV en los pacientes con ERC de una manera precisa, clínicamente efectiva y sanitariamente rentable.
It is well known that cardiovascular disease (CVD) in patients with chronic kidney disease (CKD) develops earlier and is more frequent, more severe, and shows different pathophysiological aspects and clinical manifestations compared with the non-CKD population, thus having a high medical, health and economic burden.1,2 Despite this, a group of experts assembled by the International Society of Nephrology identified recently the prevention and management of cardiovascular complications as one among several aspects of CKD that met criteria of unmet medical needs.3,4
It has been suggested that overcoming this situation requires, among other initiatives, uniting knowledge and skills between the fields of cardiology and nephrology for a better prevention and care of CVD in patients with CKD.5,6 In this conceptual framework the coordinating committee of the Working Group for Cardiorenal Medicine of the Spanish Society of Nephrology (Grupo de Trabajo de Medicina Cardiorenal de la Sociedad Española de Nefrología [S.E.N.] – CaReSEN) has written this position paper to share with the readers of Nefrología their belief that the time has come for nephrologists to incorporate, in a systematic way, cardiovascular knowledge and skills to their expertise in diagnosing and treating patients with CKD. The authors strongly believe that a new generation of nephrologists subspecialized in cardio-nephrology with an avant-garde approach to the screening, detection, diagnosis, prognosis, and management of the cardiovascular complications of patients with CKD is needed.
This article aims to provide arguments that support the notion that CKD is a multisystemic disease with a dominant cardiovascular component whose proper management exceeds conventional nephrology, as well as to propose the actions to be carried out to develop and integrate the emerging subspecialty of cardio-nephrology into the specialty of nephrology.
CVD in patients with CKD: an unmet medical needThere are multiple interactions between CVD and CKD that increase the disease burden of CKD patients and make CVD a major problem faced by nephrologists, other medical specialists and health systems. Some of the most relevant are briefly reviewed below.
Medical and health burden of CVD in CKDCurrent European guidelines on CVD prevention7 indicate that CKD should be considered as a high risk condition for CVD. Indeed, established traditional and emerging cardiovascular risk factors are more common in patients with CKD than in subjects without CKD.8 In addition, there is a continuously increasing prevalence of CKD with the clustering of multiple cardiovascular risk factors.9 CKD is highly prevalent among patients with primarily diagnosed CVD and is associated with increased risk of adverse outcomes, including progression to kidney failure.10 For instance, an analysis of over 3.4 million patients without heart failure (HF) matched to 156,743 patients with HF indicated that HF patients had a 2.12 higher risk for progression toward CKD.11 Additionally, HF patients are 2.96 times more likely to develop a rapid decline in estimated glomerular filtration rate (eGFR) slope (defined as >5mL/min/1.73m2 per year), which occurs in 22% of HF patients.11 Therefore, there is a bidirectional relationship between CKD and cardiovascular risk.
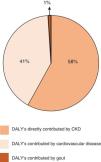
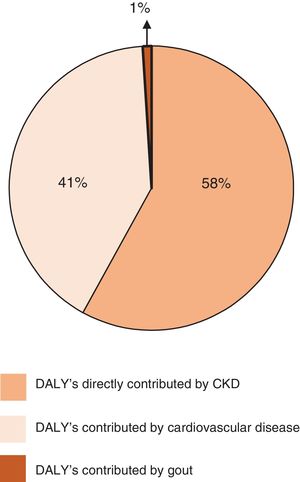
Beyond its own kidney disease-related burden, CKD has an indirect impact on global morbidity and mortality by increasing the risks associated with other major non-communicable diseases, including CVD.12 Indeed, the GBD 2017 study estimated that CKD resulted in 61.3 million Disability Adjusted Life Years (DALYs), of which approximately 58% were directly contributed by CKD, whereas approximately 41% were CVD DALYs and less than 1% were gout DALYs attributable to impaired kidney function (Fig. 1).13 Furthermore, the GBD 2013 study estimated that cardiovascular deaths attributed to CKD outnumbered kidney failure deaths throughout the world.14 Importantly, although age dominates cardiovascular risk factors and the older population has the greatest CVD burden,15 CKD young adults (25–34 years of age) have at least a 100-fold higher risk of CVD-related mortality than the general population.16 Furthermore, children with CKD and no traditional cardiovascular risk factors, have increased mortality from CVD.17
Percentages of Age-standardised Disability Adjusted Life Years (DALY's) directly contributed by chronic kidney disease (CKD), cardiovascular disease, and gout in patients with CKD in accordance with the Global Burden of Disease 2017 (adapted from Ref. 13 with permission).
