Guía de práctica clínica sobre detección y manejo de la enfermedad renal diabética: documento de consenso de la Sociedad Española de Nefrología
Más datosPara abordar todas las novedades en el manejo de las personas con diabetes mellitus (DM) y enfermedad renal crónica (ERC), el Grupo Español de Estudio de Nefropatía Diabética (GEENDIAB) bajo los auspicios de la Sociedad Española de Nefrología (SEN) ha decidido publicar una actualización de la Guía de Práctica Clínica para la detección y el manejo de la enfermedad renal diabética (ERD). Está dirigida a una amplia audiencia de clínicos que tratan la diabetes y la ERC. La terminología de la enfermedad renal en los pacientes diabéticos ha evolucionado hacia una nomenclatura más inclusiva que evita el infradiagnóstico de esta entidad. Así, los términos «diabetes y enfermedad renal» y «enfermedad renal diabética» son los propuestos en las últimas guías KDIGO 2022 para designar a todo el espectro de pacientes que pueden beneficiarse de un abordaje terapéutico integral solo diferenciado según el rango del FGe y la albuminuria.
Las recomendaciones se han dividido en 5 áreas principales de interés: Capítulo 1: Cribado y diagnóstico de la enfermedad renal diabética, Capítulo 2: Control metabólico en personas con diabetes y ERC, Capítulo 3: Control de la presión arterial en personas con enfermedad renal diabética, Capítulo 4: Tratamiento dirigido a la progresión de la ERC en personas con enfermedad renal diabética y Capítulo 5: Tratamiento antiagregante plaquetario o anticoagulante en personas con diabetes y ERC.
Para elaborar esta guía se siguieron las recomendaciones de la Organización Mundial de la Salud (OMS) para el desarrollo de guías. Se realizaron revisiones sistemáticas, con evaluación de los resultados y resúmenes de los hallazgos, y se informó de la fuerza de las recomendaciones siguiendo los perfiles de evidencia Grading of Recommendations Assessment, Development and Evaluation (GRADE).
To address all the changes in the management of people with diabetes (DM) and chronic kidney disease (CKD), under the auspices of the Spanish Society of Nephrology (SEN), the Spanish Diabetic Nephropathy Study Group (GEENDIAB) decided to publish an updated Clinical Practice Guideline for detection and management of diabetic kidney disease (DKD). It is aimed at a wide audience of clinicians treating diabetes and CKD. The terminology of kidney disease in diabetic patients has evolved towards a more inclusive nomenclature that avoids underdiagnosis of this entity. Thus, the terms “diabetes and kidney disease” and “diabetic kidney disease” are those proposed in the latest KDIGO 2022 guidelines to designate the whole spectrum of patients who can benefit from a comprehensive therapeutic approach only differentiated according to eGFR range and albuminuria.
Recommendations have been divided into five main areas of interest: Chapter 1: Screening and diagnosis of diabetic kidney disease, Chapter 2: Metabolic control in people with diabetes and CKD, Chapter 3: Blood pressure control in people with diabetic kidney disease, Chapter 4: Treatment targeting progression of CKD in people with diabetic kidney disease, and Chapter 5: Antiplatelet or anticoagulant therapy in people with diabetes and CKD.
World Health Organization (WHO) recommendations for guideline development were followed to report this guideline. Systematic reviews were carried out, with outcome ratings and summaries of findings, and we reported the strength of recommendations following the “Grading of Recommendations Assessment, Development and Evaluation” GRADE evidence profiles.
Recomendación 1.1. Se recomienda realizar una prueba anual para la detección de la enfermedad renal diabética (ERD). En los pacientes con DM tipo 1 (DM1) debe realizarse a partir de los 5 años desde el diagnóstico de la DM y en la DM tipo 2 (DM2) o en adultos con diabetes latente autoinmunitaria (LADA), desde el momento de la detección de la enfermedad. Se recomienda la medición de la presencia de albuminuria mediante la estimación del cociente albúmina/creatinina en una muestra esporádica de orina, así como la evaluación del filtrado glomerular estimado (FGe) mediante la ecuación CKD-EPI (2D).
Recomendación 1.2. Remitir al paciente con DM al nefrólogo puede ser adecuado en cualquier situación en la que el médico al cargo del paciente pueda requerir ayuda para el manejo de la enfermedad renal, de acuerdo con las recomendaciones actuales (2D).
Recomendación 1.3. Estará indicado realizar una biopsia renal en el paciente con DM: 1) cuando exista un rápido incremento de la proteinuria o proteinuria de rango nefrótico; 2) si se comprueba proteinuria >1g/día en orina de 24h, en los pacientes con DM de duración inferior a 5 años; 3) cuando se aprecie un rápido deterioro de la función renal en ausencia de retinopatía diabética; 4) si existen alteraciones en el sedimento urinario (hematíes dismórficos) no asociadas con la presencia de una infección urinaria concomitante; 5) cuando se compruebe un rápido deterioro del FGe en el paciente con DM y función renal previa estable, 6) en presencia de signos clínicos y/o analíticos de enfermedad autoinmunitaria asociada (2D).
Capítulo 2: Control metabólico en las personas con diabetes y enfermedad renal crónicaRecomendación 2.1. Los pacientes con DM2 y ERC deben ser tratados con un inhibidor del cotransportador de sodio-glucosa-2 (ISGLT2) y, si es necesario, debe instaurarse un tratamiento farmacológico adicional para mejorar el control glucémico (1B).
Recomendación 2.2. Los agonistas del receptor del péptido-1 similar al glucagón (arGLP1) se recomiendan como tratamiento farmacológico adicional, ya que han mostrado beneficios cardiovasculares y, recientemente, beneficios renales en términos de progresión de la ERC en las personas con DM2 (1B).
Capítulo 3: Control de la presión arterial en las personas con enfermedad renal diabéticaRecomendación 3.1. Se recomienda el control de la presión arterial (PA) con un objetivo de presión arterial sistólica (PAS) de <130mmHg cuando se tolere, en los pacientes con insuficiencia renal diabética. En caso contrario, se recomienda un objetivo general de PAS<140 (2C).
Recomendación 3.2. Se recomienda iniciar un inhibidor de la enzima convertidora de angiotensina (IECA) o un antagonista de los receptores de la angiotensina II (ARA-II) indistintamente en los pacientes con hipertensión y nefropatía diabética (2B).
Recomendación 3.3. Los antagonistas esteroideos de los receptores de mineralocorticoides (ARM) son probablemente útiles para el manejo de la hipertensión en los pacientes con FGe>30ml/min/1,73m2 y potasio sérico <4,8mmol/l (2D).
Recomendación 3.4. Aunque los ARM no esteroideos pueden ser útiles en el control de la PA, no los recomendamos para el manejo de la PA debido a la actual falta de evidencias (2B).
Recomendación 3.5. Debe evitarse la combinación de IECA con tratamiento con ARA-II o aliskiren en los pacientes con diabetes y ERC (2D).
Capítulo 4: Tratamiento dirigido a la progresión de la enfermedad renal crónica en las personas con enfermedad renal diabéticaRecomendación 4.1. Los pacientes con DM2 y ERC con un FGe≥20ml/min/1,73m2 deben recibir tratamiento con un inhibidor del cotransportador sodio-glucosa tipo 2, que se mantendrá hasta la fase terminal de la enfermedad renal (diálisis o trasplante renal) (1A).
Recomendación 4.2. Recomendamos que se inicie el tratamiento con un inhibidor de la enzima convertidora de la angiotensina (IECA) o un bloqueante de los receptores de la angiotensina II (ARA-II) en los pacientes con diabetes, hipertensión y albuminuria. Estos fármacos deben ser titulados hasta la dosis máxima tolerada que haya sido aprobada (1A).
Recomendación 4.3. Los pacientes con DM2, FGe≥25ml/min/1,73m2 y albuminuria aumentada (CAC >100mg/g) en tratamiento con la dosis máxima tolerada estable de inhibidores del SRA deben ser tratados con un arGLP1 con beneficio renal demostrado (1A).
Recomendación 4.4. Se propone iniciar un antagonista no esteroideo del receptor de mineralocorticoides (ARM) con beneficio renal y/o cardiovascular demostrado en los pacientes con DM2, FGe≥25ml/min/1,73m2, concentración sérica de potasio normal e incremento de albuminuria (CAC≥30mg/g), pese a recibir la dosis máxima tolerada de un inhibidor del SRA (1B).
Recomendación 4.5. Se propone mantener una ingesta proteica de 0,6-0,8g/kg (peso)/día para los pacientes con diabetes y ERC no tratados con diálisis (2C).
Capítulo 5: Tratamiento antiagregante plaquetario o anticoagulante en las personas con diabetes y enfermedad renal crónicaRecomendación 5.1. Los pacientes con diabetes mellitus tipo 1 (DM1) o diabetes mellitus tipo 2 (DM2) y enfermedad renal crónica (ERC) con enfermedad cardiovascular aterosclerótica establecida deben ser tratados con dosis bajas de ácido acetilsalicílico (75-100mg/día) para la prevención secundaria (1B).
Recomendación 5.2. Tras un síndrome coronario agudo o una intervención coronaria percutánea se recomienda el tratamiento antiplaquetario dual (con dosis bajas de ácido acetilsalicílico y un inhibidor 2Y12), seguido de una monoterapia antiplaquetaria con una duración determinada por un equipo multidisciplinar en función del perfil riesgo-beneficio (1B).
Recomendación 5.3. En los pacientes con DM1 o DM2 y ERC y antecedente de un ictus isquémico no cardioembólico o un ictus isquémico transitorio se recomienda el tratamiento antiagregante a largo plazo para reducir el riesgo de ictus recurrente (1C).
Recomendación 5.4. En los pacientes con DM1 o DM2 y ERC que han sufrido un ictus isquémico agudo no cardioembólico/ataque isquémico transitorio se deberá considerar el tratamiento dual con antiagregantes plaquetarios (con dosis bajas de ácido acetilsalicílico y un inhibidor de P2Y12) seguido de monoterapia con un antiagregante (2C).
Recomendación 5.5. En los pacientes con DM1 o DM2 y ERC en estadios 3 o superiores no existe evidencia clara de un perfil beneficio-riesgo favorable para recomendar la prescripción de ácido acetilsalicílico a dosis bajas para la prevención primaria de la enfermedad cardiovascular aterosclerótica (2C).
Recomendación 5.6. Los pacientes con fibrilación auricular no valvular, DM1 o DM2 y ERC en estadios 1-4 (dabigatrán hasta estadio 3b) deben ser tratados preferentemente con anticoagulantes orales de acción directa frente a antagonistas de la vitamina K (1B).
Recomendación 5.7. Los pacientes con tromboembolia venosa, DM1 o DM2 y ERC en estadios 1-4 (dabigatrán hasta estadio 3b) deben ser tratados preferentemente con anticoagulantes orales de acción directa frente a los antagonistas de la vitamina K (2C).
Métodos de elaboración de las guíasEl proceso de desarrollo consensuado fue dirigido por el Grupo Español de Estudio de Nefropatía Diabética (GEENDIAB) bajo los auspicios de la Sociedad Española de Nefrología (SEN).
Estas guías se ajustaron a las recomendaciones de la Organización Mundial de la Salud (OMS) para el desarrollo de guías (Apéndice 1) (material suplementario)1 y se han notificado de acuerdo con la lista de verificación para la notificación Appraisal of Guidelines for Research and Evaluation (AGREE) II2.
Las fases de ejecución de las directrices fueron las siguientes:
- 1.
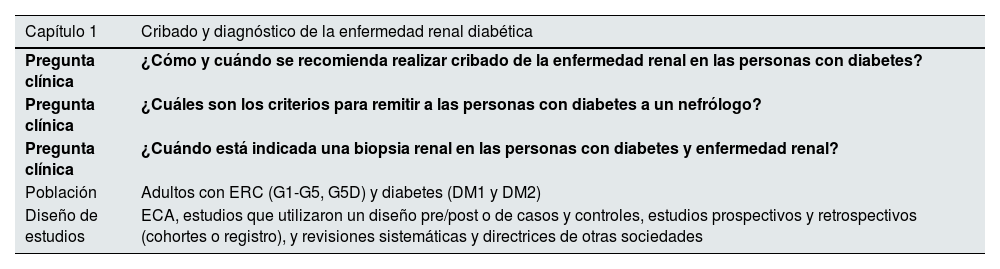
Definición del ámbito de las guías. Las preguntas clave de la guía se formularon utilizando la metodología Población, Intervención, Comparador y Resultado (PICO) (tabla 1).
Tabla 1.Preguntas clínicas y temas de esta revisión sistemática en el formato PICO
Capítulo 1 Cribado y diagnóstico de la enfermedad renal diabética Pregunta clínica ¿Cómo y cuándo se recomienda realizar cribado de la enfermedad renal en las personas con diabetes? Pregunta clínica ¿Cuáles son los criterios para remitir a las personas con diabetes a un nefrólogo? Pregunta clínica ¿Cuándo está indicada una biopsia renal en las personas con diabetes y enfermedad renal? Población Adultos con ERC (G1-G5, G5D) y diabetes (DM1 y DM2) Diseño de estudios ECA, estudios que utilizaron un diseño pre/post o de casos y controles, estudios prospectivos y retrospectivos (cohortes o registro), y revisiones sistemáticas y directrices de otras sociedades Capítulo 2 Control metabólico en las personas con diabetes y ERC Pregunta clínica En los pacientes con DM1 o DM2 y ERC, ¿cuáles son los efectos de la medicación hipoglucemiante sobre los resultados y los daños clínicamente relevantes? Población Adultos con ERC (G1-G5, G5D, G1T-G5T) y diabetes (DM1 o DM2) Intervención Tratamientos más antiguos: metformina, sulfonilureas o tiazolidinedionasTratamientos más recientes: inhibidores de la alfa-glucosidasa, AR GLP-1, inhibidores de la DPP-4, SGLT2iEn la DM1: diferentes tipos de insulina Comparador Tratamiento estándar/placebo Resultados Resultados críticos e importantes: mortalidad (todas las causas), muerte cardiovascular, muerte por causa renal, necesidad de inicio de TRR, duplicación de la creatinina sérica, nueva aparición de albuminuria >300mg/g, daño renal, acontecimientos adversos cardiovasculares mayores, insuficiencia cardíaca, infarto de miocardio, accidente cerebrovascular, abandonos del tratamiento debidos a efectos adversos, efectos adversos graves, hiperpotasemia, HbA1c (%), FGe, porcentaje de cambio con respecto al valor basal de uACR, progresión de la retinopatía diabética, cetoacidosis diabética, infecciones de las vías urinarias, efectos adversos gastrointestinales, hipoglucemia, amputaciones, fracturas Diseño de estudios ECA Capítulo 3 Control de la presión arterial en las personas con enfermedad renal diabética Pregunta clínica En los pacientes con DM1 o DM2 y ERC, ¿cuál es el objetivo de la presión arterial? Población Adultos con ERC (G1-G5, G5D, G1T-G5T) y diabetes (DM1 o DM2) Intervención Control intensivo de la presión arterial Comparador Control estándar de la presión arterial Resultados Resultados críticos e importantes: presión arterial sistólica y diastólica, necesidad de inicio de TRR, duplicación de creatinina sérica, FGe, uACR, mortalidad (todas las causas), muerte cardiovascular, insuficiencia cardíaca, infarto de miocardio, hiperpotasemia y abandonos de tratamiento por efectos adversos Diseño de estudios ECA Pregunta clínica En los pacientes con DM1 o DM2 y ERC, ¿cuáles son los efectos de los diferentes tratamientos para la hipertensión sobre los resultados y los daños clínicamente relevantes? Población Adultos con ERC (G1-G5, G5D, G1T-G5T) y diabetes (DM1 o DM2) Intervención Posibles tratamientos: ARA, IECA, ARM no esteroideos, aliskiren Comparador Otras terapias/tratamiento estándar/placebo Resultados Resultados críticos e importantes: presión arterial sistólica y diastólica, necesidad de inicio de TRR, duplicación de creatinina sérica, FGe, uACR, mortalidad (todas las causas), muerte cardiovascular, insuficiencia cardíaca, infarto de miocardio, hiperpotasemia y abandonos del tratamiento por efectos adversos Diseño de estudios ECA Capítulo 4 Tratamiento dirigido a la progresión de la ERC en las personas con enfermedad renal diabética Pregunta clínica En los pacientes con DM1 o DM2 y ERC, ¿cuáles son los efectos de los diferentes tratamientos dirigidos a la progresión de la ERC sobre los resultados y los daños clínicamente relevantes? Población Adultos con ERC (G1-G5, G5D, G1T-G5T) y diabetes (DM1 o DM2) Intervención Posibles tratamientos: ARA, IECA, ARM esteroideos y no esteroideos, aliskiren, SGLT2i, GLP1RA, GLP1 RA/GIP, DPP4i, pentoxifilina, restricción proteica Comparador Otras terapias/tratamiento estándar/placebo Resultados Resultados críticos e importantes: mortalidad (todas las causas), muerte cardiovascular, muerte por causa renal, necesidad de inicio de TRR, duplicación de la creatinina sérica, nueva aparición de albuminuria >300 mg/g, daño renal, acontecimientos adversos cardiovasculares mayores, insuficiencia cardíaca, infarto de miocardio, accidente cerebrovascular, abandonos del tratamiento debidos a efectos adversos, efectos adversos graves, hiperpotasemia, HbA1c (%), FGe, porcentaje de cambio con respecto al valor basal de uACR, progresión de la retinopatía diabética, cetoacidosis diabética, infecciones de las vías urinarias, efectos adversos gastrointestinales, hipoglucemia, amputaciones, fracturas Diseño de estudios ECA Capítulo 5 Tratamiento antiagregante plaquetario o anticoagulante en las personas con diabetes y ERC Pregunta clínica En los pacientes con DM1 o DM2 y ERC, ¿cuáles son las indicaciones y los efectos del tratamiento antiplaquetario o anticoagulante sobre los resultados clínicamente relevantes y los daños clínicamente relevantes? Población Adultos con ERC (G1-G5, G5D, G1T-G5T) y diabetes (DM1 o DM2) Intervención Tratamiento antiagregante plaquetario (ácido acetilsalicílico, inhibidores de la fosfodiesterasa: dipiridamol y cilostazol, e inhibidores de P2Y12: clopidogrel, prasugrel y ticagrelor) y anticoagulante (acenocumarol, warfarina, apixabán, rivaroxabán, edoxabán y dabigatrán) Comparador Placebo/otro tratamiento Resultados Resultados críticos e importantes: mortalidad (todas las causas), muerte cardiovascular, muerte por causa renal, infarto de miocardio, accidente cerebrovascular, abandonos del tratamiento por efectos adversos, efectos adversos graves, hemorragia Diseño de estudios ECA ARA: antagonistas de los receptores de la angiotensina II; ARM: antagonista no esteroideo del receptor de mineralocorticoides; DM1: diabetes mellitus tipo 1; DM2: diabetes mellitus tipo 2; ECA: ensayos controlados aleatorizados; ERC: enfermedad renal crónica; FGe: filtrado glomerular estimado; HbA1c: hemoglobina glicosilada; IECA: inhibidores de la enzima convertidora de la angiotensina.
