There is evidence indicating that some metabolites of arachidonic acid produced by cytochromes P450 (CYP) and epoxide hydroxylase (EPHX2), such as hydroxyeicosatetraenoic acids (HETEs), epoxyeicosatrienoic acids (EETs) or dihydroxyeicosatrienoic acids (DHETEs), play an important role in blood pressure regulation and they could contribute to the development of hypertension (HT) and kidney damage. Therefore, the main aim of the study was to evaluate whether the genetic polymorphisms of CYP2C8, CYP2C9, CYP2J2, CYP4F2, CYP4F11 and EPHX2, responsible for the formation of HETEs, EETs and DHETEs, are related to the progression of impaired renal function in a group of patients with hypertension.
Methods151 HT patients from a hospital nephrology service were included in the study. Additionally, a group of 87 normotensive subjects were involved in the study as control group. For HT patients, a general biochemistry analysis, estimated glomerular filtration rate and genotyping for different CYPs and EPHX2 variant alleles were performed.
ResultsCYP4A11 rs3890011, rs9332982 and EPHX2 rs41507953 polymorphisms, according to the dominant model, presented a high risk of impaired kidney function, with odds ratios (OR) of 2.07 (1.00–4.32; P=0.049), 3.02 (1.11–8.23; P=0.030) and 3.59 (1.37–9.41; P=0.009), respectively, and the EPHX2 rs1042032 polymorphism a greater risk according to the recessive model (OR=6.23; 95% CI=1.50–25.95; P=0.007). However, no significant differences in allele frequencies between HT patients and in normotensive subjects for any of the SNP analyzed. In addition, the patients with diagnosis of dyslipidemia (n=90) presented higher frequencies of EPHX2 K55R (rs41507953) and *35A>G (rs1042032) variants than patients without dyslipidemia, 4% vs. 14% (P=0.005) and 16 vs. 27% (P=0.02), respectively.
ConclusionsIn this study has been found higher odds of impaired renal function progression associated with rs3890011 and rs9332982 (CYP4A11) and rs41507953 and rs1042032 (EPHX2) polymorphisms, which may serve as biomarkers for improve clinical interventions aimed at avoiding or delaying, in chronic kidney disease patients, progress to end-stage kidney disease needing dialysis or kidney transplant.
Existen evidencias que muestran que algunos metabolitos del ácido araquidónico producidos por los citocromos P450 (CYP) y por la enzima epóxido hidroxilasa (EPHX2), tales como los ácidos hidroxieicosatetraenoicos (HETEs), ácidos epoxieicosatrienoicos (EETs) o ácidos dihidroxieicosatrienoicos (DHETEs), juegan un papel importante en la regulación de la presión arterial y, por tanto, podrían contribuir al desarrollo de hipertensión y daño renal. Por ello, el objetivo del estudio fue evaluar si los polimorfismos de los genes CYP2C8, CYP2C9, CYP2J2, CYP4F2, CYP4F11 y EPHX2, responsables de la formación de HETEs, EETs y DHETEs, están relacionados con la progresión de la función renal en un grupo de pacientes con hipertensión arterial (HT).
MétodosSe incluyeron en el estudio 151 pacientes de un servicio hospitalario de nefrología con diagnóstico de HT. Además, un grupo de 87 sujetos normotensos participó en el estudio como grupo de control. A los pacientes con HT se les realizó un análisis de bioquímica general, se calculó la tasa estimada de filtración glomerular y se analizaron los genotipos de los diferentes genes CYP y EPHX2 incluidos en el estudio.