CKD is associated with a huge economic burden that has a significant impact on annual health-care budget in many developed nations.18 Total costs of care increase substantially with worsening categories of eGFR and with inpatient care. More specifically, CVD-related admissions are responsible for the dominant proportion of costs at all CKD stages.19 This is not only because CVD is by far the main prevalent primary cause of hospitalization in patients with CKD (31.8% for CVD vs 8.7% for the second cause),20 but also to the costly effect of the combination of both diseases. Indeed, as shown in United Kingdom, the average estimated hospital cost of treating a major adverse cardiovascular event is substantially higher in CKD patients than in non-CKD patients.21 On the other hand, the data from the US Renal Data System on expenditures show the enhancer effect of cardiovascular complications on CKD costs.17 For instance, in the Medicare population aged 65 and older the per-person per-year costs in 2014 increased by 93% in patients with CKD and HF compared to patients with CKD alone.17 In this regard, data from Spain for the 2008-2010 period show that the direct and indirect health care costs related to HF are 58% higher in patients with eGFR values <60mL/min/1.73m2 than in patients with values ≥60mL/min/1.73m2.22
Diversity of clinical phenotypes of CVD in CKDThe high cardiovascular death rates associated with all stages of CKD reflect accelerated rates of both atherosclerosis and HF.23 Indeed, diverse community-based studies showed a robust association between lower renal function and atherosclerosis-related clinical complications, including myocardial infarction, stroke, or peripheral artery disease.23 These negative outcomes have been observed in any stage of CKD.24 Furthermore, the NEFRONA study (Observatorio Nacional de Atherosclerosis en NEFrología) that prospectively assessed the prevalence and progression of subclinical atherosclerosis confirmed an increased prevalence of this condition in patients with early and intermediate CKD stages and showed that the risk for subclinical atherosclerosis increased with CKD stage.25
Beyond the impact of atherosclerosis on cardiovascular outcomes in CKD patients, it is necessary to recognize also the contribution of non-atherosclerotic cardiac diseases (i.e., primary cardiomyopathies, valvular diseases and arrhythmias) to the bad cardiovascular prognosis associated with CKD.1,2 In accordance to the US Renal Data System, the incidence of these cardiac diseases is higher in CKD patients than in non-CKD patients, irrespectively of the influence of non-kidney related potential confounding factors.17 Either ischemic or non-ischemic cardiac diseases in CKD patients are characterized by progression to chronic congestive HF.26 Combined chronic dysfunction of kidney and heart is referred to as type 4 cardiorenal (or renocardiac) syndrome.27 Interestingly, the prevalence of HF in CKD patients has been reported close to 26%, compared to 6% among patients without CKD.17 Of note, the presence of HF reduces the probability of survival among patients both with and without CKD, but to a greater extent among those with CKD (p-value for interaction <0.0001).17 Additionally, cumulative evidence supports that when HF develops in the context of CKD, the ensuing hemodynamic alterations further compromise renal function28 facilitating the progression of CKD.11
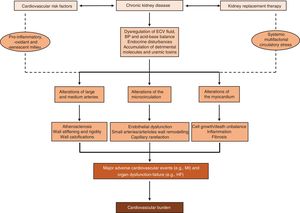
Complex pathophysiology of CVD in CKDAs demonstrated in animal models of CKD,29,30 the development and progression of CKD involve alterations in the cardiovascular system providing the pathophysiological basis for the phenotypic expression and outcomes of CVD in CKD patients. Indeed, the vascular tree and the heart undergo major structural and functional changes when kidney function declines31–33 that are exacerbated by concomitant cardiovascular risk factors34 and when kidney replacement therapy (KRT) is required.35 This facilitates atherosclerotic events (e.g., myocardial infarction)36 and organ dysfunction (e.g. HF)26 causing most of the cardiovascular burden of CKD (Fig. 2).