- 2.
Definición del comité directivo. Se seleccionó un comité de dirección específico para el asunto, formado por expertos entre los que se encontraban nefrólogos y endocrinólogos en el campo de interés, miembros del Grupo Español de Estudio de Nefropatía Diabética (GEENDIAB) y 2 metodólogos.
- 3.
Implementación de estrategias de búsqueda bibliográfica centradas en cada una de las preguntas PICO. Los estudios relevantes se obtuvieron a partir de una búsqueda bibliográfica sistemática. Se realizaron búsquedas en MEDLINE y CENTRAL (Registro Cochrane Central de Ensayos Controlados) hasta julio de 2023 (Apéndice 2) (material suplementario).
- 4.
Selección de estudios según criterios de inclusión predefinidos. Para el capítulo 1, la selección no se limitó a ensayos clínicos aleatorizados, sino que también incluyó estudios que utilizaron un diseño pre/post o de casos y controles, estudios prospectivos y retrospectivos (cohortes o registro), y revisiones sistemáticas y directrices de otras sociedades. Para el resto de capítulos solo se incluyeron ensayos controlados aleatorizados (ECA) que incluyeran a personas con diabetes y ERC. Se incluyeron revisiones y metanálisis para la búsqueda manual de bibliografía adicional.
- 5.
Extracción de datos y evaluación crítica de la bibliografía. Se utilizaron formularios estándar de extracción de datos. Para los ensayos controlados aleatorizados, el riesgo de sesgo se evaluó mediante la herramienta Cochrane de evaluación del riesgo de sesgo3; para los estudios observacionales se utilizó la herramienta ROBINS-I4.
- 6.
Realizar la síntesis de la evidencia y el metanálisis de los estudios incluidos. Los resultados explorados fueron: mortalidad por todas las causas, mortalidad cardiovascular, mortalidad por causa renal, episodios cardiovasculares individuales (infarto de miocardio, ictus, insuficiencia cardíaca), necesidad de inicio de TRR, duplicación de la creatinina sérica, nueva aparición de albuminuria >300mg/g, compuesto renal, episodios adversos cardiovasculares mayores, insuficiencia cardíaca, infarto de miocardio, accidente cerebrovascular, abandonos del tratamiento debidos a efectos adversos, efectos adversos graves, hiperpotasemia, hemoglobina glucosilada (HbA1c) (%), FGe, porcentaje de cambio con respecto al valor basal de la uACR, progresión de la retinopatía diabética, cetoacidosis diabética, infecciones urinarias, efectos adversos gastrointestinales, hipoglucemia, amputaciones, fracturas.
Los análisis de resultados se realizaron incluyendo todos los ECA. Para los resultados dicotómicos, los resultados se expresaron como cocientes de riesgos (RR) con intervalos de confianza (IC) del 95%. Cuando se utilizaron escalas de medición continuas para evaluar los efectos del tratamiento, se utilizó la diferencia de medias (MD). Los resultados de tiempo transcurrido hasta el acontecimiento se abordaron como variables continuas. Para los recuentos y las tasas, los resultados de un estudio se expresaron como RR, y los logaritmos (naturales) de los cocientes de tasas se combinaron entre los estudios mediante el método de la varianza inversa genérica. Los datos se agruparon con ayuda del modelo de efectos aleatorios.
Los estudios con múltiples grupos de intervención se analizaron con diferentes métodos: 1) utilizando solo los grupos con la intervención de interés para crear una única comparación por pares (si había 3 grupos que incluían diferentes terapias de inducción, solo se incluyó un tratamiento de inducción), y 2) incluyendo cada comparación por pares por separado, pero con los grupos de intervención compartidos divididos de forma aproximadamente uniforme entre las comparaciones. En este último caso, para los resultados dicotómicos se dividió tanto el número de episodios como el número total de pacientes y, para los resultados continuos, solo se dividió el número total de participantes y no se modificaron las medias ni las desviaciones estándar.
Se evaluaron cuidadosamente los datos numéricos importantes, como la población cribada, los pacientes aleatorizados, la población por intención de tratar (ITT), la población según tratamiento y la población por protocolo (PP). Se investigaron los abandonos, las pérdidas durante el seguimiento y las retiradas. Se evaluaron críticamente los problemas relacionados con los datos perdidos y los métodos de imputación. La heterogeneidad se analizó mediante una prueba de chi cuadrado sobre N-1 grados de libertad, con un alfa de 0,05 utilizado para la significación estadística y con la prueba de I2. Los valores de I2 del 30-60%, del 50-90% y del 75-100% corresponden a niveles moderados, sustanciales y considerables de heterogeneidad. Se utilizaron funnel plots para evaluar la posible existencia de sesgo por estudios pequeños.
Se elaboraron tablas de resumen de resultados (SoF) para incluir una descripción de la población y de la intervención y el comparador. Además, las tablas SoF incluían los resultados de la síntesis de datos como estimaciones de efectos relativos y absolutos. En estas tablas se proporciona también la clasificación de la calidad de la evidencia para cada resultado crítico e importante. Las tablas de SoF están disponibles en el Apéndice 3 (material suplementario).
- 7.
Calificación de la fuerza de las recomendaciones, basada en la calidad de la evidencia utilizando el enfoque GRADE. Para calificar las recomendaciones de las directrices se utilizó la nomenclatura GRADE (Grading of Recommendation, Assessment, Development, and Evaluation)5. La fuerza de las recomendaciones individuales se calificó como fuerte (Nivel 1) o débil (Nivel 2), y la calidad de la evidencia de apoyo se mostró como A (alta), B (moderada), C (baja) o D (muy baja) (Apéndice 4) (material suplementario).
- 8.
Finalización de las recomendaciones de las directrices y justificación de las mismas. El comité directivo integró la evidencia bibliográfica y redactó las recomendaciones calificadas y la justificación subyacente, calificó la fuerza de las recomendaciones y desarrolló puntos de práctica.
- 9.
Convocatoria de una revisión pública del borrador de las guías en diciembre 2023.
- 10.
Modificación de las guías en función de los comentarios de la revisión externa. Un comité de expertos validadores validó las recomendaciones utilizando las directrices AGREE II2.
- 11.
Finalización y publicación de la directriz.
Recomendación 1.1. Se recomienda realizar una prueba anual para la detección de la ERD. En los pacientes con DM tipo 1 (DM1) debe realizarse a partir de los 5 años desde el diagnóstico de la DM y en DM tipo 2 (DM2) o en adultos con diabetes latente autoinmunitaria (LADA), desde el momento de la detección de la enfermedad. Se recomienda la medición de la presencia de albuminuria mediante la estimación del cociente albúmina/creatinina en una muestra esporádica de orina y el FGe mediante la ecuación CKD-EPI.
Fuerza de recomendación: 2D.
Justificación: En individuos adultos, muchas guías recomiendan que la detección de la proteinuria sea realizada mediante la determinación del cociente albúmina/creatinina en orina (uACR), preferiblemente en la orina de primera hora de la mañana. La concentración de albúmina o de proteínas debe ser siempre referida a la concentración de creatinina, para minimizar el efecto del grado de hidratación (concentración urinaria). Este resultado se aproxima bastante a la pérdida en orina de 24h, si no existe una desviación excesiva en el área de superficie corporal6–9. Debemos tener en cuenta que hay una amplia variabilidad en los métodos de estimación de la albuminuria, por lo que para considerar la existencia de albuminuria patológica se requiere la estimación de más de una muestra10,11. Los factores que pueden tener influencia sobre la determinación de la albuminuria, independientemente del daño renal existente, son el ejercicio, las infecciones, la fiebre, una insuficiencia cardíaca congestiva, la menstruación, la hiperglucemia o una PA muy elevada12. Se necesitan 2 valores elevados en 3 muestras, obtenidas con separación de al menos 3 meses, para considerar que la presencia de albuminuria es significativa. Es recomendable obtener el cociente urinario proteína/creatinina en los pacientes con sospecha de patología renal intersticial (síndrome de Sjögren, nefrotoxicidad por agentes antirretrovirales, etc.), dado que en dichas situaciones la proteinuria es debida principalmente a proteínas tubulares de bajo peso molecular, diferentes a la albúmina. La existencia de una disociación significativa entre la uACR y el cociente proteína/creatinina también debería señalar la posibilidad de la presencia de cadenas ligeras libres en la orina (proteinuria de Bence-Jones) o de inmunoglobulinas (como en el síndrome nefrótico impuro).
Por otra parte, para medir el filtrado glomerular (FG) existen diferentes ecuaciones: aclaramiento de creatinina con orina de 24h, aclaramiento de creatinina mediante la fórmula de Cockcroft-Gault, estimación mediante MDRD (Modification of Diet in Renal Diseases), EKFC o CKD-EPI (Chronic Kidney Disease Epidemiology) basadas en la creatinina plasmática (2009) o CKD-EPI creatinina-cistatina (2021), medición del FG por métodos isotópicos o mediante iohexol. Actualmente el método usado con mayor frecuencia es la ecuación CKD-EPI, que está prácticamente implantada en todos los laboratorios de los hospitales y centros de salud13,14. Los métodos de estimación del FGe mediante ecuaciones basadas en la cistatina tienen el inconveniente de su mayor coste y no están implantados en los laboratorios de forma rutinaria, aunque serían muy útiles en poblaciones de edad avanzada o en los pacientes con patologías asociadas a la ERC, como insuficiencia cardíaca, cáncer, malnutrición o cirrosis hepática, y su estimación es aconsejada por algunas guías de práctica clínica. Los métodos de estimación isotópicos pueden ser realizados solamente en centros hospitalarios15,16. Es muy posible que en un futuro muy próximo se aconseje la medición del FG mediante iohexol, que técnicamente parece factible pero cuya aplicación aún no está ampliamente difundida17.
Existen otros métodos para evaluar la enfermedad renal en los pacientes con DM: a) ecografía reno-vesical: algunos estudios señalan que los datos aportados por métodos ecográficos pueden conducir a la sospecha de la presencia de nefropatía, así como para establecer un diagnóstico diferencial con otras causas de enfermedad renal18,19, o b) otros biomarcadores para la detección precoz de enfermedad renal en los pacientes con DM20–28, tales como marcadores de inflamación, disfunción endotelial o tubular, marcadores en estudios genéticos Genome Wide Association Studies (GWAS), y otros (cistatina, NAG, NGAL, KIM-1, IL-6, Netrin-1, trombospondina-2, glicanos urinarios, exosomas urinarios, VEGF, galectina-3, GDF-15, TNF-alfa soluble). En la actualidad, muchos grupos de investigación están trabajando para poner en marcha baterías de biomarcadores o sistemas combinados que incluyen múltiples datos (gradient boosting machines), pero que por ahora son difíciles de aplicar en práctica clínica diaria29–37. Por todo ello creemos necesario generar más evidencia. Nuestra recomendación es que se apliquen marcadores bien contrastados y reconocidos, aunque puedan ser subrogados.
Recomendación 1.2. Derivar al paciente con DM al nefrólogo puede ser adecuado en cualquier situación en la que el médico al cargo del paciente pueda requerir ayuda para el manejo de la enfermedad renal, de acuerdo con las recomendaciones actuales.
Fuerza de recomendación: 2D.
Justificación: Las situaciones en las que es recomendable una evaluación por nefrología son:
- 1.
Albuminuria o uACR>300mg/g mantenidos en 2 controles sucesivos9.
- 2.
FGe reducido: hasta la fecha, todos los documentos de consenso y Guías de Práctica Clínica recomiendan derivar al paciente con DM cuando el FGe <30ml/min/1,73m2 o cuando el uACR>300mg/g. Sin embargo, publicaciones recientes proponen que dicha derivación sea más precoz, para permitir un manejo compartido con atención primaria y con los especialistas implicados en el cuidado del paciente con DM-1 y DM-238, como una práctica óptima adecuada para la prevención más temprana de la ERD.
- 3.
Deterioro rápido de la función renal: debe considerarse que un paciente con DM presenta progresión del daño renal cuando se constate un deterioro del FGe>5ml/min/año o >10ml/min en 5 años. Se considera que existe progresión con base en 2 aspectos: progresión a un estadio o categoría más grave de deterioro de la función renal (estadio KDIGO 1-5) o albuminuria (<30, 30-299, >300mg/g). También se considera que existe progresión cuando el porcentaje de deterioro del FGe cae más del 25% con respecto al valor basal o cuando el incremento en el uACR es superior al 50%. No obstante, es conveniente recordar que un reciente documento de consenso internacional para definir los criterios de progresión señala como posibles criterios subrogados de «progresión» descensos del 30, 40, 50 o 57% en el FGe dependiendo de factores tales como la elección del punto de partida basal del FGe, o la repercusión de intervenciones que puedan comportar efectos agudos sobre la estimación del FGe39.
- 4.
Control deficiente de la PA: PAS>140mmHg y/o PA diastólica >85mmHg a pesar de un tratamiento antihipertensivo adecuado o hipertensión arterial resistente (≥180/110mmHg) o de un abordaje con 3 fármacos antihipertensivos a dosis máximas toleradas, siendo uno de ellos un diurético.
- 5.
Anemia de causa renal con hemoglobina (Hb<10g/dl) que requiera tratamiento con agentes estimulantes de la eritropoyesis, después de excluir otras causas de anemia (déficit de hierro, folatos o cobalaminas)40.
- 6.
Trastornos del equilibrio ácido-base, principalmente acidosis metabólica no controlada.
- 7.
Deterioro de la función renal después de iniciar tratamiento con inhibidores del sistema renina-angiotensina-aldosterona (iSRAA) o de inhibidores del transportador renal de Na-glucosa (iSGLT2), con disminución mantenida y no reversible del FGe igual o superior al 30% con respecto al valor basal o hiperpotasemia ≥5,5mEq/l no controlada41.
- 8.
Duda razonable de que la afectación renal pueda ser debida a enfermedad renal no diabética. En este caso se establecerá un diagnóstico diferencial. El paciente debe ser derivado al nefrólogo para evaluación en casos de sedimento urinario activo (presencia de hematuria), ausencia de retinopatía diabética, corta duración de la DM con adecuado control de la HbA1c, asociación de sintomatología sistémica que incremente la sospecha de otras patologías asociadas, disfunción renal rápida o rápido incremento de la proteinuria o presencia de síndrome nefrótico.
