Chronic kidney disease (CKD) refers to abnormalities of kidney structure and/or function over time with implications for health.1 The global prevalence of kidney failure is uncertain. In the year 2017, the global burden of kidney failure was estimated to be 0.07%, or approximately 5.3million people.2 Kidney failure may be treated with supportive care, dialytic therapies and kidney transplantation.3 Haemodialysis (HD) is the most popular modality of kidney replacement therapy (KRT) worldwide.4–7 Haemodialysis services are available in many African countries but not affordable or accessible to the large majority of resident candidates.8 Outcomes of patients initiated on HD may include recovery of some kidney function which might not require dependency on HD, dependency on HD, transitioning to kidney allograft transplantation or death. We set out to document clinical characteristics and the twelve-month outcomes of adult patients initiated on incident HD for treatment of end stage kidney disease (ESKD) at the Kenyatta National Hospital in Nairobi-Kenya. This was a prospective observation study. Patients were enrolled between April and September 2022 and followed up for twelve months from October 2022 to September 2023.
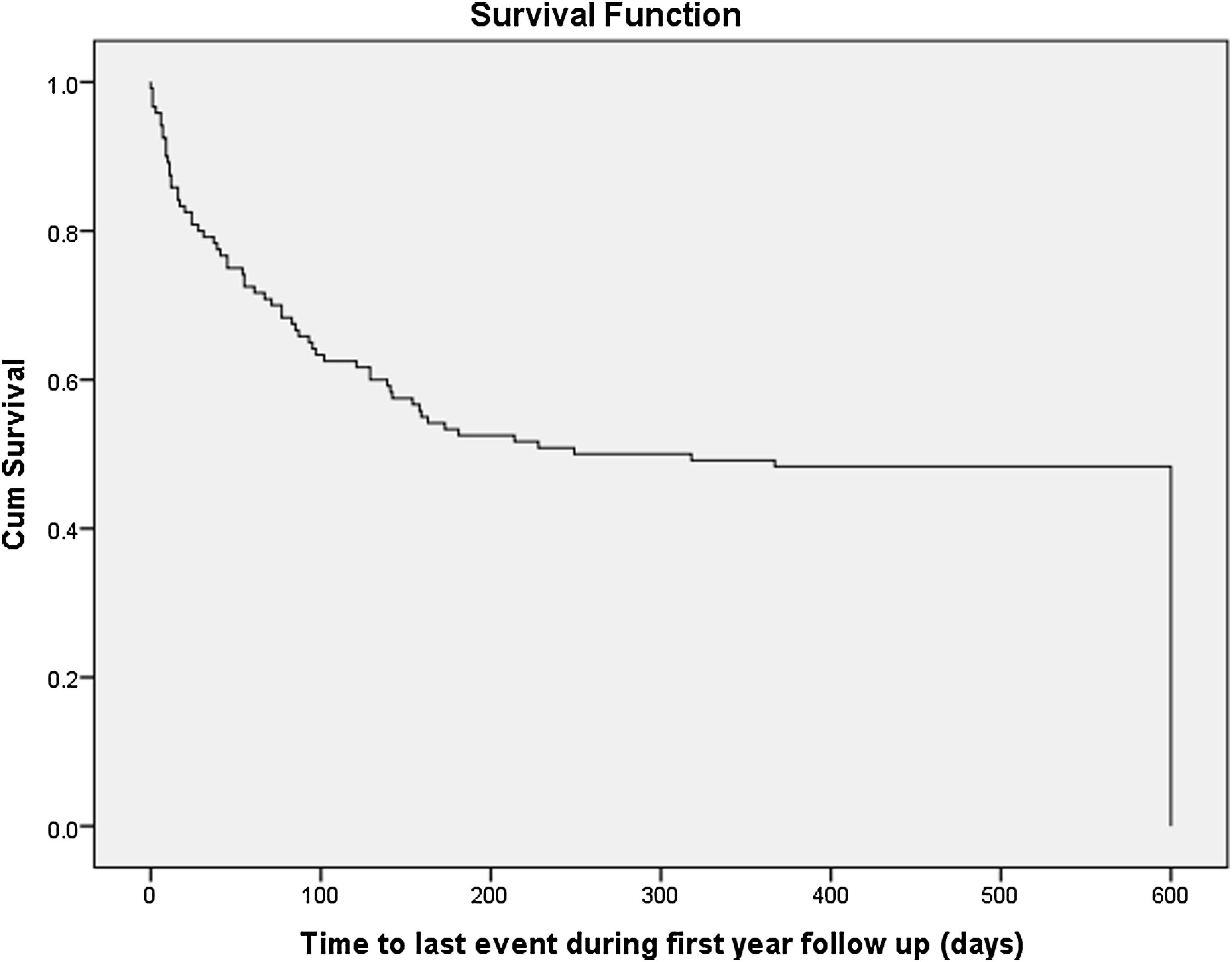
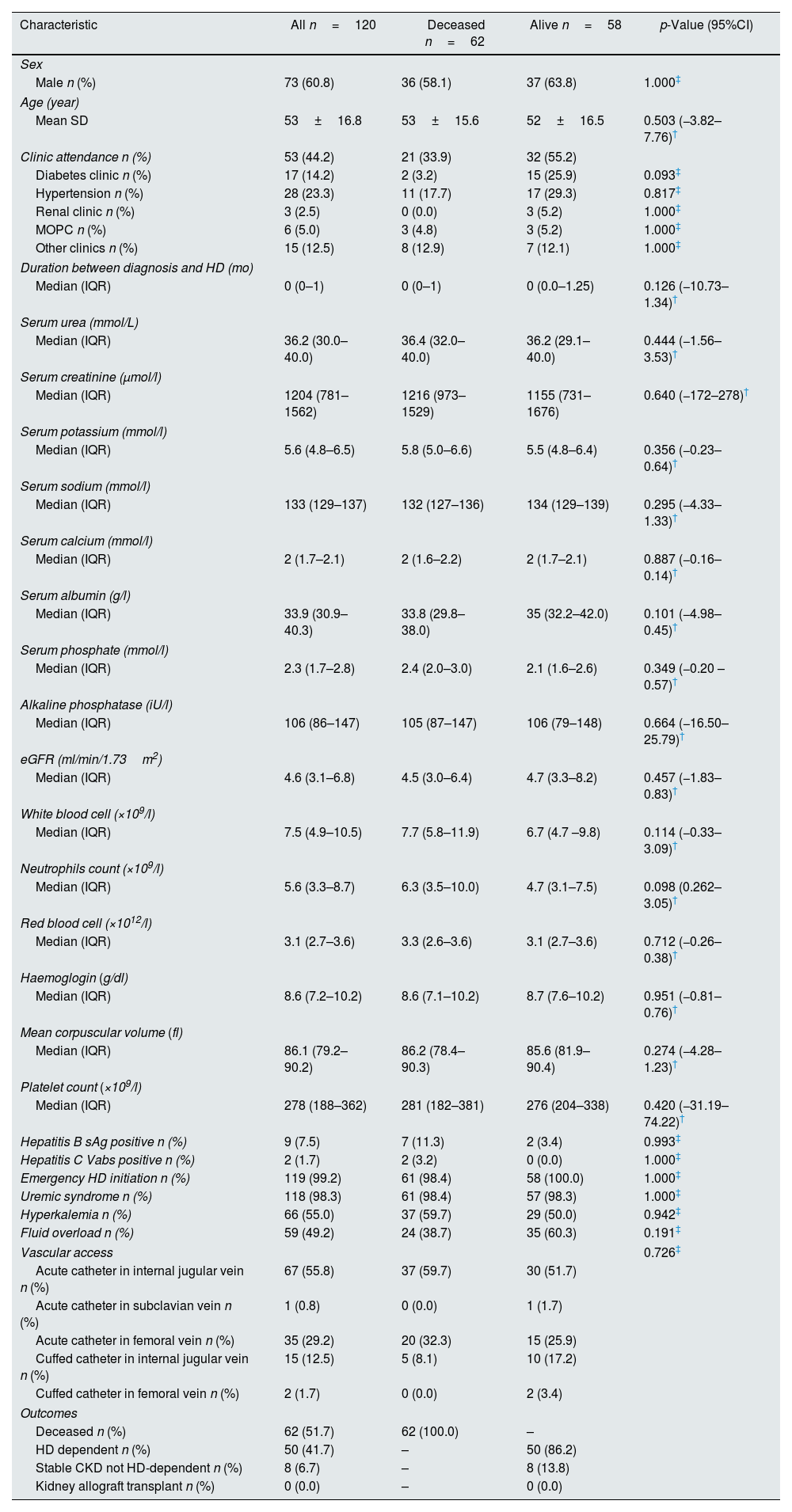