Although the pathophysiology of CVD in CKD has been extensively studied, there are some emerging issues that are worth considering. Most research in CKD-related vascular disease has been devoted to macrovascular complications. However, a recent review of all publications evaluating structure and function of the microcirculation in humans and animals with CKD found that capillary rarefaction resulting in a significant decrease in microvascular density, is a quintessential finding.37 For instance, the median capillary density was reduced by 24% in the heart in animal models of CKD38 and by 12% and 16% in necropsy studies in CKD patients and patients on dialysis, respectively.39 As shown experimentally, CKD results in the loss of coherent vessel systems distal to smaller arterioles, generating a typical heterogeneous pattern with avascular patches, resulting in a dysfunctional endothelium with diminished perfusion, shunting and tissue hypoxia.40 Therefore, microvascular disease is a principal pathogenic factor in the progression of CKD and the development of widespread severe organ dysfunction and multimorbidity in CKD patients.
Beyond the classically recognized macroscopic left ventricular hypertrophy (LVH) in patients with CKD, myocardial interstitial fibrosis (MIF) is a frequent finding in endomyocardial biopsies and necropsy studies in these patients.41 Indeed, clinical and population-based pathological post-mortem studies have shown that patients with CKD have significantly more interstitial fibrotic tissue in the heart compared with subjects without CKD, and that the stage of CKD directly correlates with the extent of MIF, thus being the more severe fibrosis found in patients with kidney failure and patients on KRT.39,42 Interestingly, coronary microvascular rarefaction and MIF are closely correlated in CKD patients.39 Recent scientific work has identified causal connections between CKD and MIF.43 Furthermore, it has been proposed that MIF may play a key role in the development of HF in CKD patients.44 In this framework, limited data and a large potential for impact make it necessary to develop optimal strategies for the diagnosis of MIF in CKD patients, as well as strategies aimed at individualized prevention and management of MIF in patients with kidney failure and on KRT.45
Finally, patients with CKD are at high risk for acute kidney injury (AKI) and accumulating evidence supports the notion that cardiovascular damage due to AKI leads to poor long-term cardiovascular outcomes,46 independent of or intertwined with the cardiovascular risks associated with CKD itself. Indeed, a 2017 meta-analysis of 25 studies involving a total of 254,408 patients, including 55,150 with AKI,47 showed that AKI was associated with an 86% increase in the risk of death from cardiovascular causes during a median follow-up of 1.4 years, a 58% increase in the risk of chronic HF during 2.9 years of follow-up, a 40% increase in the risk of acute myocardial infarction during 2.3 years of follow-up, and a 15% increase in the risk of stroke over a period of 2.7 years.47 Beyond AKI-associated hydroelectrolytic disturbances that promote acute cardiac dysfunction, defined as type 3 cardiorenal syndrome, AKI induces structural myocardial damage, with low-grade inflammation and cellular apoptosis and necrosis developing within days or weeks after an event and MIF developing months or years later.48 Thus the cardiovascular impact of CKD is further reinforced in conditions of abrupt decline of kidney function whatever its cause.