ResultadosLos polimorfismos rs3890011, rs9332982 del gen CYP4A11 y rs41507953 del gen EPHX2 presentaron un alto riesgo de alteración de la función renal con unos odds ratio (OR) de 2.07 (1.00–4.32; P = 0.049), 3.02 (1.11–8.23; P = 0.030) and 3.59 (1.37–9.41; P = 0.009), respectivamente, así como el polimorfismo rs1042032 del gen EPHX2 presentó también un mayor riesgo (OR = 6.23; 95% CI = 1.50–25.95; P = 0.007). Sin embargo, no se observaron diferencias significativas en las frecuencias alélicas entre pacientes con HT y sujetos normotensos para ninguno de los polimorfismos analizados. Además, los pacientes con dislipidemia (n=90) presentaron una mayor frecuencia de las variantes EPHX2K55R (rs41507953) y *35A>G (rs1042032) que los pacientes sin dislipidemia, 4 vs. 14% (p=0,005) y 16 vs. 27% (p=0,02), respectivamente.
ConclusionesEn este estudio se han encontrado mayores probabilidades de progresión de la función renal asociadas a los polimorfismos rs3890011 y rs9332982 del gen CYP4A11 y rs41507953 y rs1042032 del gen EPHX2, que podrían servir como biomarcadores para mejorar las intervenciones clínicas destinadas a evitar o retrasar, en pacientes con enfermedad renal crónica, el avance de la enfermedad renal hacia la etapa terminal en la que necesitarían diálisis o trasplante de riñón.
Chronic kidney disease (CKD) is described as a condition with gradual loss of kidney function. It is generally defined by persistent (lasting more than 3 months) abnormality of kidney function as measured by estimated glomerular filtration rate (eGFR) levels lowers than 60ml/min/1.73m2. Using the eGFR, CKD is divided into six stages of worsening progression1 updated by Kidney Disease Improving Global Outcomes (KDIGO).2
CKD is considered a serious health problem worldwide, given its social and economic consequences1,3 and an emerging cardiovascular risk factor, being independently related to a higher incidence of cardiovascular events.4 Moreover, the progression of CKD has a significant impact on the quality of life of patients and the need ultimate for renal replacement therapy represents a significant consumption of resources for health systems.3,5
About 10% of people worldwide have CKD, though the incidence and prevalence of CKD differ significantly.1,6 This prevalence is currently increasing, fundamentally due to the increased incidence of diabetes mellitus (DM) and hypertension (HT), and the ageing of population. Thus, early identification of patients susceptible to developing CKD is of great importance to decrease progression and minimize cardiovascular morbidity.