Recomendación 1.3. La indicación de realizar una biopsia renal en el paciente con DM tendrá lugar en las siguientes situaciones: 1) cuando exista un rápido incremento de la proteinuria o proteinuria de rango nefrótico; 2) si se comprueba proteinuria >1g/día en orina de 24h, en los pacientes con DM de duración inferior a 5 años; 3) cuando exista un rápido deterioro de la función renal en ausencia de retinopatía diabética; 4) cuando existan alteraciones en el sedimento urinario (hematíes dismórficos) no asociadas con la presencia de una infección urinaria concomitante; 5) si se comprueba un rápido deterioro del FGe en el paciente con DM y función renal previa estable, 6) cuando existan signos clínicos y/o analíticos de enfermedad autoinmunitaria asociada42,43.
Fuerza de recomendación: 2D.
Justificación: Algunos estudios han descrito que diversas situaciones pueden estar asociadas con la presencia de lesiones renales no debidas a la DM: elevación de PA sistólica, adecuado control de HbA1c en pacientes con una corta duración de la DM y ausencia de retinopatía diabética44. La retinopatía diabética ofrece alta sensibilidad (87%) y especificidad (93%) para predecir lesiones histológicas más graves de ERD. No obstante hay que añadir que no todos los estudios muestran resultados similares, pero algunos de ellos describen la presencia de lesiones compatibles con ERD en ausencia de retinopatía diabética45.
La presencia de una enfermedad renal no debida a la DM puede conducir a tratamientos diferentes dependiendo de la patología subyacente y, por lo tanto, a un pronóstico evolutivo diferente. La progresión hacia un estadio de enfermedad renal más avanzado suele ser mayor en los pacientes con enfermedad renal de causa diabética (44%), en comparación con las formas mixtas (diabética+no diabética) (18%) o con las de causa no diabética (12%). Fiorentino et al.46 han publicado un metanálisis de 48 estudios de biopsias renales en los pacientes con DM, entre ellos un total de 4.876 biopsias. Dicho metanálisis ha mostrado una gran variabilidad en la prevalencia de nefropatía diabética (del 6,5 al 94%), así como de nefropatías no diabéticas (del 3 al 83%) y de formas mixtas (del 4 al 45%). La conclusión es que el diagnóstico de enfermedad no diabética en pacientes con DM es muy elevado, en cuyo caso la nefropatía IgA es la causa más frecuente (del 3 al 59%).
Ante la indicación de una biopsia renal en el paciente con DM, es muy oportuno considerar el riesgo/beneficio de tal indicación de forma individualizada.
Capítulo 2: Control metabólico en las personas con diabetes y enfermedad renal crónicaComo prevención primaria, el control metabólico estricto es la intervención más eficaz para conseguir nefroprotección, tanto en la DM1 como en la DM247. Cuanto menor sea el valor de HbA1c obtenido, menor será el riesgo de albuminuria, ya que el control metabólico estricto disminuye el riesgo de aparición y progresión de la ERC en las personas con diabetes. En la DM2, un mejor control glucémico también se asocia a un menor número de complicaciones microangiopáticas y a una menor progresión de la albuminuria: en prevención secundaria, un control glucémico estricto puede disminuir la progresión de la albuminuria. Se recomienda una HbA1c<7%; de forma individualizada, podrían considerarse objetivos inferiores al 6,5% en los pacientes con una esperanza de vida larga, siempre que puedan alcanzarse con fármacos hipoglucemiantes sin riesgo de hipoglucemias. Del mismo modo, objetivos menos estrictos (<8%) son válidos en los pacientes con antecedentes de hipoglucemia grave, corta esperanza de vida o complicaciones microvasculares o macrovasculares extensas que requieran tratamiento con insulina, glinidas o sulfonilureas. No obstante, el objetivo de HbA1c debe adaptarse al posible riesgo de hipoglucemia de los fármacos antihiperglucemiantes prescritos. El uso de la hipoglucemia con monitorización continua de la glucosa podría prevenir potencialmente la hipoglucemia.
Las intervenciones sobre el estilo de vida deben ser una parte importante de la atención a las personas con diabetes y ERC y han de reforzarse, ya que la ingesta reducida de sodio, el ejercicio físico y el abandono del tabaco son piedras angulares del tratamiento. En cualquier caso, la mayoría de los pacientes necesitarán asesoramiento dietético y fármacos seleccionados para un abordaje global de la enfermedad.
Recomendación 2.1. Los pacientes con DM2 y ERC deben ser tratados con un inhibidor del cotransportador de sodio-glucosa-2 (SGLT2i) y, si es necesario, con el tratamiento farmacológico adicional necesario para mejorar el control glucémico (tabla S2.1).
Fuerza de la recomendación: 1B.
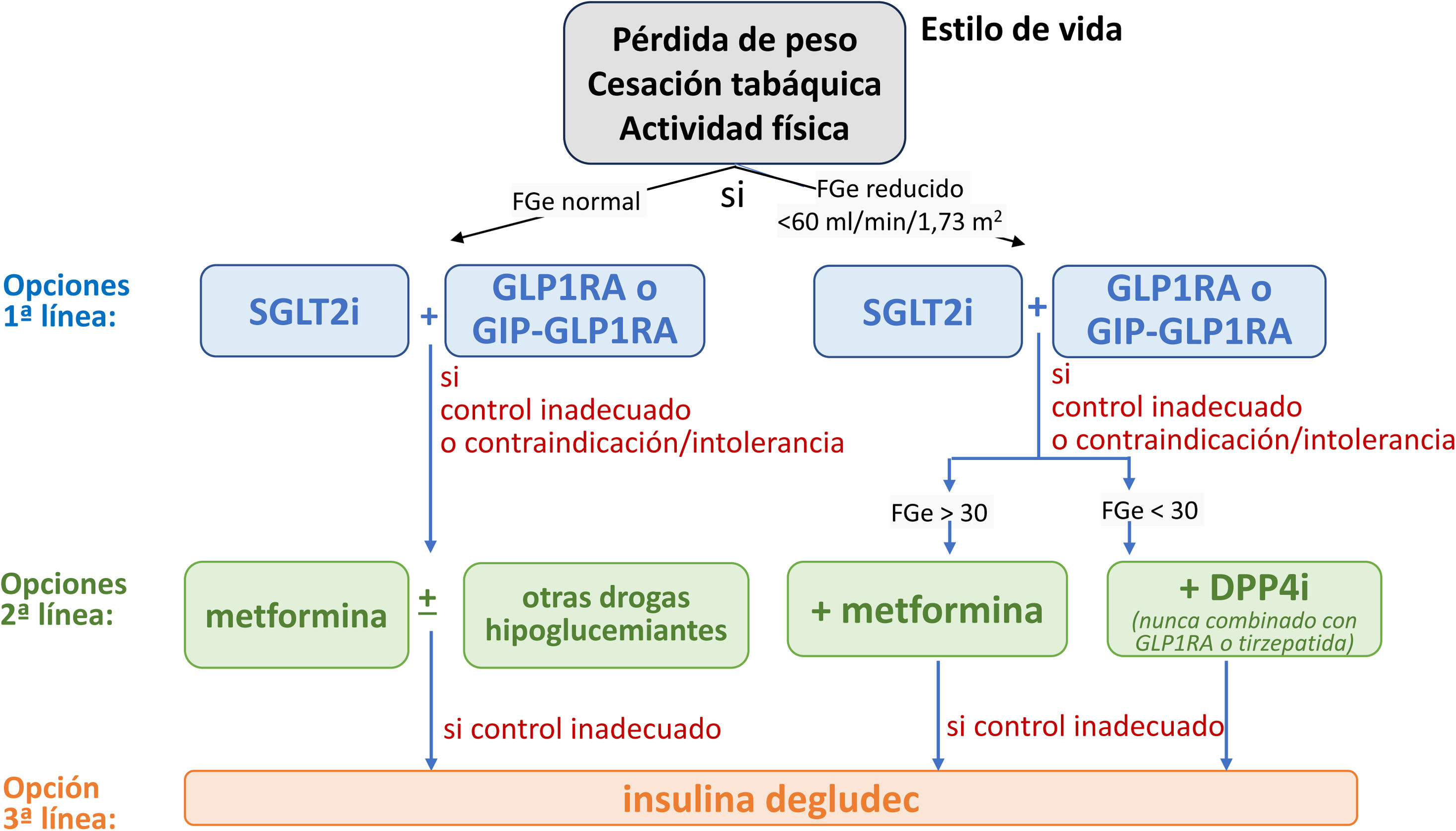
Justificación: En los últimos años, la aparición de los SGLT2i representa un gran avance en la base de evidencias sobre la protección cardiorrenal en la ERC. Los SGLT2i deberían utilizarse como tratamiento de primera línea para la mayor parte de la población en función del FGe (fig. 1), ya que los SGLT2i han mostrado capacidad para disminuir la aparición, la progresión y los episodios cardiovasculares adversos mayores (MACE) de la ERC en los pacientes con DM2, independientemente de su efecto sobre el control glucémico.
Tratamiento farmacológico para el control metabólico en los pacientes con diabetes tipo 2 y enfermedad renal crónica. DPP4i: inhibidores de la dipeptidil peptidasa-4; FGe: filtrado glomerular estimado; GIP-GLP1RA: agonista dual de los receptores del polipéptido insulinotrópico dependiente de la glucosa (GIP) y del péptido similar al glucagón-1 (GLP-1); GLP1 AR: agonista del receptor del péptido-1 similar al glucagón; SGLT2i: inhibidor del cotransportador 2 de sodio-glucosa.
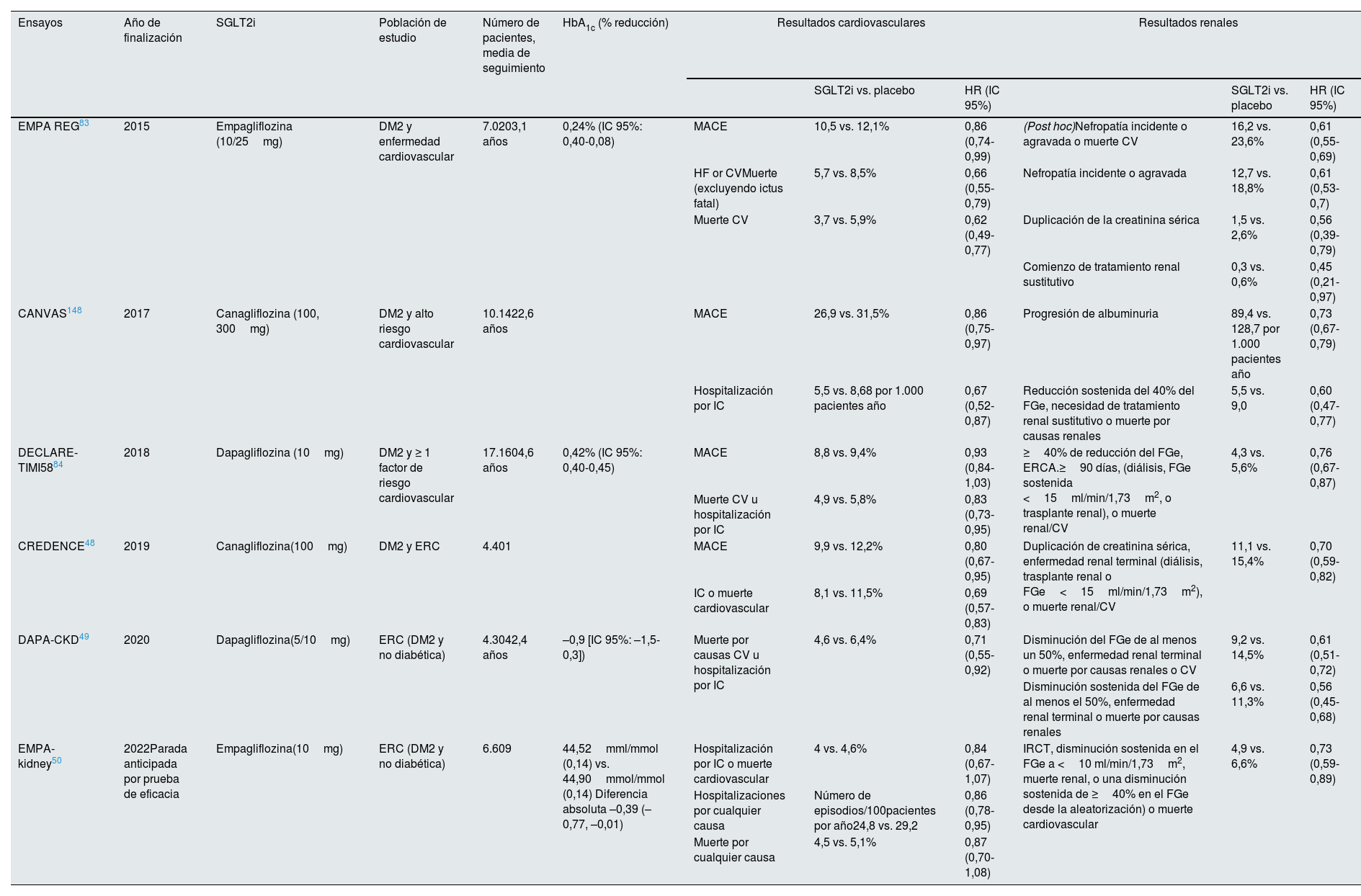
Los SGLT2i disminuyen la hiperglucemia al aumentar la excreción urinaria de glucosa, ya que el cotransportador SGLT2 es responsable del 90% de la reabsorción de glucosa en el túbulo proximal. Primero se descubrió que los SGLT2i tenían efectos cardiovasculares y renoprotectores en ensayos de seguridad cardiovascular, en los que la nefroprotección era un criterio de valoración secundario. En abril de 2019 se publicó el estudio CREDENCE48, el primer ensayo clínico que investigó los efectos de los SGLT2i en los pacientes con DM y ERC (FGe≥30ml/min/1,73m2 y albuminuria ≥ 300mg/g) con objetivos renales primarios. La canagliflozina redujo en un 30% la incidencia de acontecimientos renales (ERC avanzada, duplicación de la creatinina sérica o muerte renal o cardiovascular). La magnitud del beneficio hizo que el ensayo se interrumpiera prematuramente. El ensayo Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD49) incluyó participantes con y sin ERT2, y mostró beneficios cardiorrenales en ambos grupos. EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin)50 estudió el efecto de otro SGLT2i, empagliflozina, y mostró resultados similares en una población con ERC más amplia, confirmando así el beneficio de la inhibición de SGLT2 sobre el riesgo de progresión de la enfermedad renal o de muerte por causas cardiovasculares en la ERC diabética y no diabética en un espectro más amplio de pacientes. Este estudio incluyó a los pacientes sin albuminuria, anteriormente infrarrepresentados en la mayoría de los ensayos. Reveló que los agentes SGLT2i tienen beneficios basados en la evidencia en la reducción de la tasa de progresión de la ERC a insuficiencia renal. En resumen, los SGLT2i deben prescribirse a los pacientes elegibles para abordar la carga global de la nefropatía diabética, la ERC y sus complicaciones cardiovasculares independientemente del control glucémico, ya que la mejoría de la HbA1c es bastante moderada en los pacientes con un FGe bajo (tabla 2).