Underdiagnosis and undertreatment of CVD in CKDCVD is systematically underdiagnosed in CKD patients as the diagnostic sensitivities and specificities of clinical manifestations and non-invasive tests are questionable in this population. Two examples (e.g. coronary artery disease and HF) may illustrate this issue. The classic triad of ischemic symptoms, elevated cardiac biomarkers, and electrocardiographic changes is frequently absent in CKD patients suffering from acute coronary syndrome, who are more likely to present with systolic or diastolic dysfunction causing HF symptoms, or with syncope.49 Exercise electrocardiography is limited by lack of specificity of the ST-segment response and by inability of many CKD patients to exercise to a diagnostic workload.50 The accuracy of exercise and of pharmacological myocardial perfusion imaging is reduced in CKD patients compared with the general population, and sensitivities and specificities <80% have been reported.51 Conversely, creatine kinase MB isoform and cardiac troponins may be elevated in the absence of true myocardial necrosis, possibly because of myocardial apoptosis or small coronary vessel disease that develops in CKD.52
As regards to HF, its diagnosis as defined by the European Society of Cardiology (ESC)53 has limitations when applied to CKD patients. First, almost all patients with kidney failure develop signs and symptoms consistent with fluid overload due to inability of the severely diseased kidneys to excrete sodium and water independently of the presence or absence of cardiac dysfunction.26 Second, structural heart disease, for instance LVH, is highly prevalent in patients with CKD, the prevalence increasing progressively with the loss of renal function and thus being present in more than 80% of patients with kidney failure.54 Finally, CKD is one of the numerous causes of elevated natriuretic peptides that may weaken their diagnostic utility in HF.55
Patients with advanced CKD or kidney failure and patients on long-term dialysis are usually excluded from cardiovascular clinical trials conducted in the general population or in at-risk populations.56 This excluding behavior also extends into kidney transplant patients.57 It is noteworthy that regardless of the exclusion criteria (either serum creatinine levels above a threshold or eGFR values below a threshold) the percentage of trials that exclude patients with CKD has increased in recent years.58 This is particularly relevant for the use of HF modifying therapies in patients with any stage of CKD or on KRT and concomitant HF either with reduced or with preserved ejection fraction (Fig. 3).59 In addition, there are no universally agreed selection criteria and cardiovascular outcomes for trials conducted specifically in these populations.60,61 As a consequence, the treatment of CVD in patients with advanced CKD stages and patients on KRT is not based on evidence, but rather empirical with variable screening protocols and lack of consensus on optimal management which collectively gives rise to undertreatment that, in turn, facilitates the high cardiovascular burden in CKD patients, namely in those with HF.62
Summary of data from published systematic reviews reporting the percent of cardiovascular trials that excluded patients with heart failure (HF) and chronic kidney disease (CKD) (i.e., any stage of CKD or on kidney replacement therapy [KRT]) from HF clinical trials. Panel A. Presents the data in HF patients not separated according to ejection fraction (EF) values. Panel B. Presents the data of trials in which HF patients were stratified according to EF values in patients with HF with reduced EF (HFrEF), mid-range EF (HFmrEF) and preserved EF (HFpEF) (adapted with permission from Ref. 59).
An additional factor that further reinforces the relationship between CKD and CVD is the high cardiovascular morbidity and mortality of patients on KRT. The prevalence of any CVD in people on prevalent dialysis exceeds 60%, being well above that of patients with stage 5 CKD,17 with no global clear-cut differences between patients on hemodialysis and patients on peritoneal dialysis, although this aspect may depend of the type of CVD to be considered.17,63,64 Data from a systematic review and meta-analysis indicate that the incidence of de novo CVD is also similar in the two dialytic therapies.65 On the other hand, CVD accounts for >50% of deaths in people on either modality of dialysis,16,17,64 and CVD mortality remains up to 30 times higher in dialysis than in the general population.16 Analysis of available studies comparing cardiovascular mortality between the two dialysis modalities show discrepant results with either similar mortality for either modality65 or significantly lower mortality for hemodialysis compared to peritoneal dialysis.66 Given the inability to perform randomized studies, the existence of confounders cannot be ruled out.