There were 120 patients enrolled into the study. The mean age was 53 years. Three in every five patients were males. Less than half reported to have had attendance to a regular clinic before initiation of HD, though the duration was very short. The medians for serum urea, creatinine, potassium, sodium, and calcium, albumin, phosphate, alkaline phosphatase, white blood cell counts, neutrophils, red blood cells, hemoglobin concentration, mean corpuscular volume and platelet counts are shown in Table 1. The median estimated glomerular filtration rate (eGFR) at the start of HD was 4.6mL/min/1.73m2. Almost all the patients were initiated HD as emergency. The indications for HD-start were uremic syndromes in 118 (98.2%), hyperkalemia in 66 (55.0%) and fluid overload in 59 (49.2%). The most popular vascular access for HD was acute HD catheter placed in internal jugular veins in 67 (55.8%) patients. By the twelfth month, 62/120 (51.7%) were deceased, 50/120 (41.7%) were dependent on HD while 8/120 (6.7%) had regained significant kidney function and did not require to continue with HD, while none had received kidney allograft transplant. There were no differences in the characteristics of those who were deceased when compared with those who survived (Table 1). About 41 (34.2%) of patients died within the first three months after initiation of HD treatment. The median survival duration was 7 weeks (1.0–18.0). Kaplan–Meier curve (Fig. 1) shows the survival from initiation of HD up to the twelfth month.