Estudios con SGLT2i en los pacientes con DM2 y ERC
| Ensayos | Año de finalización | SGLT2i | Población de estudio | Número de pacientes, media de seguimiento | HbA1c (% reducción) | Resultados cardiovasculares | Resultados renales | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SGLT2i vs. placebo | HR (IC 95%) | SGLT2i vs. placebo | HR (IC 95%) | ||||||||
| EMPA REG83 | 2015 | Empagliflozina (10/25mg) | DM2 y enfermedad cardiovascular | 7.0203,1 años | 0,24% (IC 95%: 0,40-0,08) | MACE | 10,5 vs. 12,1% | 0,86 (0,74-0,99) | (Post hoc)Nefropatía incidente o agravada o muerte CV | 16,2 vs. 23,6% | 0,61 (0,55-0,69) |
| HF or CVMuerte (excluyendo ictus fatal) | 5,7 vs. 8,5% | 0,66 (0,55-0,79) | Nefropatía incidente o agravada | 12,7 vs. 18,8% | 0,61 (0,53-0,7) | ||||||
| Muerte CV | 3,7 vs. 5,9% | 0,62 (0,49-0,77) | Duplicación de la creatinina sérica | 1,5 vs. 2,6% | 0,56 (0,39-0,79) | ||||||
| Comienzo de tratamiento renal sustitutivo | 0,3 vs. 0,6% | 0,45 (0,21-0,97) | |||||||||
| CANVAS148 | 2017 | Canagliflozina (100, 300mg) | DM2 y alto riesgo cardiovascular | 10.1422,6 años | MACE | 26,9 vs. 31,5% | 0,86 (0,75-0,97) | Progresión de albuminuria | 89,4 vs. 128,7 por 1.000 pacientes año | 0,73 (0,67-0,79) | |
| Hospitalización por IC | 5,5 vs. 8,68 por 1.000 pacientes año | 0,67 (0,52-0,87) | Reducción sostenida del 40% del FGe, necesidad de tratamiento renal sustitutivo o muerte por causas renales | 5,5 vs. 9,0 | 0,60 (0,47-0,77) | ||||||
| DECLARE-TIMI5884 | 2018 | Dapagliflozina (10mg) | DM2 y ≥ 1 factor de riesgo cardiovascular | 17.1604,6 años | 0,42% (IC 95%: 0,40-0,45) | MACE | 8,8 vs. 9,4% | 0,93 (0,84-1,03) | ≥40% de reducción del FGe, ERCA.≥90 días, (diálisis, FGe sostenida <15ml/min/1,73m2, o trasplante renal), o muerte renal/CV | 4,3 vs. 5,6% | 0,76 (0,67-0,87) |
| Muerte CV u hospitalización por IC | 4,9 vs. 5,8% | 0,83 (0,73-0,95) | |||||||||
| CREDENCE48 | 2019 | Canagliflozina(100mg) | DM2 y ERC | 4.401 | MACE | 9,9 vs. 12,2% | 0,80 (0,67-0,95) | Duplicación de creatinina sérica, enfermedad renal terminal (diálisis, trasplante renal o FGe<15ml/min/1,73m2), o muerte renal/CV | 11,1 vs. 15,4% | 0,70 (0,59-0,82) | |
| IC o muerte cardiovascular | 8,1 vs. 11,5% | 0,69 (0,57-0,83) | |||||||||
| DAPA-CKD49 | 2020 | Dapagliflozina(5/10mg) | ERC (DM2 y no diabética) | 4.3042,4 años | –0,9 [IC 95%: –1,5-0,3]) | Muerte por causas CV u hospitalización por IC | 4,6 vs. 6,4% | 0,71 (0,55-0,92) | Disminución del FGe de al menos un 50%, enfermedad renal terminal o muerte por causas renales o CV | 9,2 vs. 14,5% | 0,61 (0,51-0,72) |
| Disminución sostenida del FGe de al menos el 50%, enfermedad renal terminal o muerte por causas renales | 6,6 vs. 11,3% | 0,56 (0,45-0,68) | |||||||||
| EMPA-kidney50 | 2022Parada anticipada por prueba de eficacia | Empagliflozina(10mg) | ERC (DM2 y no diabética) | 6.609 | 44,52mml/mmol (0,14) vs. 44,90mmol/mmol (0,14) Diferencia absoluta –0,39 (–0,77, –0,01) | Hospitalización por IC o muerte cardiovascular | 4 vs. 4,6% | 0,84 (0,67-1,07) | IRCT, disminución sostenida en el FGe a <10 ml/min/1,73m2, muerte renal, o una disminución sostenida de ≥40% en el FGe desde la aleatorización) o muerte cardiovascular | 4,9 vs. 6,6% | 0,73 (0,59-0,89) |
| Hospitalizaciones por cualquier causa | Número de episodios/100pacientes por año24,8 vs. 29,2 | 0,86 (0,78-0,95) | |||||||||
| Muerte por cualquier causa | 4,5 vs. 5,1% | 0,87 (0,70-1,08) | |||||||||
IC 95%: intervalo de confianza al 95%; CV: cardiovascular; DM2: diabetes mellitus tipo 2; ERC: enfermedad renal crónica; FGe: filtrado glomerular estimado; IC: insuficiencia cardíaca; IRCT: insuficiencia renal crónica terminal; MACE: acontecimientos cardiovasculares adversos mayores (por sus siglas en inglés).
Esta guía recomienda el uso de SGLT2i como agentes nefroprotectores en los pacientes con DM2 y un FGe >20ml/min/1,73m2, independientemente del uso de metformina. La administración de SGLT2i debe continuarse hasta la insuficiencia renal terminal (diálisis o trasplante renal). Dado su mecanismo de acción, los efectos protectores reno- y cardiovasculares persisten incluso cuando el FG disminuye <45ml/min/1,73m2, donde el efecto sobre la disminución de la glucemia es mínimo.
Recomendación 2.2. Los agonistas del receptor del péptido-1 similar al glucagón se recomiendan como tratamiento farmacológico adicional, ya que han mostrado beneficios cardiovasculares y, recientemente, beneficios renales en términos de progresión de la ERC en personas con DM2 (tablas S2.2-S2.10).
Fuerza de la recomendación: 1B.
Justificación: Los GLP1RA proporcionan protección cardiovascular en los pacientes con ERC. Además, tanto los SGLT2i como los GLP1RA han mostrado beneficios cardio-renal-metabólicos tanto en los pacientes con metformina como sin ella51 (el tratamiento con GLP1RA, principalmente semaglutida, ha mostrado beneficios CV y renales), además de ser seguros en los pacientes con ERC, incluso con un FGe de solo 15ml/min/1,73m2.
Los GLP1RA actuales son análogos de GLP-1, hormonas incretinas de origen intestinal que favorecen la secreción de insulina estimulando los receptores GLP1 y disminuyen la secreción de glucagón tras una comida estimulando los receptores GLP1 pancreáticos. Inducen la pérdida de peso, aumentan la sensación de saciedad e inicialmente ralentizan el vaciado gástrico. Los GLP1RA también han mostrado capacidad para disminuir la PA y la albuminuria en ECA. Los estudios preclínicos proponen que los GLP1RA regulan la inflamación renal. Los GLP1RA inhibieron la producción de IL-6 y TNF-α estimulada por AGE en células mesangiales y las ratas diabéticas tratadas con GLP1RA mostraron inhibición de la activación renal de NF-κB, disminución de los factores proinflamatorios (TNF-α, IL-1β y CCL-2) y reducción del estrés oxidativo. La información sobre las acciones antiinflamatorias de GLP1RA en la ERC es limitada. A este respecto, REMODEL52 evaluará los mecanismos antiinflamatorios de protección renal mediante semaglutida53.
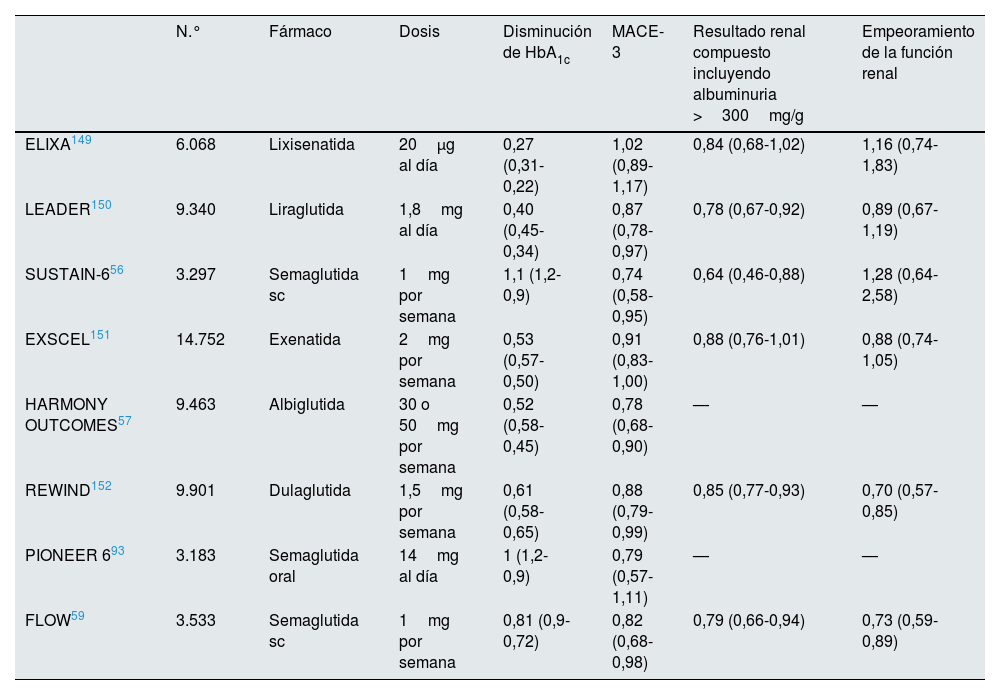
Ensayos de seguridad cardiovascular como REWIND54 (dulaglutida), LEADER (dulaglutida)55, SUSTAIN6 (semaglutida)56, HARMONY57 (albiglutida) y AMPLITUDE (efpeglenatida)58 pusieron de relieve una reducción del riesgo de episodios de ECV incluso en los pacientes con función renal disminuida. Estos ensayos han mostrado en los principales resultados secundarios renales una disminución de la albuminuria y una menor pérdida de FG sobre todo por la disminución de la albuminuria en poblaciones con DM2 y ERC, pero los cambios en el control de la glucosa, el peso o la PA solo explican un 10-25% de los beneficios renales, lo que apunta a efectos adicionales de estos fármacos sobre la protección renal. En este sentido, los ECA en curso están tratando de abordar los mecanismos de protección renal en la nefropatía diabética52 con semaglutida subcutánea (tabla 3).
Ensayos clínicos aleatorizados con GLP1RA en los pacientes con DM2 y ERC
| N.° | Fármaco | Dosis | Disminución de HbA1c | MACE-3 | Resultado renal compuesto incluyendo albuminuria >300mg/g | Empeoramiento de la función renal | |
|---|---|---|---|---|---|---|---|
| ELIXA149 | 6.068 | Lixisenatida | 20μg al día | 0,27 (0,31-0,22) | 1,02 (0,89-1,17) | 0,84 (0,68-1,02) | 1,16 (0,74-1,83) |
| LEADER150 | 9.340 | Liraglutida | 1,8mg al día | 0,40 (0,45-0,34) | 0,87 (0,78-0,97) | 0,78 (0,67-0,92) | 0,89 (0,67-1,19) |
| SUSTAIN-656 | 3.297 | Semaglutida sc | 1mg por semana | 1,1 (1,2-0,9) | 0,74 (0,58-0,95) | 0,64 (0,46-0,88) | 1,28 (0,64-2,58) |
| EXSCEL151 | 14.752 | Exenatida | 2mg por semana | 0,53 (0,57-0,50) | 0,91 (0,83-1,00) | 0,88 (0,76-1,01) | 0,88 (0,74-1,05) |
| HARMONY OUTCOMES57 | 9.463 | Albiglutida | 30 o 50mg por semana | 0,52 (0,58-0,45) | 0,78 (0,68-0,90) | — | — |
| REWIND152 | 9.901 | Dulaglutida | 1,5mg por semana | 0,61 (0,58-0,65) | 0,88 (0,79-0,99) | 0,85 (0,77-0,93) | 0,70 (0,57-0,85) |
| PIONEER 693 | 3.183 | Semaglutida oral | 14mg al día | 1 (1,2-0,9) | 0,79 (0,57-1,11) | — | — |
| FLOW59 | 3.533 | Semaglutida sc | 1mg por semana | 0,81 (0,9-0,72) | 0,82 (0,68-0,98) | 0,79 (0,66-0,94) | 0,73 (0,59-0,89) |
DM2: diabetes mellitus tipo 2; ERC: enfermedad renal crónica.
El estudio FLOW (Evaluate Renal Function with Semaglutide Once Weekly)59 es el primer ensayo clínico de GLP1RA con un criterio de valoración renal como resultado primario utilizando semaglutida subcutánea en dosis de 1mg una vez a la semana. En él se evaluó el efecto de la semaglutida en 3.533 participantes con DM2, FGe de 25-75ml/min/1,73m2 y albuminuria de 100-5.000mg/g. Se interrumpió prematuramente después de que el análisis intermedio mostrara su eficacia. En términos de control metabólico, la semaglutida tuvo un mayor efecto en la reducción de la HbA1c (–0,87 vs. –0,06%, diferencia estimada: –0,81 [IC 95%: –0,9-0,72%]).
Tirzepatida es un agonista dual del polipéptido insulinotrópico dependiente de la glucosa (GIP) y de los receptores GLP1 (twincretin). Actualmente, la tirzepatida es el fármaco más eficaz en el control glucémico y la pérdida de peso en los pacientes con diabetes de tipo 2, mostrando superioridad en los ensayos clínicos frente a la semaglutida 1mg o las insulinas basales y sin riesgo de hipoglucemia. A pesar de estas pruebas, el fármaco aún no ha recibido la aprobación de la Food and Drug Administration (FDA). También mejora otros factores de riesgo cardiorrenal (PA, colesterol LDL y albuminuria) en poblaciones con diabetes de tipo 2 u obesidad. El ensayo SURPASS-460 estudió el efecto de la tirzepatida en participantes con DM2 y alto riesgo cardiovascular. En comparación con la insulina glargina, mejoró un criterio de valoración renal secundario compuesto preespecificado (disminución del FGe≥40% con respecto al valor basal, muerte renal, insuficiencia renal o albuminuria de nueva aparición >300mg/g), aunque la reducción del riesgo se debió principalmente a la disminución de la albuminuria >300mg/g. La tirzepatida también ralentizó el descenso del FGe, pero que sepamos, hasta ahora no hay ECA que hayan evaluado este fármaco en un ensayo con criterios de valoración renales como resultados primarios.
Los inhibidores de la DPP-4 reducen moderadamente la glucemia con un bajo riesgo de hipoglucemia y pueden utilizarse en los pacientes frágiles o con intolerancia o contraindicaciones a los GLP-1RA, pero no han mostrado una mejora de los resultados renales o cardiovasculares. No deben utilizarse en combinación con GLP-1RA.
La metformina no debe utilizarse en los pacientes con un FGe inferior a 30ml/min/1,73m2 debido al riesgo de acidosis láctica secundaria y se aplicará con precaución en pacientes con un FGe entre 30-44ml/min/1,73m2, reduciendo el fármaco a un máximo de 1.000mg/día. Los inhibidores de la DPP-4, los GLP-1RA y los SGLT2i pueden prescribirse en pacientes con ERC avanzada. El efecto antihiperglucémico de las 2 primeras clases se mantiene en esta población, y aunque este efecto se pierde parcialmente con los SGLT-2i, también se recomiendan por su beneficio CV y renal.
No se aconseja el tratamiento con sulfonilureas o glinidas en pacientes con un FG inferior, ya que pueden inducir hipoglucemia.
La insulina y las dosis altas de glitazonas deben evitarse en la medida de lo posible en personas con ERC y DM2, ya que disminuyen la natriuresis y aumentan la retención de líquidos61. Si se requiere tratamiento con insulina, la dosis debe ajustarse y disminuirse en caso de progresión de la ERC, debido a su eliminación renal retardada61,62. Si el paciente requiere insulina, se recomienda el tratamiento basal con análogos de insulina, debido al menor riesgo de hipoglucemia. En un ensayo de seguridad CV (DEVOTE), la insulina degludec mostró un menor riesgo de hipoglucemia grave frente a glargina U100 en los pacientes con DM2 y alto riesgo CV (incluyendo a los pacientes con ERC)55.
A la luz de las nuevas evidencias y resultados en protección renal y cardiovascular, las recomendaciones no pueden hacerse solo sobre el control glucémico e irían más allá de la intervención metabólica, ya que los nuevos grupos terapéuticos actúan sobre varios aspectos.
La tabla 4 y la figura 1 resumen los puntos clave sobre el tratamiento de las personas con diabetes y ERC.