Although the severity of CVD is reduced after kidney transplantation, it still remains the leading cause of premature patient and allograft loss, as well as a source of significant morbidity.67,68 Whereas all major phenotypes of CKD-associated CVD are represented in the kidney transplant recipient population, pre-existing risk factors for cardiovascular disease are amplified by superimposed cardio-metabolic derangements after transplantation such as the metabolic effects of immunosuppressive regimens, as well as the detrimental impact of allograft dysfunction.69
Developing the subspecialty of cardio-nephrologyIt is undeniable that the high burden and complexity of CVD in CKD patients requires dedicated and highly committed cardiovascular care which, in turn, requires both a demanding training plan and an adequate clinical environment.
The need of the subspecialtyNephrology is facing a period of remarkable and unprecedented change. Despite the successful scientific, clinical and health policy developments produced during the last years, significant barriers exist to ensure a robust pipeline of well-qualified nephrologists. There is widespread agreement, however, that any initiative to reassert the ‘appeal’ of nephrology must include significant focus on reinvigorating the trainee experience before and during fellowship.70 Indeed, current nephrology fellows perceive several gaps in training. Innovation in education and training is needed to better prepare future nephrologists for the growing challenges of kidney care.71 As the clinical complexity of nephrology is well recognized72 the interface between nephrology and other fields of medicine continues to expand, thus providing opportunities for subspecialization.73
In this conceptual framework, the members of the Western Europe Regional Board, International Society of Nephrology have recently proposed a training program in the subspecialty of cardio-nephrology as one of the potential goals for European nephrology in the future.6 The proposal is based in the notion that CVD in CKD patients represents a challenge and opportunity to develop strategies to enhance the skills and capabilities of current and, namely, future nephrologists.
In parallel with this proposal, the American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology have endorsed the need for a dedicated cardiorenal interdisciplinary team that spearheads early identification of patients with the dual burden of heart and kidney disease and jointly manages appropriate clinical interventions across the inpatient and outpatient settings.5 This collaborative would also oversee cross-training among nephrology and cardiology fellows and nursing and allied healthcare providers in both specialties to foster a deeper understanding of the intricacies of cardiorenal cross-talk.
S.E.N. commitment to cardio-nephrologyIn October 2019 the CaReSEN created the cardiorenal medicine working group (CaReSEN) taking into account four major arguments: (i) there is a critical need for guidelines and best clinical practice models from the S.E.N. geared specifically toward cardiorenal medicine outcomes and for research funding to focus on the needs of future cardiovascular therapies in CKD patients; (ii) implementation of national task forces that emphasize quality improvement measures in cardiorenal disease and the introduction of national quality benchmarks for cardiorenal outcomes will help reduce the morbidity, mortality, and economic burden of CVD in CKD patients; (iii) implementing cross-specialty educational programs across all levels in cardiovascular medicine and nephrology will help train future nephrologists who have the ability to diagnose, treat, and prevent the disease burden associated with CVD and CKD in a precise, clinically effective, and cost-favorable manner; and (iv) as it is a reality that for years the specialty of nephrology faces major fellowship recruitment challenges in our country and worldwide, the potentiation of subspecialties as cardio-nephrology can help instill new interest and enthusiasm in choosing nephrology as a career amongst trainee physicians.73–75
In accordance with these arguments, the main objective of the CaReSEN is to develop the subspecialty of cardio-nephrology within the specialty of nephrology. To achieve this objective, the CaReSEN has proposed a series of initiatives (Table 1). It is crucial to identify the pending priorities of nephrology in the following cardiorenal fields: (i) care (e.g. creation of multidisciplinary teams between nephrologists and cardiologists with shared clinical protocols); (ii) education (e.g. modification of the undergraduate and postgraduate Medicine study plans, modification of the training program ‘médicos internos y residentes’ [MIR] in nephrology, and modification of the continuous training of nephrologists to stimulate the cardiorenal vision of nephrological diseases); and (iii) research (e.g. facilitation of the development of cardiorenal translational research, and of clinical trials that include or focus on patients with advanced stages of CKD, patients with kidney failure, and patients on KRT).