Characteristic of the all patients started on haemodialysis and comparison of those deceased with those who were alive.
| Characteristic | All n=120 | Deceased n=62 | Alive n=58 | p-Value (95%CI) |
|---|---|---|---|---|
| Sex | ||||
| Male n (%) | 73 (60.8) | 36 (58.1) | 37 (63.8) | 1.000‡ |
| Age (year) | ||||
| Mean SD | 53±16.8 | 53±15.6 | 52±16.5 | 0.503 (−3.82–7.76)† |
| Clinic attendance n (%) | 53 (44.2) | 21 (33.9) | 32 (55.2) | |
| Diabetes clinic n (%) | 17 (14.2) | 2 (3.2) | 15 (25.9) | 0.093‡ |
| Hypertension n (%) | 28 (23.3) | 11 (17.7) | 17 (29.3) | 0.817‡ |
| Renal clinic n (%) | 3 (2.5) | 0 (0.0) | 3 (5.2) | 1.000‡ |
| MOPC n (%) | 6 (5.0) | 3 (4.8) | 3 (5.2) | 1.000‡ |
| Other clinics n (%) | 15 (12.5) | 8 (12.9) | 7 (12.1) | 1.000‡ |
| Duration between diagnosis and HD (mo) | ||||
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0.0–1.25) | 0.126 (−10.73–1.34)† |
| Serum urea (mmol/L) | ||||
| Median (IQR) | 36.2 (30.0–40.0) | 36.4 (32.0–40.0) | 36.2 (29.1–40.0) | 0.444 (−1.56–3.53)† |
| Serum creatinine (μmol/l) | ||||
| Median (IQR) | 1204 (781–1562) | 1216 (973–1529) | 1155 (731–1676) | 0.640 (−172–278)† |
| Serum potassium (mmol/l) | ||||
| Median (IQR) | 5.6 (4.8–6.5) | 5.8 (5.0–6.6) | 5.5 (4.8–6.4) | 0.356 (−0.23–0.64)† |
| Serum sodium (mmol/l) | ||||
| Median (IQR) | 133 (129–137) | 132 (127–136) | 134 (129–139) | 0.295 (−4.33–1.33)† |
| Serum calcium (mmol/l) | ||||
| Median (IQR) | 2 (1.7–2.1) | 2 (1.6–2.2) | 2 (1.7–2.1) | 0.887 (−0.16–0.14)† |
| Serum albumin (g/l) | ||||
| Median (IQR) | 33.9 (30.9–40.3) | 33.8 (29.8–38.0) | 35 (32.2–42.0) | 0.101 (−4.98–0.45)† |
| Serum phosphate (mmol/l) | ||||
| Median (IQR) | 2.3 (1.7–2.8) | 2.4 (2.0–3.0) | 2.1 (1.6–2.6) | 0.349 (−0.20 –0.57)† |
| Alkaline phosphatase (iU/l) | ||||
| Median (IQR) | 106 (86–147) | 105 (87–147) | 106 (79–148) | 0.664 (−16.50–25.79)† |
| eGFR (ml/min/1.73m2) | ||||
| Median (IQR) | 4.6 (3.1–6.8) | 4.5 (3.0–6.4) | 4.7 (3.3–8.2) | 0.457 (−1.83–0.83)† |
| White blood cell (×109/l) | ||||
| Median (IQR) | 7.5 (4.9–10.5) | 7.7 (5.8–11.9) | 6.7 (4.7 –9.8) | 0.114 (−0.33–3.09)† |
| Neutrophils count (×109/l) | ||||
| Median (IQR) | 5.6 (3.3–8.7) | 6.3 (3.5–10.0) | 4.7 (3.1–7.5) | 0.098 (0.262–3.05)† |
| Red blood cell (×1012/l) | ||||
| Median (IQR) | 3.1 (2.7–3.6) | 3.3 (2.6–3.6) | 3.1 (2.7–3.6) | 0.712 (−0.26–0.38)† |
| Haemoglogin (g/dl) | ||||
| Median (IQR) | 8.6 (7.2–10.2) | 8.6 (7.1–10.2) | 8.7 (7.6–10.2) | 0.951 (−0.81–0.76)† |
| Mean corpuscular volume (fl) | ||||
| Median (IQR) | 86.1 (79.2–90.2) | 86.2 (78.4–90.3) | 85.6 (81.9–90.4) | 0.274 (−4.28–1.23)† |
| Platelet count (×109/l) | ||||
| Median (IQR) | 278 (188–362) | 281 (182–381) | 276 (204–338) | 0.420 (−31.19–74.22)† |