Puntos clave que resumen el tratamiento para el control metabólico en los pacientes con DM2 y ERC
| 1. Los SGLT-2i son fármacos antihiperglucemiantes con probados efectos protectores cardiovasculares y renales en los pacientes con DM2, ERC e ICC. |
| 2. Los GLP1RA son fármacos antihiperglucemiantes que también han mostrado beneficios renales en los pacientes con ERC y DM2. |
| 3. El resto de clases farmacológicas (incluida la metformina) no han mostrado de forma concluyente una reducción de episodios renales en los pacientes con DM2. |
| 4. Los SGLT2i y GLP1RA recomendados son aquellos que han mostrado reducción de episodios renales en ensayos clínicos en DM2 diseñados con este objetivo (canagliflozina, dapagliflozina, empagliflozina, semaglutida). |
| 5. Los SGLT2i tienen un efecto antihiperglucémico débil con reducción del FGe, por lo que se recomienda su combinación con otras clases terapéuticas (preferiblemente con GLP-1RA) en los pacientes con ERC G3A en adelante. |
| 6. El GLP-1RA mantiene su eficacia antihiperglucémica en los pacientes con ERC avanzada. |
| 7. Si el paciente no tiene un control glucémico adecuado con la combinación SGLT2i/GLP1RA (o bien contraindicación o intolerancia a alguno de ellos), se prescribirá metformina, siempre que FG>30. Los DPP4i son una alternativa con un efecto antihiperglucémico débil en los pacientes con contraindicación o intolerancia a GLP-1RA. |
| 8. La tirzepatida, un agonista dual GLP-1/GIP, es el fármaco que hasta el momento ha mostrado mayor eficacia antihiperglucémica en los pacientes con DM2 y puede ser una alternativa a GLP-1RA en los pacientes con ERC para mejorar el control glucémico, aunque todavía no hay estudios publicados con criterios de valoración renal y este fármaco aún no ha sido aprobado por la FDA. |
| 9. GLP1RA, DPP4i y tirzepatida no tienen un efecto aditivo o sinérgico, por lo que no deben prescribirse simultáneamente. |
| 10. Si el paciente requiere insulina, se recomienda la insulinoterapia basal con análogos de insulina, debido al menor riesgo de hipoglucemia. La insulina degludec mostró en un ensayo de seguridad CV (DEVOTE) un menor riesgo de hipoglucemia grave frente a glargina U100 en los pacientes con DM2 y alto riesgo CV (incluyendo a los pacientes con ERC). |
DM2: diabetes mellitus tipo 2; ERC: enfermedad renal crónica; FDA: Food and Drug Administration; FG: filtrado glomerular; FGe: filtrado glomerular estimado.
Recomendación 3.1. Se recomienda el control de la presión arterial (PA) con un objetivo de presión arterial sistólica (PAS) de <130mmHg cuando se tolere, en los pacientes con insuficiencia renal diabética. En caso contrario, se recomienda un objetivo general de PAS<140mmHg (tabla S3.1).
Fuerza de la recomendación: 2C.
Justificación: El ensayo SPRINT ha mostrado que el control intensivo de la PA, definido como un objetivo de PAS<120mmHg, reduce los episodios cardiovasculares y la mortalidad por todas las causas en los pacientes con ERC63. Sin embargo, el ensayo SPRINT incluyó exclusivamente a participantes que no tenían diabetes y los beneficios observados en el ensayo SPRINT no son evidentes en los estudios que incluyen a pacientes con diabetes. Las pruebas específicas sobre el objetivo de control de la PA en los pacientes con ERC y diabetes son muy limitadas, y las evidencias proceden de ensayos clínicos que incluyen a pacientes con DM, tanto con enfermedad renal como sin ella.
Tras revisar los ensayos ACCORD BP57, ADVANCE58 y ABCD59, en los que participaron pacientes diabéticos con y sin ERC, el control intensivo de la PA podría conllevar una variación mínima o nula de la mortalidad por todas las causas y cardiovascular en comparación con el control estándar de la PA55,63,64. Algunos estudios examinaron los episodios cardiovasculares64,65, y el control intensivo de la PA podría no asociarse a mejores resultados. Cuando los episodios cardiovasculares se evaluaron por separado, el control intensivo de la PA en los pacientes con ERC y diabetes puede producir poca o ninguna diferencia en el ictus65–67 y la insuficiencia cardíaca65–67. Sin embargo, dicho control podría disminuir el riesgo de infarto de miocardio57–59, aunque la calidad de las pruebas es moderada. El ensayo ACCORD BP sí mostró una reducción significativa del ictus en el grupo de control intensivo de la PA.
Por lo tanto, para generar recomendaciones para la ERC y la DM2, deben tenerse en cuenta ciertas características de los ensayos55,63,64. Debemos considerar que la mayoría de los pacientes incluidos en estos ensayos presentaban albuminuria como manifestación de enfermedad renal. En la mayoría de ellos, los valores medios de creatinina eran normales y con un FGe bien conservado64–66. En el ensayo ACCORD BP solo se incluyeron los pacientes con DM2, y se excluyeron los individuos con un nivel de creatinina sérica superior a 1,5mg/dl (132,6μmol/l). En el ensayo ADVANCE, los pacientes podían presentar albuminuria, aunque no era obligatorio, y la media de creatinina sérica fue de 87μmol/l en ambos grupos. El ensayo ABCD incluyó a los pacientes normotensos con diabetes sin tratamiento para la hipertensión y la media del aclaramiento de creatinina fue >80ml/min en ambos grupos. En cuanto a la enfermedad renal, se excluyó a los pacientes que recibían diálisis y/o tenían una creatinina sérica superior a 3mg/dl. En Estacio et al.64, 129 pacientes con diabetes tipo 2 y PA entre 140/80 y 90mmHg sin albuminuria significativa fueron aleatorizados para un tratamiento intensivo de la PA (objetivo de PA diastólica de 75mmHg) con valsartán, y para un tratamiento moderado de la PA (PA diastólica entre 80 y 90mmHg), inicialmente con placebo.
La evolución demográfica de los pacientes y la disponibilidad de estas nuevas opciones terapéuticas subrayan la necesidad de ensayos clínicos contemporáneos. Estos ensayos deben investigar objetivos de PA adaptados a las necesidades matizadas de los pacientes con ERC y diabetes, teniendo en cuenta el espectro más amplio de comorbilidades y el potencial de mejora de los resultados con nuevos tratamientos.
En los pacientes con ERC y diabetes, el control de la PA es especialmente importante debido al riesgo agravado de enfermedad cardiovascular y a la progresión de la insuficiencia renal. Históricamente, los objetivos de presión arterial en estas poblaciones se basaban en una gama más reducida de estudios clínicos que quizá no engloben plenamente la complejidad de los perfiles de los pacientes que se observan hoy en día, como la mayor prevalencia de obesidad y síndrome metabólico. La introducción de nuevos agentes farmacoterapéuticos, como los inhibidores de SGLT2, los agonistas de los receptores de GLP-1 y los antagonistas de los receptores de mineralocorticoides, ha ampliado significativamente las opciones de tratamiento. Estos agentes ofrecen ventajas que van más allá de la reducción de la PA, como la mejora de los resultados cardiovasculares y la ralentización de la progresión de la ERC.
La evolución demográfica de los pacientes y la disponibilidad de estas nuevas opciones terapéuticas ponen de manifiesto la necesidad de ensayos clínicos más contemporáneos. Estos ensayos deben investigar objetivos de PA adaptados a las necesidades personalizadas de los pacientes con ERC y diabetes, teniendo en cuenta el espectro más amplio de comorbilidades y el potencial de mejora de los resultados con los nuevos tratamientos.
Recomendación 3.2. Recomendamos iniciar IECA o ARA-II indistintamente para los pacientes con hipertensión y ERD (tablas S3.2, S3.3).
Fuerza de la recomendación: 2B.
Recomendación 3.3. Los ARM esteroideos son probablemente útiles para el manejo de la hipertensión en los pacientes con FGe >30ml/min/1,73m2 y potasio sérico <4,8mmol/l (tabla S3.4).
Fuerza de la recomendación: 2D.
Recomendación 3.4. Aunque los ARM no esteroideos pueden ser útiles en el control de la PA, no los recomendamos para el manejo de la PA (tabla S3.5).
Fuerza de la recomendación: 2B.
Justificación: Los diferentes efectos de los antagonistas de los receptores de la angiotensina II (ARA) sobre el control de la PA en comparación con los inhibidores de la enzima convertidora de la angiotensina (IECA) no están bien definidos66,67.
Solo 2 ECA con un escaso número de participantes evaluaron este resultado y los autores mostraron una tendencia hacia un mejor control de la PA en los pacientes tratados con ARA-II en comparación con IECA67–71.
En cuanto al control de la PA, los ARA-II pueden reducir la PA sistólica, pero pueden producir una ligera reducción o ninguna diferencia en la PA diastólica en comparación con el tratamiento estándar72–74. En el RENAAL72, el ORIENT73 y el Irbesartán Diabetic Trial74, el resultado primario fue el renal, compuesto por la duplicación de la creatinina sérica basal, la aparición de enfermedad renal terminal, la necesidad de diálisis crónica y/o trasplante y la muerte por cualquier causa. Sobre la base de estos 3 ECA72–74, los ARA pueden ser beneficiosos en cuanto a los resultados renales en comparación con el tratamiento estándar, a pesar del control similar de la PA entre los grupos.
En cuanto a los resultados cardiovasculares, los ARA-II probablemente disminuyen el riesgo de insuficiencia cardíaca e infarto de miocardio en comparación con el placebo o el tratamiento estándar72,73, pero los ARA-II no redujeron el riesgo de mortalidad por todas las causas72–74.
Aunque la calidad de la evidencia es baja debido al grave riesgo de inconsistencia e imprecisión, los ARA-II pueden dar lugar a un riesgo, desde ligeramente superior a ninguna diferencia, en la hiperpotasemia en comparación con el tratamiento estándar73,74.
Además, las evidencias con respecto al uso de ARM esteroideos en los pacientes con diabetes y proteinuria son muy limitadas, ya que solo unos pocos estudios a pequeña escala75,76 han analizado esta cuestión. Sobre la base de 86 pacientes y con una calidad de la evidencia baja debido al grave riesgo de sesgo y a la imprecisión, los ARM esteroideos pueden reducir tanto la PAS como la PAD y pueden disminuir la albuminuria en comparación con el tratamiento estándar74–76.
Dado que la reducción de la PA relacionada con los ARM no esteroideos fue moderada utilizando tanto finerenona en el estudio FIDELIO-DKD77 como esaxerenona78, se planteó la hipótesis de que en el efecto beneficioso sobre los resultados cardiorrenales influían principalmente las vías no hemodinámicas. El ensayo ARTS-DN79 se diseñó para profundizar en el efecto de finerenona sobre la albuminuria y evaluó los efectos del tratamiento sobre la monitorización ambulatoria de la PA en 24h en un subconjunto de 240 participantes. En este grupo de pacientes, la monitorización ambulatoria de la PA en 24h se midió al comienzo del estudio, 60 días después del inicio de finerenona y en la última visita durante el tratamiento. Estos ensayos proponen que los ARM no esteroideos pueden reducir la PAS y ligeramente la PAD en comparación con el tratamiento estándar76,77.
En cuanto a los episodios adversos, los ARM no esteroideos probablemente aumentan el riesgo de interrupción del tratamiento debido a efectos secundarios, con una calidad de la evidencia moderada (RR: 1,25 (1,03-1,52))77,78. La adición de estos fármacos a un paciente que ya recibe IECA/ARA-II aumenta el riesgo de hiperpotasemia77,78, lo que pone de relieve la importancia de controlar periódicamente los valores séricos de potasio en estos pacientes.
Recomendación 3.5. Debe evitarse la combinación de IECA con tratamiento con ARA-II o aliskiren en los pacientes con diabetes y ERC (tabla S3.6 y S3.7, tabla S4.4).
Fuerza de la recomendación: 2D.
Justificación: La evidencia publicada sobre el uso de aliskiren como tratamiento antihipertensivo en los pacientes con diabetes y ERC es muy limitada. Dos80,81 de los 3 estudios analizados comparan el uso de este fármaco con el ARA, mientras que en el tercer estudio, los pacientes recibieron o bien aliskiren o bien ARA82. El aliskiren puede reducir ligeramente la PAS y PAD en comparación con el tratamiento estándar, pero las pruebas son muy inciertas.
Capítulo 4: Tratamiento dirigido a la progresión de la enfermedad renal crónica en las personas con enfermedad renal diabéticaRecomendación 4.1. Los pacientes con DM2 y ERC con un FGe≥20ml/min/1,73m2 deben ser tratados con un inhibidor del cotransportador sodio-glucosa tipo 2, que se mantendrá hasta la fase terminal de la enfermedad renal (diálisis o trasplante renal) (tabla S4.7) (fig. 2).
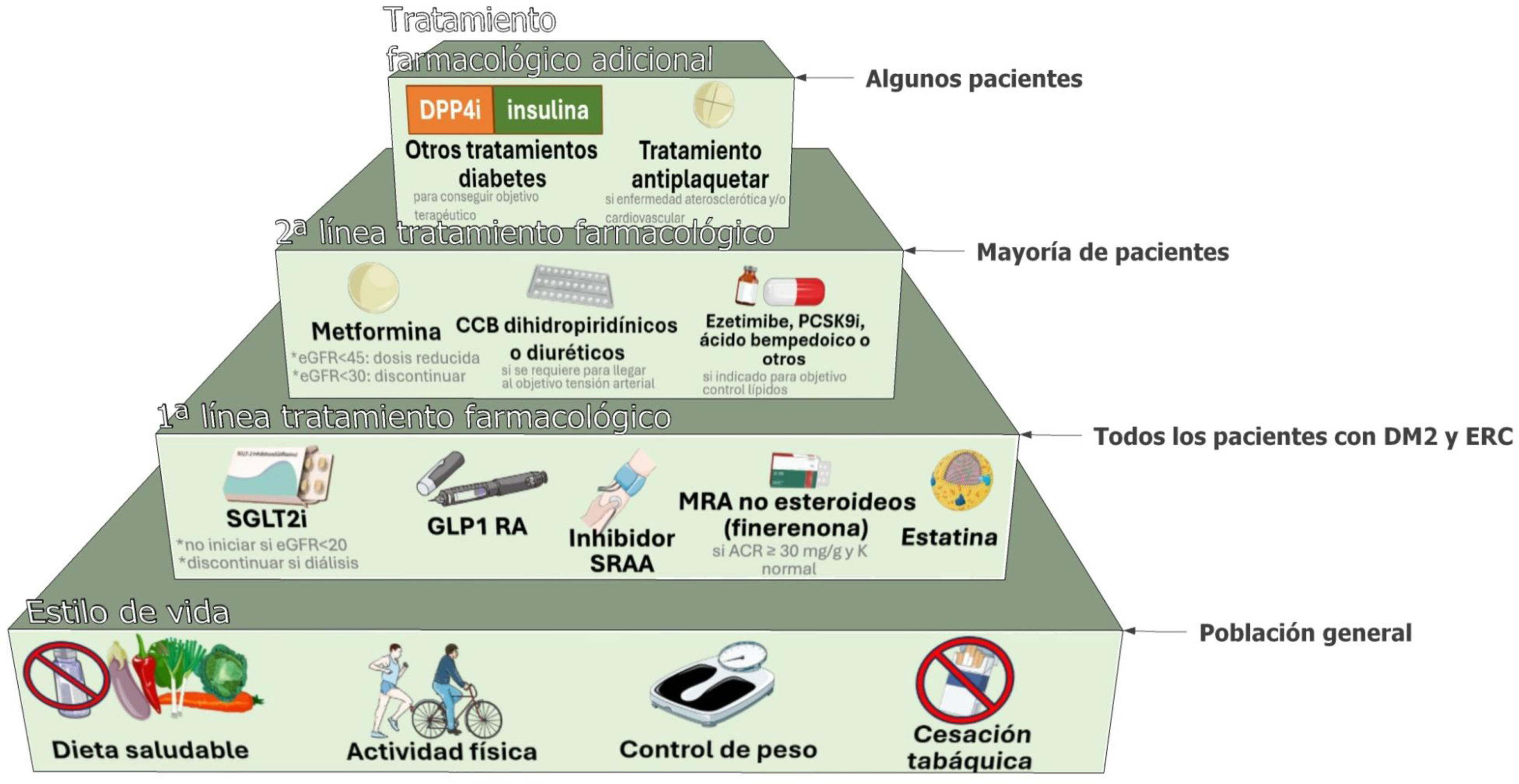
Tratamiento general de los pacientes con diabetes tipo 2 y enfermedad renal crónica (ERC).