Main initiatives proposed by CaReSEN to launch the subspecialty of cardio-nephrology.
| Identify the pending priorities of nephrology in the fields of cardiorenal care, cardiorenal education and cardiorenal research |
| Cooperate with national and international medical associations related to cardiorenal medicine |
| Promote collaborations between public institutions for the development of the aforementioned priorities |
| Collaborate with private institutions in medical training, translational research and health dissemination related to cardiorenal medicine |
| Cooperate with kidney patient organizations in initiatives aimed at education in cardiovascular issues |
It is also mandatory to promote collaborations at two different levels: (i) public institutions (e.g. clinical centers, academic institutions, research centers, and health, scientific and educational administrations); and (ii) scientific societies (e.g. national and international medical societies with background in the implementation of cardiorenal medicine). In this regard, the CaReSEN is planning to share its actions with the EUropean REnal and CArdiovascular Medicine (EURECA-m) Working Group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) that was created in 2010 with the mission to promote collaborations among European centers and medical specialties pursuing education and research in the overlapping area of cardiovascular and renal medicine.76
Finally, it also seems convenient to interact with private institutions involved in the dissemination of the nature and actions of the subspecialty to facilitate its integration into real world healthcare. In this regard, one specific objective would be to explore with kidney patients associations the creation of a ‘Cardiorenal School’ aimed at educating them in the prevention and management of the cardiovascular aspects of their disease. The role of national nonprofit global organizations fostering these initiatives, as it is the case of the Cardio Renal Society of America,77 deserves to be considered.
Integrating cardio-nephrology training into nephrology trainingCurrent general nephrology training, while helpful for providing the core knowledge and skills needed for managing patients with CKD and AKI, does not seem to be sufficient for covering the rapidly evolving field of cardiorenal medicine. Therefore, a proposal was made some years ago for an innovative program to enhance the education of both cardiology and nephrology training.78 This program is meant to shape the foundation for a more comprehensive view of cardiorenal medicine than the conventional nephrology fellowship training.
Depending on the scope of practice of the teaching facility, the 6-month educational cardiorenal program can be offered as a cumulative block of elective time of the nephrology training, preferably during the second half of the 4-year period of fellowship. Alternatively, it can be offered as a “specialty track” such as those currently offered by a number of centers in other areas of nephrology.
A variety of topics related to cardiorenal medicine can be considered for inclusion in the program, relating to both acute and chronic processes as well as the inpatient or outpatient setting (Table 2).74 While the core knowledge would remain the same to ensure consistency, the interest of the trainee as well as the expertise and clinical focus area of the attending physicians would supplement and guide the specific subject materials covered. Obviously, the patient population as well as any related local disciplines and programs (e.g. the presence of an active cardiovascular surgery service or an extracorporeal membrane oxygenation program) will affect the educational content and value of the program.
Some illustrative examples of topics to cover in the cardiorenal program by the nephrologist trainee.
| In the setting of diagnosis |
| Assessment of volume status |
| Use of bioimpedance and point-of-care ultrasound |
| Focused diagnosis of heart failure |
| Knowledge of specific echocardiographic and blood biomarker criteria in CKD patients |
| In the setting of treatment |
| Therapy of fluid overload |
| Handling of diuretic resistance and use of ultrafiltration with dialysis techniques |
| Personalization of cardiovascular therapy |
| Knowing the impact of kidney failure and KRT on cardiovascular drug pharmacology |
| In the setting of prevention |
| Prevention of cardio-renal disease onset and progression |
| Management of common risk factors |
| Prevention of AKI in the setting of cardiovascular diagnostic procedures |
| Application of protocols to minimize contrast-associated risk |
| Prevention of WRF in the setting of cardiovascular surgical procedures |
| Application of protocols to minimize hemodynamic instability-associated risk |
| In the setting of decision making |
| Decisions on procedures and interventions |
| Delivering effective clinical care while ensuring adequate use of resources (e.g., indication of cardioprotective instead of conventional HD) |
| End-of-life decisions |
| Recognition of cardiac terminal cases (e.g., indications of comprehensive conservative treatment instead of KRT) |
CKD, chronic kidney disease; KRT, kidney replacement therapy; AKI, acute kidney injury; WRF, worsening renal function; HD, hemodialysis.