| Hepatitis B sAg positive n (%) | 9 (7.5) | 7 (11.3) | 2 (3.4) | 0.993‡ |
| Hepatitis C Vabs positive n (%) | 2 (1.7) | 2 (3.2) | 0 (0.0) | 1.000‡ |
| Emergency HD initiation n (%) | 119 (99.2) | 61 (98.4) | 58 (100.0) | 1.000‡ |
| Uremic syndrome n (%) | 118 (98.3) | 61 (98.4) | 57 (98.3) | 1.000‡ |
| Hyperkalemia n (%) | 66 (55.0) | 37 (59.7) | 29 (50.0) | 0.942‡ |
| Fluid overload n (%) | 59 (49.2) | 24 (38.7) | 35 (60.3) | 0.191‡ |
| Vascular access | 0.726‡ | |||
| Acute catheter in internal jugular vein n (%) | 67 (55.8) | 37 (59.7) | 30 (51.7) | |
| Acute catheter in subclavian vein n (%) | 1 (0.8) | 0 (0.0) | 1 (1.7) | |
| Acute catheter in femoral vein n (%) | 35 (29.2) | 20 (32.3) | 15 (25.9) | |
| Cuffed catheter in internal jugular vein n (%) | 15 (12.5) | 5 (8.1) | 10 (17.2) | |
| Cuffed catheter in femoral vein n (%) | 2 (1.7) | 0 (0.0) | 2 (3.4) | |
| Outcomes | ||||
| Deceased n (%) | 62 (51.7) | 62 (100.0) | – | |
| HD dependent n (%) | 50 (41.7) | – | 50 (86.2) | |
| Stable CKD not HD-dependent n (%) | 8 (6.7) | – | 8 (13.8) | |
| Kidney allograft transplant n (%) | 0 (0.0) | – | 0 (0.0) | |
CI, confidence interval; eGFR, estimated glomerular filtration rate; HD, haemodialysis; IQR, interquartile range; mo, month; MOPC, medical outpatient clinic; n, number; sAg, surface antigen; SD, standard deviation; Vabs, virus antibodies.
Despite the fact that only about 0.1–0.2% of the general population suffers from ESKD, treatment of kidney failure absorbs up to 5–7% of total health-care budgets in most regions.9 It is therefore necessary to evaluate the impact of this costly intervention on the treated patients. Outcomes are some of the measurable impacts. Mortality was high for this cohort, the highest being in the first three months. Mortality in patients who are initiated on HD has remained high despite advances in technology incorporated to treatment.10,11 None of the studied characteristics predicted the risk of death in ours study. In conclusion, the mortality of adults treated with HD for ESKD is high despite the fact that HD treatment is a very expensive venture. It is reasonable to propose further researches in our setting which delve deeper in the socio-cultural determinants of outcomes of patients with CKD treated with dialysis. This is because there seems to be unstudied factors that are contributory to mortality in this patients’ population.
FundingThis work was supported by the Kenyetta National Hospital Research and Programs.
Conflict of interestNone.
We would like to thank the staff and Management of Kenyatta National Hospital Renal Department for their co-operation during this study.