Los pacientes con diabetes y ERC deben recibir un enfoque holístico para evitar complicaciones cardiovasculares. Este abordaje debe contemplar cambios en el estilo de vida centrados en la nutrición, con especial atención al control del peso, la práctica regular de ejercicio físico y el abandono del hábito tabáquico, añadiendo el uso de fármacos de primera línea, según las características clínicas de cada paciente y priorizando aquellos con beneficio demostrado desde el punto de vista cardiorrenal. El control glucémico se basa en el tratamiento con insulina en la diabetes mellitus tipo 1 (DM1), una combinación de inhibidores del cotransportador 2 de sodio-glucosa (SGLT2i) y GLP1 RA, especialmente semaglutida 1mg una vez a la semana, para la diabetes mellitus tipo 2 (DM2). Los iSGLT2 deben iniciarse cuando el filtrado glomerular estimado (eGFR) sea superior a 20ml/min por 1,73m2 y continuarse hasta iniciar el tratamiento con diálisis o trasplante. Como tratamiento de segunda línea se debería añadir metformina si el control glucémico es inadecuado, y cuando el eGFR es superior a 30ml/min por 1,73m2, ajustando las dosis de metformina cuando el FGe estuviera entre 30-45ml/min por 1,73m2. Los inhibidores de la enzima convertidora de la angiotensina (IECA) o los antagonistas de los receptores de la angiotensina II (ARA) deben ser los fármacos de primera línea para la hipertensión cuando hay albuminuria. De lo contrario, también pueden considerarse los antagonistas del calcio dihidropiridínicos (CCB) o un diurético. A menudo son necesarias las 3 clases para alcanzar los objetivos de PA. El control adecuado de los lípidos con diferentes grupos farmacológicos es crucial, y se recomienda el uso de estatinas para la mayoría de los pacientes con DM1 o DM2 y ERC. Un antagonista no esteroideo de los receptores de mineralocorticoides (ns-MRA) como la finerenona puede añadirse al tratamiento de primera línea para los casos con DM2 y alto riesgo de progresión de la ERC y de episodios cardiovasculares, dependiendo de la albuminuria del paciente (>30mg/g). En función de las características del paciente, diferentes grupos farmacológicos aumentan el arsenal terapéutico para mejorar el control metabólico de los pacientes, entre ellos los inhibidores de la dipeptidil peptidasa-4 (DPP4i) o la insulina. También pueden utilizarse otras terapias adicionales, como los antagonistas de los receptores mineralocorticoides esteroideos, para alcanzar los objetivos de PA si los valores de potasio (K) lo permiten. En general, el ácido acetilsalicílico debe utilizarse de por vida para la prevención secundaria en los pacientes con problemas cardiovasculares establecidos, y puede considerarse en prevención primaria en aquellos con alto riesgo de problemas cardiovasculares ateroscleróticos.
ARA-II: antagonistas de los receptores de la angiotensina II; CCB: antagonistas del calcio; DPP4i: inhibidores de la dipeptidil peptidasa-4; eGFR: filtrado glomerular estimado; GLP1 AR: agonista del receptor del péptido-1 similar al glucagón; IECA: inhibidores de la enzima convertidora de la angiotensina; MRA: antagonista del receptor de mineralocorticoides; PCSK9i: inhibidor de la proproteína convertasa subtilisina/kexina tipo 9; SGLT2i: inhibidor del cotransportador 2 de sodio-glucosa; SRAA: antagonistas sistema renina-angiotensina.
Fuerza de la recomendación: 1A.
Justificación: Los pacientes con DM2 y ERC tienen un mayor riesgo de progresión a insuficiencia renal. Actualmente, existen pruebas consistentes que confirman que los iSGLT2 confieren efectos protectores renales significativos en estos pacientes.
El potencial de los iSGLT2 para modificar el riesgo de progresión de la ERC se puso de relieve por primera vez en un subanálisis del ensayo EMPA-REG OUTCOME en los pacientes con DM2 y enfermedad cardiovascular establecida83. Los análisis de la evolución del FGe mostraron una reducción de la pérdida FG estimado con el tiempo, lo que se tradujo en un descenso del 46% en el riesgo de progresión de la enfermedad renal (ERT, muerte renal y duplicación de la creatinina sérica). También se observaron beneficios con canaglifozina48 y dapaglifozina84, pero no con ertuglifozina85.
Posteriormente se diseñaron 3 ensayos clínicos específicos para comprobar el efecto de los iSGLT2 en la progresión de la ERC: CREDENCE48, DAPA-CKD49 y EMPA-KIDNEY50. Los resultados de estos estudios han confirmado definitivamente los beneficios renoprotectores de los iSGLT2 en los pacientes con DM2 y ERC, y en un subestudio de DAPA-CKD86 los que mantuvieron el tratamiento tras el inicio de diálisis tuvieron menos mortalidad que los que lo suspendieron. CREDENCE reclutó a los pacientes con DM2, un FGe de 30-90ml/min/1,73m2 y un CAC de 300-5.000mg/g en tratamiento con un IECA o un ARA-II. La canagliflozina redujo el riesgo del objetivo primario compuesto (duplicación sostenida de la creatinina sérica, ERT o muerte por causas renales o cardiovasculares) en un 30% en comparación con el placebo (HR=0,70; IC 95%: 0,59-0,82). Es importante señalar que se produjo una reducción del riesgo de progresión de la enfermedad renal, incluida la ERT. El riesgo de diálisis de mantenimiento, trasplante renal o muerte renal disminuyó significativamente en un 28%. DAPA-CKD incluyó a personas con y sin DM2. Los criterios de inclusión fueron un FGe de 25-75ml/min/1,73m2 y un CAC de 200-5.000mg/g y tratamiento con una dosis estable de un IECA o ARA-II durante ≥4 semanas. La dapagliflozina mostró una reducción del resultado primario compuesto (descenso sostenido del 50% en el FGe, ERT o muerte por causas renales o cardiovasculares) del 39% en comparación con el placebo (HR=0,61; IC 95%: 0,51-0,72). Es importante destacar que estas reducciones del riesgo relativo volvieron a ser evidentes para el componente de progresión de la enfermedad renal del compuesto primario. EMPA-KIDNEY reclutó un amplio perfil de participantes (con y sin DM2) en riesgo de progresión de la ERC utilizando como criterios de inclusión un FGe de 20-45ml/min/1,73m2 (sin indicación respecto al CAC) o un FGe≥45 a <90ml/min/1,73m2 con un CAC≥200mg/g (o ratio proteína/creatinina ≥300mg/g). EMPA-KIDNEY mostró una reducción del 28% en el objetivo primario compuesto de progresión de la enfermedad renal (disminución sostenida del FGe a <10ml/min/1,73m2, reducción sostenida del FGe≥40% con respecto al valor basal, ERT o muerte por causas renales) o muerte por causas cardiovasculares (HR=0,72; IC 95%: 0,64-0,82). También se observaron efectos similares para los componentes individuales de la progresión de la enfermedad renal.
En un metanálisis exhaustivo de grandes ensayos clínicos aleatorizados con iSGLT2 se adoptó una definición unificada de progresión de la enfermedad renal como una reducción sostenida del FGe ≥ 50% desde la aleatorización, fallo renal o muerte por insuficiencia renal87. Los resultados mostraron una reducción global del 37% del riesgo de progresión de la enfermedad renal (HR=0,63; IC 95%: 0,58-0,69), que fue similar entre los participantes con y sin DM2. En los pacientes con diabetes, el HR para el resultado de progresión de la enfermedad renal fue de 0,64 (IC 95%: 0,52-0,79) en CREDENCE, 0,57 (IC 95%: 0,45-0,73) en DAPA-CKD, y 0,55 (IC 95%: 0,44-0,71) en EMPA-KIDNEY.
Recomendación 4.2. Recomendamos que se inicie el tratamiento con un inhibidor de la enzima convertidora de la angiotensina (IECA) o un bloqueante de los receptores de la angiotensina II (ARA-II) en los pacientes con diabetes, hipertensión y albuminuria. Estos fármacos deben ser titulados hasta la dosis máxima tolerada que haya sido aprobada (tabla S4.1-3).
Fuerza de la recomendación: 1A.
Justificación: La piedra angular del tratamiento de la ERC en los pacientes con DM2 ha sido el uso de inhibidores del SRA, con varios ensayos clínicos aleatorizados que mostraron la reducción de la progresión de la ERC y del riesgo de desenlaces renales en sujetos de alto riesgo con albuminuria moderada o gravemente aumentada.
Los ensayos clínicos IRMA-2 (Irbesartan in patients with type 2 diabetes and albuminuria)88 e INNOVATION (The incipient to overt: Angiotensin II blocker, Telmisartan, investigation on type 2 diabetic nephropathy)89 se diseñaron para comprobar si el bloqueo del SRA reducía el riesgo de progresión de la ERC en la diabetes, definida como el desarrollo de albuminuria gravemente aumentada (CAC>300mg/g). En estos estudios participaron los pacientes con DM2 y albuminuria moderadamente elevada (CAC entre 30 y 300mg/g). El estudio IRMA-2 mostró que el tratamiento con el ARA-II irbesartán reducía de forma dosis/dependiente el riesgo de progresión de la ERC. La dosis más alta de 300mg/día se asoció a una reducción del riesgo de casi 3 veces a los 2 años de seguimiento, resultado que fue independiente del efecto hipotensor del irbesartán. Por otra parte, en el ensayo INNOVATION con el ARA-II telmisartán se observó una menor tasa de transición a nefropatía manifiesta con respecto al placebo tras un año de seguimiento. En este estudio, el efecto beneficioso del telmisartán en la ralentización de la progresión a nefropatía manifiesta también fue independiente de la reducción de la PA con telmisartán.
En cuanto al beneficio del bloqueo del SRA en los pacientes con albuminuria muy elevada, este se comprobó en 2 ensayos clínicos en los que participaron los pacientes con una excreción de albúmina en orina ≥300mg/día. En el ensayo RENAAL (The Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan)72 se asignó aleatoriamente a 1.513 pacientes a recibir losartán o placebo una vez al día, junto con el tratamiento antihipertensivo convencional necesario (excluidos IECA y ARA-II). El objetivo primario compuesto de duplicación de la concentración sérica de creatinina, insuficiencia renal terminal o muerte se redujo en un 16% en los pacientes tratados con losartán según el análisis por ITT (p=0,02), efecto que se mantuvo tras ajustar según el valor de la PA. Los componentes individuales del criterio de valoración compuesto primario que evaluaba la progresión de la enfermedad renal mostraron un beneficio significativo, con una reducción del riesgo de duplicación de la concentración sérica de creatinina del 25% (p<0,01) y del riesgo de enfermedad renal terminal del 28% (p=0,002) en el grupo de losartán en comparación con el grupo placebo. Además, entre los pacientes que continuaron recibiendo el tratamiento asignado al estudio según el análisis por protocolo, el losartán confirió una reducción del 22% en el riesgo de la variable principal de valoración compuesta (p<0,01)90.
A diferencia del estudio RENAAL, el ensayo clínico IDNT (Irbesartan Diabetic Nephropathy Trial) incluyó un comparador activo además de un placebo. Este estudio reclutó a 1.715 pacientes con DM2 y edades comprendidas entre 30 y 70 años, hipertensión arterial y excreción urinaria de proteínas ≥ 900mg/24h, que fueron aleatorizados para recibir tratamiento con irbesartán, amlodipino o placebo74. El criterio de valoración primario fue la combinación de la duplicación de la creatinina sérica basal, la aparición de enfermedad renal terminal (inicio de diálisis, trasplante renal o concentración de creatinina sérica ≥6,0mg/dl) o la muerte por cualquier causa. El riesgo relativo del criterio de valoración primario en los grupos placebo y amlodipino no difirió significativamente. Sin embargo, el tratamiento con irbesartán se asoció con una reducción de riesgo de un 20% de la variable principal de valoración compuesta con respecto al grupo placebo (p=0,02) y una reducción de un 23% con respecto al grupo amlodipino (p=0,006). El riesgo de duplicación de la concentración sérica de creatinina fue un 33% menor en el grupo de irbesartán que en el de placebo (p=0,003) y un 37% menor en el grupo de irbesartán que en el de amlodipino (p<0,001). El riesgo relativo de ERT en los pacientes que recibieron irbesartán fue un 23% inferior al de los otros 2 grupos (p=0,07 para ambas comparaciones). Estas diferencias fueron independientes de la PA alcanzada. La concentración sérica de creatinina aumentó un 24% más lentamente en el grupo de irbesartán que en el de placebo (p=0,008) y un 21% más lentamente que en el de amlodipino (p=0,02).
No existe evidencia en relación con los resultados ni una eficacia superior al comparar el tratamiento con IECA con respecto al tratamiento con ARA-II. Así pues, cualquiera de los 2 agentes puede utilizarse en el tratamiento de los pacientes con DM2 y ERC, y la elección entre IECA y ARA-II dependerá de otros factores (preferencias del paciente, coste, perfil de efectos secundarios, etc.)91,92.
Recomendación 4.3. Los pacientes con DM2, FGe≥25ml/min/1,73m2 y albuminuria aumentada (CAC>100mg/g) en tratamiento con la dosis máxima tolerada estable de inhibidores del SRA deben ser tratados con un arGLP1 con beneficio renal demostrado (tabla S4.8).
Fuerza de la recomendación: 1A.
Justificación: Existen nuevas evidencias en relación con las propiedades nefroprotectoras del arGLP1 semaglutida. En un análisis post hoc de los ensayos SUSTAIN 6/PIONEER 6 que incluía datos agrupados de 6.480 participantes con alto riesgo cardiovascular, se observó una diferencia significativa en el efecto estimado del tratamiento (semaglutida vs. placebo) sobre la pendiente del FGe: 0,59ml/min/1,73m2 (IC 95%: 0,29-0,89)56,93. Este efecto fue numéricamente mayor en los sujetos con un FGe entre 30 y 60ml/min por 1,73m2 (1,06ml/min/1,73m2; IC 95%: 0,45-1,67), pero sin interacción significativa para el efecto del tratamiento por subgrupos.
Esta hipótesis de que la semaglutida puede reducir la disminución del FGe y tener beneficios protectores renales se ha evaluado en un ensayo clínico específico que investiga los efectos de la semaglutida subcutánea una vez a la semana (1mg) en una población de los pacientes con DM2 y ERC con alto riesgo de progresión de la enfermedad renal. El estudio FLOW59, que se interrumpió prematuramente tras el análisis intermedio, mostró eficacia tras reclutar a 3.533 adultos con DM2 y enfermedad renal (definida por un FGe de 25 a 75ml/min/1,73m2, con un CAC superior a 300 e inferior a 5.000mg/g si el FGe era ≥50ml/min/1,73m2 o un CAC>100 y <5.000mg/g si el FGe era de 25 a menos de 50ml/min/1,73m2), en tratamiento con una dosis máxima estable de inhibidores del SRA.
Los resultados mostraron una reducción del riesgo relativo del 24% del objetivo primario compuesto en el grupo de semaglutida con respecto al grupo placebo (HR=0,76; IC 95%: 0,66-0,88; p=0,0003), con resultados similares para un compuesto de los componentes específicos renales del resultado primario (HR=0,79; IC 95%: 0,66-0,94). Además, hubo 3 resultados secundarios confirmatorios clave, que se evaluaron mediante un enfoque de pruebas jerárquicas preespecificado: la tasa anual de cambio en el FGe desde la aleatorización hasta el final del estudio (pendiente total del FGe); un compuesto de infarto de miocardio no mortal, ictus no mortal o muerte por causa cardiovascular (MACE, episodios cardiovasculares mayores); y muerte por cualquier causa. Todos los resultados de estos desenlaces confirmatorios fueron favorables a la semaglutida: la pendiente total del FGe mostró una reducción menor en 1,16ml/min/1,73m2/año (p<0,001); el riesgo de MACE fue un 18% menor (HR=0,82; IC 95%: 0,68-0,98; p=0,029); y el riesgo de muerte por cualquier causa fue un 20% menor (HR=0,80; IC 95%: 0,67-0,95; p=0,01).
Recomendación 4.4. Proponemos iniciar un antagonista no esteroideo del receptor de mineralocorticoides (ARM) con beneficio renal y/o cardiovascular demostrado en los pacientes con DM2, FGe≥25ml/min/1,73m2, concentración sérica de potasio normal (K ≤ 5,1 mmol/l), e incremento de albuminuria (CAC≥30mg/g) a pesar de recibir la dosis máxima tolerada de un inhibidor del SRA (tabla S4.6).
Fuerza de la recomendación: 1A.
Justificación: Puede añadirse un ARM no esteroideo al tratamiento de primera línea de los pacientes con DM2 y alto riesgo residual de progresión de la enfermedad renal, evidenciado por albuminuria persistente (CAC≥30mg/g). La elección de un ARM no esteroideo debe priorizar los agentes con beneficios renales o cardiovasculares documentados, siendo la finerenona actualmente el único ARM no esteroideo con dichos beneficios clínicos demostrados.
Las pruebas iniciales de la eficacia clínica de la finerenona para mejorar la función renal y ralentizar el empeoramiento de la ERC proceden del ensayo FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease)77. En este ensayo en fase 3, aleatorizado, en doble ciego, multicéntrico y controlado con placebo, se asignó aleatoriamente a 5.734 adultos con DM2 y ERC para recibir finerenona o placebo. Los pacientes elegibles tenían un FGe de 25 a menos de 60ml/min/1,73m2, un CAC de 30 a menos de 300mg/g y retinopatía diabética, o bien tenían un FGe de 25 a menos de 75ml/min/1,73m2 y un CAC de 300 a 5.000mg/g. Todos los participantes fueron tratados con un bloqueo optimizado del SRA antes de la aleatorización. El objetivo primario fue el tiempo transcurrido hasta el primer episodio de un criterio de valoración compuesto consistente en un descenso sostenido de al menos el 40% en el FGe con respecto al valor basal durante al menos 4 semanas, la aparición de fallo renal (definido como un FGe inferior a 15ml/min/1,73m2 o enfermedad renal terminal (inicio de diálisis mantenida durante ≥90 días o trasplante renal), o muerte renal. Los resultados mostraron que la incidencia del resultado compuesto primario fue significativamente menor en el grupo de finerenona que en el de placebo (17,8 vs. 21,1%, respectivamente) (HR=0,82; IC 95%: 0,73-0,93; p=0,001). Además, las incidencias de los componentes individuales del objetivo primario fueron consistentemente inferiores con finerenona que con placebo.