The long-range view is for the nephrologist to develop and utilize competence in electrocardiography, echocardiography, vascular and lung ultrasound, and also physiologic studies such as body composition and impedance. While the classically referred studies would still be best performed by experienced specialists in the respective fields, advanced cardiorenal-trained nephrologists would gain competence in performing and interpreting some studies (e.g., point-of-care ultrasound)79 and generate real-time data relevant to patient care.
Knowledge of the relevant consensus guidelines of medical professional societies and initiatives (e.g. the ESC guidelines for the diagnosis and treatment of acute and chronic HF,53 and the Acute Dialysis Quality Initiative XI Workgroup functional classification system of HF in patients with kidney failure80) should be part of this educational program. A better understanding of the role of cardiorenal interactions in CKD and HF in symptom development, disease progression and prognosis (e.g., evaluation of kidney function throughout the HF trajectory,81 and impact of HF pathophysiology82 and treatment26 on the continuum of cardiac dysfunction in CKD) needs also be considered.
To enhance academic skills, the trainees will be required to develop a clinical research project in cardiorenal medicine. The research could focus on three fundamental thematic axes: (i) the search for new mechanisms involved in the crosstalk between the diseased kidney and the cardiovascular system83,84; (ii) the development of clinical studies aimed at increasing evidence based-practice in the diagnosis and treatment of CVD in patients with kidney failure and patients with KRT85; and (iii) the investigation of the impact of different cardiorenal multidisciplinary models on care outcomes and utilization of health care resources.86
As difficulties to providing any new educational cardiorenal program usually include a lack of external funding and faculty time constraints, integrating cardio-nephrology training into nephrology training should be done, at least in its beginning, with the use of already existing resources and with a minimal time commitment from faculty members. This said, it is important to remark that program-specific mentors should exist to ensure curriculum development and ensures trainees have an adequate experience.
Collaboration with the Spanish Society of Cardiology (SEC)It is undeniable that patients with combined CKD and CVD are complex and difficult to manage, and it is clear that CKD is the most important predictor of cardiovascular outcomes in all areas of cardiology and that CVD is the leading cause of death in kidney patients. Although these are the most important and obvious clinical reasons for collaborative care between nephrologists and cardiologists, there have been obstacles to collaboration. For instance, the customary training in cardiology never really focused on areas outside of CVD. The same is true for nephrology and CKD, although a more comprehensive education has always been part of this specialty because of the impact of systemic disease on the kidneys.87 Thus, specialists are often consulted for a procedure (e.g., cardiac catheterization or initiation of dialysis) with little collaboration on addressing the cardiorenal health of the patient.88 A second obstacle to an interdisciplinary collaboration may reside in the desire to maintain control of acute patients while chronic and hopeless patients are “left to the others”. Although it might be true that one specialist has advanced knowledge and skills concerning certain acute disorders (e.g. acute coronary syndrome or AKI) while the other is an expert in a given therapy for advanced patients (e.g. LV assist support or hemodialysis),89 the truth is that a combined path of diagnosis and care can only come from a multidisciplinary approach in which cardiologists and nephrologists cooperate mutually and respectfully. Two examples support the advantages of a multidisciplinary approach when treating cardiorenal patients. On the one hand, fluid overload resulting in intravascular and tissue congestion is a prominent finding in both decompensated HF90 and late stages of CKD,91 namely when the two conditions coexist, that should be treated with a novel vision not limited simply to forcing diuresis, but to normalize the extracellular volume and its distribution between tissues and blood vessels, without worsening heart or kidney function. On the other hand, accumulating evidence suggest that thanks to sodium-glucose cotransporter 2 (SGLT2) inhibitors the heart and the kidney work better together, thus offering a unique opportunity for clinicians to treat cardiac and renal disease in concert rather than in discord.92,93
Therefore, the time has come for the collaboration between nephrologists and cardiologists in two major areas: (i) the development of a common educational cardiorenal program to enhance the curriculums and the clinical training in both cardiology and nephrology, thus facilitating the creation of subspecialists in cardio-nephrology78; and (ii) the development of cardiorenal clinical units in which teams of cardiologists and nephrologists participate in paired consultation and co-management of cardiorenal patients based on common protocols and goals collected in clinical practice guidelines to maximize all chances for organ and patient recovery.94 Some specific aims to be accomplished in this collaborative educational and clinical initiative are presented in Table 3. In this sense, the S.E.N. and the SEC have initiated an institutional collaboration through a team of nephrologists and cardiologists to develop these two areas. Of note, this collaboration is open to the involvement of other medical specialties also dealing with patients eventually presenting with cardiorenal problems (e.g., internists and intensivists).
Specific objectives to achieve in the cardiorenal collaboration of the S.E.N.and the SEC.
| To develop fully combined educational programs resulting in board certification in cardio-nephrology |
| To provide that nephrology fellows spend at least 6 months in a cardiology department learning the diagnostic approach to and the therapeutic management of cardiac patients and the point of view of the cardiologist on patients in the cardiorenal interface |
| To provide that cardiology fellows spend at least 6 months in a nephrology department learning the diagnostic approach to and the therapeutic management of kidney patients and the point of view of the nephrologist on patients in the cardiorenal interface |
| To develop multidisciplinary clinical units of cardiorenal medicine allocated to the combined management of cardiorenal patients with special emphasis on the diagnosis and management of heart failure in kidney failure and viceversa, and the indication, prescription, and modality of delivery of extracorporeal support therapies |
| To create a joint platform to perform basic, translational, preclinical, and clinical studies in cardiorenal areas allowing cardiology and nephrology faculties to develop careers in cardio-nephrology |
S.E.N., Sociedad Española de Nefrología; SEC, Sociedad Española de Cardiología.
Almost two centuries have passed since Sir Richard Bright identified alterations in cardiac structure in patients with advanced stages of CKD,95 thus the interdependence between CKD and CVD has been part of the history of nephrology. Due to its large clinical consequences and its impact and on public health and society, the time has come to professionalize the management of this interdependence, enabling the training of subspecialists in cardio-nephrology within the specialty of nephrology. The S.E.N. is committed to this and through the CaReSEN is developing a training plan for subspecialists in cardio-nephrology to which it welcomes its members as well as other medical specialists also related to the CKD–CVD binomial.








![Summary of data from published systematic reviews reporting the percent of cardiovascular trials that excluded patients with heart failure (HF) and chronic kidney disease (CKD) (i.e., any stage of CKD or on kidney replacement therapy [KRT]) from HF clinical trials. Panel A. Presents the data in HF patients not separated according to ejection fraction (EF) values. Panel B. Presents the data of trials in which HF patients were stratified according to EF values in patients with HF with reduced EF (HFrEF), mid-range EF (HFmrEF) and preserved EF (HFpEF) (adapted with permission from Ref. 59). Summary of data from published systematic reviews reporting the percent of cardiovascular trials that excluded patients with heart failure (HF) and chronic kidney disease (CKD) (i.e., any stage of CKD or on kidney replacement therapy [KRT]) from HF clinical trials. Panel A. Presents the data in HF patients not separated according to ejection fraction (EF) values. Panel B. Presents the data of trials in which HF patients were stratified according to EF values in patients with HF with reduced EF (HFrEF), mid-range EF (HFmrEF) and preserved EF (HFpEF) (adapted with permission from Ref. 59).](https://static.elsevier.es/multimedia/02116995/0000004100000004/v1_202107230533/S0211699521000679/v1_202107230533/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)