Se obtuvo una evidencia clínica adicional a partir de los estudios FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease)94 y FIDELITY (Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis)95. El estudio FIGARO incluyó a los pacientes adultos con DM2 y ERC en estadios 1 o 2 (FGe≥60ml/min/1,73m2) con un CAC de 300 a 5.000mg/g o ERC en estadios 2 a 4 (FGe 25-90ml/min/1,73m2) con un CAC de 30 a menos de 300mg/g. De forma similar a FIDELIO-DKD, el bloqueo del SRA se optimizó en todos los pacientes antes de la aleatorización. El objetivo primario fue cardiovascular (un compuesto de mortalidad por causas cardiovasculares, infarto de miocardio no mortal, ictus no mortal u hospitalización por insuficiencia cardíaca), mientras que el primer objetivo secundario fue un compuesto de una disminución sostenida del FGe≥40% con respecto al valor basal, fallo renal o muerte renal. Se aleatorizó a un total de 7.437 pacientes. La incidencia del resultado primario fue significativamente menor en el grupo de finerenona (HR=0,87; IC 95%: 0,76-0,98; p=0,03), siendo el beneficio debido principalmente a una menor incidencia de hospitalización por insuficiencia cardíaca. La incidencia del objetivo secundario fue del 9,5% en el grupo de finerenona y del 10,8% en el grupo placebo (HR=0,87; IC 95%: 0,76-1,01), aunque la diferencia no llegó a alcanzar la significación estadística. El análisis de los componentes del objetivo secundario mostró una incidencia de insuficiencia renal terminal del 0,9% en el grupo de finerenona y del 1,3% en el grupo placebo (HR=0,64; IC 95%: 0,41-0,99). El resultado renal compuesto de fallo renal, una disminución sostenida desde el valor basal de al menos el 57% en el FGe o muerte renal se produjo en el 2,9% de los pacientes del grupo de finerenona y en el 3,8% del grupo placebo (HR=0,77; IC 95%: 0,60-0,99).
Por último, el estudio FIDELITY fue un análisis agrupado preespecificado de FIDELIO-DKD y FIGARO-DKD cuyo objetivo era proporcionar estimaciones más sólidas de la eficacia y seguridad de la finerenona en todo el espectro de los pacientes con ERC y DM2. Este estudio incluyó a un total de 13.171 sujetos y mostró que los pacientes que recibían finerenona tenían un menor riesgo de presentar el resultado cardiovascular compuesto de muerte cardiovascular, IM no mortal, ictus no mortal u hospitalización por IC (HR=0,86; IC 95%: 0,78-0,95), así como un riesgo inferior de presentar el resultado renal compuesto de aparición de fallo renal, disminución sostenida del FGe≥57% o muerte renal (HR=0,77; IC 95%: 0,67-0,88). Entre los componentes del resultado renal se observó una reducción del 20% en el riesgo de ERC terminal (HR=0,80; IC 95%: 0,64-0,99).
Recomendación 4.5. Se propone mantener una ingesta proteica de 0,6-0,8g/kg (peso)/día para los pacientes con diabetes y ERC no tratados con diálisis (tabla S4.12).
Fuerza de la recomendación: 2C.
Justificación: Los primeros estudios en animales mostraron que la ingesta elevada de proteínas contribuye al desarrollo de un aumento de la presión intraglomerular y de la hiperfiltración glomerular, lo que a su vez provoca daños tubulointersticiales y glomeruloesclerosis96. Sobre esta base, estudios clínicos han mostrado que una ingesta reducida de proteínas en la dieta disminuye la hiperfiltración glomerular y ralentiza la progresión de la ERC en comparación con una ingesta estándar de proteínas de 0,8g/kg/día97–99. Sin embargo, estos estudios incluyeron principalmente los pacientes con ERC avanzada y sujetos sin diabetes100, mientras que en los pacientes con diabetes y ERC faltan estudios clínicos que comparen diferentes niveles de contenido proteico en la dieta. Aunque 2 metaanálisis muestran un pequeño impacto beneficioso de la dieta baja en proteínas sobre el descenso del FGe101,102, la elevada heterogeneidad de los estudios (tipo de diabetes, estadios de ERC, tipos de intervenciones, duración y adherencia a las indicaciones) no permite dar recomendaciones sólidas. Así pues, consideramos aconsejable aplicar las directrices KDIGO relativas a la ingesta diaria de proteínas a los pacientes con diabetes y ERC que no reciben diálisis (0,6-0,8g/kg/día), mientras que en los pacientes diabéticos con ERC que reciben diálisis debería aconsejarse una ingesta de proteínas en la dieta >1,2g/kg de peso corporal al día103.
Finalmente, es importante señalar los peligros potenciales de una reducción excesiva de la ingesta proteica en personas diabéticas a menos de 0,6g/kg/día. Esta restricción proteica puede provocar una disminución de la calidad de vida, un aumento del riesgo de episodios de hipoglucemia, una pérdida de peso inadecuada y malnutrición.
La tabla 4 resume los puntos clave sobre el manejo de los pacientes con DM2 y ERC.
Capítulo 5: Tratamiento antiagregante plaquetario o anticoagulante en las personas con diabetes y enfermedad renal crónicaRecomendación 5.1. Los pacientes con DM1 o DM2 y ERC con enfermedad cardiovascular aterosclerótica establecida deben ser tratados con dosis bajas de ácido acetilsalicílico (75-100mg/día) para la prevención secundaria.
Fuerza de la recomendación: 1B.
Recomendación 5.2. Tras un síndrome coronario agudo o una intervención coronaria percutánea, se recomienda el tratamiento antiplaquetario dual (con dosis bajas de ácido acetilsalicílico y un inhibidor 2Y12), seguido de una monoterapia antiplaquetaria con una duración determinada por un equipo multidisciplinar en función del perfil riesgo-beneficio.
Fuerza de la recomendación: 1B.
Recomendación 5.3. En los pacientes con DM1 o DM2 y ERC y antecedente de un ictus isquémico no cardioembólico o un ictus isquémico transitorio, se recomienda el tratamiento antiagregante a largo plazo para reducir el riesgo de ictus recurrente.
Fuerza de la recomendación: 1C.
Recomendación 5.4. En los pacientes con DM1 o DM2 y ERC que han sufrido un ictus isquémico agudo no cardioembólico/ataque isquémico transitorio, se deberá considerar el tratamiento dual con antiagregantes plaquetarios (con dosis bajas de ácido acetilsalicílico y un inhibidor de P2Y12) seguido de monoterapia con un antiagregante.
Fuerza de la recomendación: 2C.
Recomendación 5.5. En los pacientes con DM1 o DM2 y ERC en estadios 3 o superiores, no existe evidencia clara de un perfil beneficio-riesgo favorable para recomendar la prescripción de ácido acetilsalicílico a dosis bajas para la prevención primaria de la enfermedad cardiovascular aterosclerótica.
Fuerza de la recomendación: 2C.
Justificación: De acuerdo con las guías actuales y la evidencia disponible, en los pacientes con diabetes, ERC y enfermedad cardiovascular aterosclerótica, la prevención secundaria de enfermedad cardiovascular aterosclerótica debe realizarse mediante ácido acetilsalicílico a dosis bajas (75-100mg/día)104–108. El reciente análisis post hoc del ensayo ADAPTABLE entre el subgrupo de pacientes con diabetes mostró que no había diferencias en cuanto a la eficacia y la seguridad de la dosis baja (81 vs. 325mg/día) o el tipo (con recubrimiento entérico frente a sin recubrimiento) de ácido acetilsalicílico, aunque no podía excluirse una reducción de las hemorragias con el ácido acetilsalicílico con recubrimiento entérico. En este estudio, los pacientes diabéticos mostraron un mayor riesgo de hemorragia que los no diabéticos, aunque no se realizó un subanálisis según la función renal basal109.
Las guías clínicas recomiendan que tras un síndrome coronario agudo o una intervención coronaria percutánea, se administre tratamiento antiagregante plaquetario dual (dosis bajas de ácido acetilsalicílico más un inhibidor de P2Y12)2,4,5. Sin embargo, debe evaluarse cuidadosamente la duración óptima del tratamiento antiagregante plaquetario dual en los pacientes con diabetes y ERC, especialmente en aquellos con ERC avanzada (FGe<30ml/min/1,73m2), debido a su mayor riesgo de hemorragia110,111. Los pacientes con diabetes, ERC y un FGe reducido presentan un mayor riesgo de acontecimientos cardiovasculares tras un síndrome coronario agudo o una intervención coronaria percutánea110,112–114, así como un mayor riesgo de hemorragia110,112–114. Sin embargo, son escasos los ensayos que han analizado la eficacia y la seguridad del tratamiento antiagregante plaquetario doble en esta población. En un análisis post hoc del ensayo PLATO (Platelet Inhibition and Patient Outcomes), que incluyó a los pacientes con síndrome coronario agudo aleatorizándolos para ticagrelor frente a clopidogrel, el ticagrelor redujo la incidencia del objetivo primario compuesto (muerte cardiovascular, infarto de miocardio o ictus en un período de 12 meses) de forma uniforme en todos los subgrupos de pacientes con diabetes y/o ERC, pero con una mayor reducción del riesgo absoluto en DM+/ERC+, mientras que no se observó un mayor riesgo de hemorragia grave con ticagrelor en comparación con clopidogrel en el subgrupo de pacientes con DM+/ERC+114. En un análisis post hoc del ensayo GLOBAL-LEADERS, que incluyó a los pacientes sometidos a intervención coronaria percutánea, se analizaron los efectos de un tratamiento antiagregante plaquetario dual durante un mes, seguido de 23 meses de monoterapia con ticagrelor, frente a un tratamiento antiagregante plaquetario dual de 12 meses seguido de 12 meses de ácido acetilsalicílico en solitario según el estado de DM/ERC. Entre los pacientes DM+/ERC+, la monoterapia con ticagrelor no se asoció a menores tasas de muerte por cualquier causa, nuevo infarto de miocardio con onda Q o complicaciones hemorrágicas mayores. Sin embargo, sí se asoció a menores tasas de la variable de valoración compuesta orientada al paciente (muerte por todas las causas, cualquier ictus, infarto de miocardio notificado in situ y cualquier revascularización) y de acontecimientos clínicos adversos netos (una combinación de la variable de valoración compuesta orientada al paciente con acontecimientos hemorrágicos de tipo 3 o 5 del Bleeding Academic Research Consortium). No obstante, los autores afirmaron que estos resultados deben considerarse generadores de hipótesis112. En un análisis post hoc del ensayo TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention), que realizó 3 meses de tratamiento antiagregante plaquetario dual con ticagrelor y ácido acetilsalicílico tras una intervención coronaria percutánea, los pacientes libres de acontecimientos fueron asignados aleatoriamente a ácido acetilsalicílico o placebo además de ticagrelor durante 12 meses. Los autores concluyeron que, en los pacientes DM+/ERC+, la monoterapia con ticagrelor reducía el riesgo de hemorragia sin aumentar significativamente los episodios isquémicos en comparación con ticagrelor más ácido acetilsalicílico. Sin embargo, esta población presentó tasas numéricamente superiores de episodios isquémicos113. De forma similar, el ensayo THEMIS (Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study) mostró que en los pacientes con enfermedad arterial coronaria estable y DM pero sin antecedentes de infarto de miocardio o ictus, la combinación de ticagrelor con ácido acetilsalicílico redujo los riesgos de episodios isquémicos. Sin embargo, este beneficio fue contrarrestado por un aumento de las complicaciones hemorrágicas. A pesar de la ausencia de interacción por FGe, los pacientes con FGe reducido tendieron a obtener menores beneficios en términos de eficacia y un mayor riesgo de hemorragia115. En el subgrupo de pacientes del ensayo THEMIS que habían sido sometidos a una intervención coronaria percutánea previa, se observó que la combinación de ticagrelor y ácido acetilsalicílico tenía un beneficio clínico neto. Sin embargo, a pesar de que no hubo una interacción significativa, los beneficios fueron menores entre los pacientes con ERC en estadio 3 o superior116.
En el estudio PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis In Myocardial Infarction 54), la adición de ticagrelor al ácido acetilsalicílico redujo el riesgo de episodios isquémicos recurrentes, incluida la muerte cardiovascular y por enfermedad coronaria en los pacientes con DM e infarto de miocardio previo, pero la combinación también se asoció con un riesgo más elevado de hemorragia mayor en la escala TIMI117 y no se realizó ningún subanálisis por función renal. En el ensayo CHARISMA, los pacientes con enfermedad cardiovascular aterosclerótica establecida (sintomáticos) o múltiples factores de riesgo de enfermedad aterosclerótica (asintomáticos), pero sin síndrome coronario agudo activo, fueron asignados aleatoriamente a recibir clopidogrel más ácido acetilsalicílico o placebo más ácido acetilsalicílico. Los pacientes con diabetes y nefropatía que recibieron clopidogrel no presentaron un mayor riesgo de hemorragia, pero experimentaron un riesgo significativamente mayor de mortalidad cardiovascular y global en comparación con el grupo placebo. Esto apunta a que el clopidogrel puede ser perjudicial en los pacientes con diabetes y ERC118.
Del mismo modo, en las guías de prevención del ictus en los pacientes con un ictus/ataque isquémico transitorio no cardioembólico reciente, se recomienda la estrategia de tratamiento antiagregante plaquetario doble con ácido acetilsalicílico y clopidogrel durante 21 días, mientras que se desaconseja el tratamiento antiagregante plaquetario doble durante más de 3 meses105,106,119. En el ensayo THALES (The Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and ASA for Prevention of Stroke and Death) realizado en los pacientes con un ictus isquémico agudo no cardioembólico de leve a moderado o un accidente isquémico transitorio que no fueron sometidos a trombólisis intravenosa o endovascular, el riesgo del compuesto de ictus o muerte en un plazo de 30 días fue menor con ticagrelor-ácido acetilsalicílico que con ácido acetilsalicílico en solitario, pero no se apreciaron diferencias en la incidencia de discapacidad entre los 2 grupos y las hemorragias graves fueron más frecuentes con ticagrelor. Además, en el análisis por subgrupos, el criterio de valoración de la eficacia pareció insignificante entre los pacientes con diabetes, y no se realizó ningún análisis por FGe120.
En cuanto a la prevención secundaria a largo plazo del ictus isquémico en los pacientes con diabetes y ERC, no se encontraron evidencias procedentes de ensayos controlados aleatorizados, y se propone seguir las guías para la población general que recomiendan el uso de antiagregantes plaquetarios para reducir el riesgo de recurrencia del ictus105,106.
Aunque algunas guías establecen que el ácido acetilsalicílico puede ser considerado para la prevención primaria entre individuos de alto riesgo, incluyendo a los pacientes con DM, basándose en el mayor riesgo de enfermedad cardiovascular aterosclerótica2,4,5, esto debe equilibrarse con su mayor riesgo de hemorragia, incluida la disfunción plaquetaria asociada con la reducción del FGe121. El ensayo ASCEND (A Study of Cardiovascular Events iN Diabetes) asignó aleatoriamente a los pacientes con diabetes y sin enfermedad cardiovascular evidente a ácido acetilsalicílico 100mg diarios o placebo. Durante un seguimiento medio de 7,4 años, se produjo una reducción significativa del 12% en la variable principal de eficacia, aunque un aumento de las hemorragias graves en el grupo del ácido acetilsalicílico, en el que la mayoría de los casos fueron hemorragias gastrointestinales y otras hemorragias extracraneales. Así pues, se llegó a la conclusión de que los beneficios absolutos se veían contrarrestados en gran medida por el riesgo de hemorragia, con un cociente entre el número necesario para tratar y el número necesario para dañar (NNT/NND) de 0,8122. Sin embargo, no se ha comunicado ningún análisis post hoc de este estudio estratificado por la presencia de ERC. Entre los pacientes con diabetes y ERC, no existen pruebas sólidas de un perfil beneficio-riesgo favorable a partir de los resultados de los análisis post hoc de los ensayos controlados aleatorizados. El JAPAD (Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes) fue un ensayo prospectivo, aleatorizado y abierto en el que participaron los pacientes con DM2 sin antecedentes de enfermedad cardiovascular aterosclerótica (n=2.539). El criterio de valoración primario fue un compuesto de muerte súbita (muerte por causas coronarias, cerebrovasculares y aórticas); infarto agudo de miocardio no mortal; angina inestable; angina de esfuerzo de reciente aparición; ictus isquémico y hemorrágico no mortal; accidente isquémico transitorio; o enfermedad vascular aórtica y periférica no mortal. Tras ajustar por diversas variables, las dosis bajas de ácido acetilsalicílico no redujeron significativamente la variable principal de valoración en los pacientes con un FGe<60ml/min/1,73m2,123 lo que se confirmó en el seguimiento a 10 años del estudio (JPAD2124) en el subconjunto de pacientes con un FGe<60ml/min/1,73m2. Sin embargo, aumentó el riesgo de hemorragia gastrointestinal en toda la población. Más recientemente, el estudio TIPS-3 (International Polycap Study 3) que asignó aleatoriamente pacientes a ácido acetilsalicílico (75mg/día) o placebo en prevención primaria, se observó que aunque el ácido acetilsalicílico no redujo la tasa de muerte cardiovascular, infarto de miocardio o ictus en el conjunto de la población, los pacientes con diabetes y aquellos con un FGe<60ml/min/1,73m2 tendieron a mostrar cierto beneficio (aunque la p para la interacción no fue significativa, el número de pacientes con FGe reducido solo supuso el 17,2% de la población y además no se realizó un análisis post hoc de los pacientes con diabetes y FGe reducido)125,126. Por lo tanto, existen pruebas limitadas del beneficio del ácido acetilsalicílico a dosis bajas en términos de eficacia y seguridad para la prevención primaria de la enfermedad cardiovascular aterosclerótica en los pacientes con diabetes y ERC en estadio 3 o superior.
Una limitación de estos estudios es que se excluyó a los pacientes con ERC avanzada o en diálisis, lo que limita la generalización de los resultados a esta población.
Según las nuevas guías 2024 de la American Diabetes Association (ADA), debe considerarse la combinación de ácido acetilsalicílico más dosis bajas de rivaroxabán en los pacientes con diabetes y enfermedad arterial coronaria y/o enfermedad arterial periférica estables y bajo riesgo de hemorragia para prevenir episodios adversos mayores en las extremidades y cardiovasculares104 basándose en los resultados positivos del COMPASS (subgrupo de pacientes con diabetes)127 y VOYAGER-PAD128, a pesar del mayor riesgo de hemorragia con esta combinación. Sin embargo, no se han comunicado datos sobre la eficacia y la seguridad entre los pacientes con diabetes y ERC en estadio 3 o superior. Aun así, en el ensayo COMPASS original no se observó ninguna interacción en términos de eficacia y seguridad por FGe reducido o presencia de diabetes129. Sin embargo, en el ensayo VOYAGER PAD se observó una tendencia a un menor beneficio en los pacientes diabéticos, así como un riesgo de hemorragia más elevado entre los pacientes diabéticos y los pacientes con un FGe reducido128. Además, los pacientes con ERC en estadio 5 o 5D fueron excluidos de estos ensayos, lo que limita la extrapolación de los resultados a este subgrupo de pacientes con diabetes y ERC.
Recomendación 5.6. Los pacientes con fibrilación auricular no valvular, DM1 o DM2 y ERC en estadios 1-4 (dabigatrán hasta estadio 3b) deben ser tratados preferentemente con anticoagulantes orales de acción directa frente a antagonistas de la vitamina K.
Fuerza de la recomendación: 1B.
Justificación: La fibrilación auricular es la arritmia más frecuente en todo el mundo y aumenta sustancialmente el riesgo de ictus y episodios tromboembólicos130. Tanto la DM como la ERC se asocian a un mayor riesgo de desarrollar fibrilación auricular en comparación con la población general130–132. Además, la presencia de DM y/o ERC en los pacientes con fibrilación auricular aumenta el riesgo de episodios tromboembólicos133–136, así como los riesgos de mortalidad y hemorragia136,137.
La puntuación más utilizada para estimar el riesgo tromboembólico y la indicación de anticoagulación oral es la CHA2DS2-VASc130 y la DM es uno de los ítems de la puntuación, por lo que la mayoría de los pacientes con diabetes y ERC indicarán anticoagulación oral. El riesgo de hemorragia bajo anticoagulación suele estimarse con la puntuación HAS-BLED que incluye la disfunción renal como ítem130.
Actualmente existen 2 clases de anticoagulantes orales comercializados: los antagonistas de la vitamina K (warfarina, acenocumarol, etc.) y los anticoagulantes orales directos o de acción directa (apixabán, rivaroxabán, edoxabán y dabigatrán). La ERC afecta a la biodisponibilidad y la farmacocinética de los anticoagulantes orales directos, ya que todos ellos son excretados, al menos parcialmente, por los riñones (el aclaramiento renal del dabigatrán es de alrededor del 80%; el del edoxabán, del 50%; el del rivaroxabán, del 33%, y el del apixabán, del 27%) y pueden requerir ajustes de la dosis en los pacientes con un FGe reducido. No se recomienda su indicación en los pacientes con ERC en estadio ≥4 (dabigatrán) o ERC en estadio 5 (edoxabán, rivaroxabán o apixabán)138. Ningún ensayo clínico ha evaluado su uso en los pacientes con diabetes y ERC. Sin embargo, análisis post hoc o metanálisis de ensayos clínicos aleatorizados han mostrado la eficacia y seguridad de los anticoagulantes orales directos frente a los antagonistas de la vitamina K en los pacientes con diabetes y en la población con ERC. Además, ambos metanálisis han mostrado un menor riesgo de episodios tromboembólicos, hemorragia intracerebral y muerte, con un riesgo similar en hemorragias mayores frente a los antagonistas de la vitamina K hasta estadios avanzados de ERC136,139.
Por otra parte, los antagonistas de la vitamina K muestran un menor tiempo en rango terapéutico en la ERC que empeora a medida que progresa la enfermedad, lo que se asocia a un mayor riesgo de episodios tromboembólicos y hemorrágicos138,140. Además, los datos derivados de ensayos clínicos aleatorizados con dabigatrán y rivaroxabán apuntan a que los anticoagulantes orales directos podrían tener un beneficio en términos de reducción de la progresión de la ERC38,39. En los estadios 5 y 5D de la ERC no se recomiendan los anticoagulantes orales directos ni los antagonistas de la vitamina K130 porque la eficacia y la seguridad en esta población se basan en un número escaso de ensayos clínicos aleatorizados y las directrices no ofrecen recomendaciones claras en este contexto138. Además, los anticoagulantes orales directos no están indicados en los pacientes con arritmias valvulares y/o prótesis valvulares cardíacas130.
Recomendación 5.7. Los pacientes con DM1 o DM2 y ERC en estadios 1-4 (dabigatrán hasta estadio 3b) con tromboembolia venosa deben ser tratados preferentemente con anticoagulantes orales de acción directa frente a los antagonistas de la vitamina K (2C).
Fuerza de la recomendación: 2C.
Justificación: La tromboembolia venosa, que incluye la trombosis venosa profunda y la embolia pulmonar, también es una indicación de anticoagulación141,142. Existe un mayor riesgo de tromboembolia venosa tanto en los pacientes con diabetes143 como en los que padecen ERC144, en los que el riesgo de tromboembolia venosa aumenta a medida que disminuye la función renal144. Además, la DM y la ERC son factores de riesgo de tromboembolia venosa recurrente44,45. Entre las trombosis venosas profundas, la trombosis venosa profunda no provocada se refiere a la trombosis venosa en ausencia de factores de riesgo identificables. Del mismo modo, la trombosis venosa profunda se produce en presencia de dichos factores de riesgo, y puede clasificarse a su vez como transitoria o persistente. La naturaleza provocada o no provocada de la trombosis venosa profunda, así como la cronicidad de cualquier factor de riesgo provocador (transitorio o persistente), tiene importantes implicaciones pronósticas y terapéuticas, ya que el riesgo de recurrencia y los regímenes de anticoagulación difieren en consecuencia141. La trombosis venosa profunda requiere anticoagulación con heparina no fraccionada, fondaparinux, heparinas de bajo peso molecular, anticoagulantes orales directos o antagonistas de la vitamina K. Entre los anticoagulantes orales directos, el apixabán y el rivaroxabán pueden iniciarse sin anticoagulación parenteral inicial141.
Entre los pacientes con embolia pulmonar, los de alto riesgo requieren tratamiento con heparina no fraccionada en la fase aguda. Aun así, entre los de riesgo intermedio o bajo, cuando está indicada la anticoagulación oral, se prefiere un anticoagulante oral directo (dabigatrán, rivaroxabán, apixabán o edoxabán) a los antagonistas de la vitamina K41.
Los anticoagulantes orales directos proporcionan una eficacia similar y un menor riesgo de hemorragia mayor y hemorragia intracraneal o hemorragia mortal que las heparinas de bajo peso molecular y los antagonistas de la vitamina K; el beneficio también se observó en la población con aclaramiento de creatinina reducido145,146, y aunque los ensayos se realizaron en los pacientes con aclaramientos de creatinina de hasta 25-30ml/min/1,73m2, existen pruebas para algunos de ellos de su seguridad en los pacientes con aclaramientos de creatinina de hasta 15ml/min/1,73m2,147. Sin embargo, los anticoagulantes orales directos no se recomiendan en los pacientes con tromboembolia venosa y ERC avanzada, embarazo, lactancia o síndrome antifosfolípido142.
La duración óptima de la anticoagulación oral dependerá del tipo de trombosis venosa profunda (provocada o no provocada), de la duración del factor de riesgo (transitorio o persistente) y del riesgo de recurrencia de la tromboembolia venosa141,142. Sin embargo, en esta revisión no se encontraron datos sobre los pacientes con diabetes y ERC y FGe reducido.
ConclusionesLos pacientes con diabetes y ERC deben ser tratados según las recomendaciones más actualizadas.
La mayor parte de esta guía se basa en evidencias de alta calidad. Especialmente para los tratamientos farmacológicos, se han evaluado muchos datos procedentes de ensayos clínicos aleatorizados.
Las principales limitaciones de esta guía son que, dado que la investigación en el campo de la diabetes sigue activa, se esperan datos adicionales sobre enfoques existentes y novedosos. Otra limitación es que no hemos cubierto el capítulo de dislipidemias ni embarazo, ya que no estaba previsto en el planteamiento inicial, por lo que recomendamos remitirse a las guías publicadas de otras sociedades científicas. Además, el elevado coste y otras limitaciones de recursos en los sistemas sanitarios limitarán la aplicación de algunas recomendaciones en individuos y poblaciones de forma generalizada.
Las Guías de Práctica Clínica seguirán evolucionando. Es probable que en un futuro próximo se necesiten nuevas directrices centradas en el diagnóstico y el tratamiento de las personas con diabetes y ERC.
AutoríaNM participó en el diseño y realización de la investigación, el análisis de los datos y la redacción del artículo. LO intervino en la realización de la investigación, la recogida y análisis de datos y la redacción del artículo. AMC, MG, JLG, MJS, BFF, MQ, DRE, JFN participaron en la recogida de datos y en la redacción del artículo. CG, PG, JG, PM, MJP, NS, RS tomaron parte en la recogida y el análisis de los datos. Todos los autores aprobaron la versión final del artículo. AMC, JLG, MJS y BFF contribuyeron a partes iguales al concepto y diseño.
FinanciaciónEsta investigación ha sido apoyada por una beca de la Fundación SENEFRO (Sociedad Española de Nefrología, GPC-DC/TP S.E.N. 2019) y una beca Mundipharma.
Conflicto de interesesAMC ha recibido honorarios como conferenciante de Bayer, Boëhringer-Ingelheim, Lilly, Novo-Nordisk, Esteve Laboratorio y Merck-Sharp-Dhôme, y ha participado en consejos asesores de Boëhringer-Ingelheim, Lilly y Merck-Sharp-Dhôme. JLG ha sido asesor de consejos científicos de AstraZeneca, Bayer y Novo Nordisk; ha impartido conferencias para AstraZeneca, Boehringer Ingelheim, Esteve, Bayer, Eli Lilly and Company, Bayer, Astellas y Novo Nordisk y ha realizado actividades de investigación para AstraZeneca. MS recibió subvenciones o contratos de Boehringer, ISCIII y Marató TV3; honorarios por conferencias de NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Fresenius y Travere Therapeutics; apoyo para asistir a reuniones de Travere; participación en un consejo de seguridad de datos, consejo de supervisión o consejo asesor de NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Bayer, GE Healthcare y Travere Therapeutics. BFF ha recibido subvenciones de Esteve y Astrazeneca y honorarios por consultoría, ponencias o viajes de Astrazeneca, Bayer, Menarini, Novo-Nordisk Boehringer-Lilly, Amgen y Mundipharma. JJGM es asesor en consejos científicos para Astra-Zeneca, Bayer, Janssen Pharmaceuticals, Eli Lilly and Company, Menarini y Novo-Nordisk; ha impartido conferencias para Abbott, Amarin, Astra-Zeneca, Boehringer Ingelheim Pharmaceuticals Inc, Janssen Pharmaceuticals, Eli Lilly and Company, Menarini, Mundipharma Pharmaceuticals, Novo-Nordisk y Roche Pharma, y actividades de investigación para Astra-Zeneca, Eli Lilly and Company, Mundipharma Pharmaceuticals y NovoNordisk. MPM ha recibido asesoramiento, honorarios como ponente o apoyo para viajes de Astellas, AstraZeneca, Boehringer Ingelheim, CSL Vifor, Lilly, Menarini y Novo Nordisk. RS ha recibido honorarios por consultoría, ponencias o viajes de AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, NovoNordisk y Vifor Pharma. CGC ha recibido ayudas para viajes y honorarios de congresos de AstraZeneca, Esteve, NovoNordisk, Boehringer Ingelheim Lilly, Astellas, Otsuka, Novartis, Astellas y Baxter; ha impartido conferencias científicas y participado en consejos asesores organizados por AstraZeneca, Boehringer Ingelheim Lilly, Mundipharma, Esteve, Otsuka y NovoNordisk. PM ha recibido honorarios como consultora y/o ponente de CSL Vifor, Fresenius Kabi, Abbot, Baxter, Palex y Medtronic. JFNG ha recibido subvenciones de Abbvie, Bionet Medical, Boehringer Ingelheim, Sanofi-Genzyme, Shire y CSL Vifor, así como honorarios por consultoría, ponencias o viajes de Astrazeneca, Amgen, Bayer, Bionet Medical, Boehringer Ingelheim, Eli Lilly, Esteve, GlaxoSmithKline, Janssen, Menarini, MSD, Mundipharma, Novartis, Novo-Nordisk, Sanofi-Genzyme, Servier y CSL Vifor. NS es asesor en consejos científicos para Astra-Zeneca, Eli Lilly and Company, Menarini y Novo-Nordisk; ha impartido conferencias para Astra-Zeneca, Boehringer Ingelheim, Eli Lilly and Company, Menarini, Mundipharma Pharmaceuticals, Novo-Nordisk.
Información sobre el suplementoEste artículo forma parte del suplemento titulado “Guía de práctica clínica sobre detección y manejo de la enfermedad renal diabética: informe de consenso de la Sociedad Española de Nefrología”, patrocinado por la Sociedad Española de Nefrología, con la colaboración de financiación de Mundipharma.
Estamos especialmente agradecidos a los miembros del Grupo de Trabajo por su pericia a lo largo de todo el proceso de revisión bibliográfica, extracción de datos, participación en reuniones y redacción y edición críticas de los enunciados y la justificación, que han hecho posible la publicación de esta guía. Por último, y en nombre del Grupo de Trabajo, agradecemos la cuidadosa evaluación del borrador de la guía por parte de revisores externos. El Grupo de Trabajo tuvo en cuenta todos los valiosos comentarios realizados y, en su caso, los cambios sugeridos se incorporaron a la publicación final. Las siguientes personas aportaron sus comentarios durante la revisión pública del documento de las guías: Cigarran Guldris, Secundino; de Alvaro Moreno, Fernando; Egido de los Rios, Jesús; Llorente Gomez de Segura, Iñaki; Marques Vidas, María; Morillas Ariño, Carlos; Ortiz Arduan, Alberto; Robles Perez-Monteoliva; Nicolás Roberto.
Este artículo forma parte del suplemento titulado «Guía de práctica clínica sobre detección y manejo de la enfermedad renal diabética: documento de consenso de la Sociedad Española de Nefrología», que ha sido financiado por la Sociedad Española de Nefrología, con patrocinio de Mundipharma.