Individual susceptibility and factors related to the progression of kidney function impairment are involved in the development of CKD.1 HT is a factor that is related to the onset of kidney damage and, at the same time, to its progression. Multiple observational studies have shown the relationship between HT and progression and appearance of CKD.7–10 In the PREVEND study (Prevention of Renal and Vascular End-stage Disease) was observed that the presence of HT is a risk factor associated with the progression of CKD, independently of baseline renal function, age, and urinary albumin excretion.8 In addition, an observational study found that higher systolic blood pressure (BP) was independently associated with a higher risk of CKD progression among patients with established CKD.9
The mechanism that would explain this relationship would be changes in the tone of the afferent arteriole, influenced by both the myogenic reflex and the tubuloglomerular feedback, which have an important role in protecting the glomerulus from changes in systemic blood pressure (BP), preventing the development of intraglomerular HT. Dysfunction of these mechanisms leads to impaired autoregulation, such that increases in systemic pressure will be associated with increases in intraglomerular pressure, predisposing to the development of kidney injury.11 These self-regulatory mechanisms maintain renal blood flow and glomerular filtration rate. The main mechanism in the dense-tubuloglomerular macula is to induce changes in afferent arteriolar resistance to change blood flow and filtration rate. In addition, at the tubular level there are mechanisms with feed-back action, which protect from HT barotrauma to the glomeruli through the stabilization of sodium excretion.12 These autoregulation mechanisms can be altered facilitating the progression of chronic kidney disease in pathology such as HT, DM and CKD.11,13
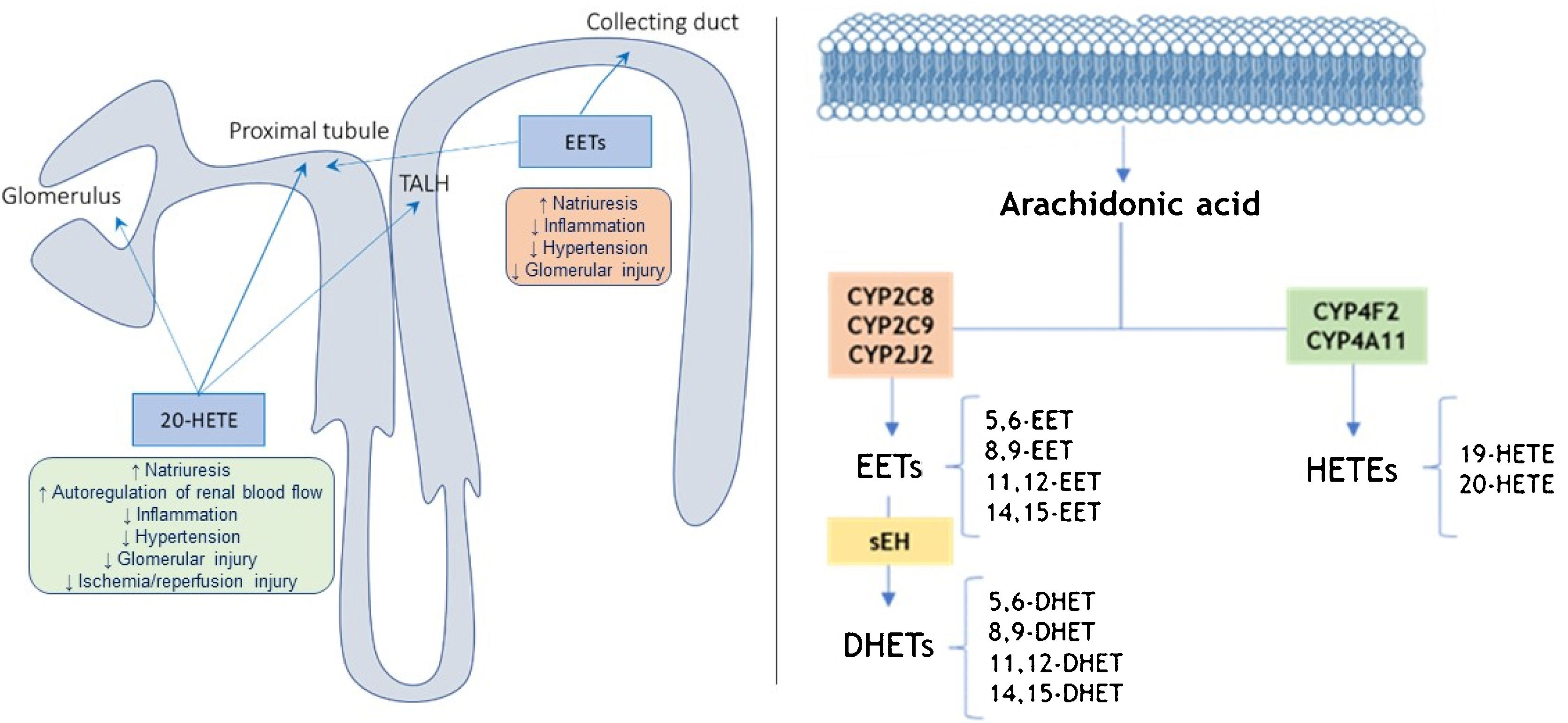
The CYP metabolites of arachidonic acid (AA), which are synthesized at the level of the proximal tubule and the thick ascending loop of Henle (TALH), serving as second messengers in the regulation of sodium transport (Fig. 1).14,15 Thus, it is likely that CYP metabolites of AA contribute to the changes in renal function and vascular tone14,16 and this could condition an altered response in pathologies such as HT, DM, hepatorenal syndrome and pregnancy.16
Location and action site of 20-HETEs and EETs in the nephron (left) and pathways of metabolism of arachidonic acid by CYP enzymes and (right).
Adapted from11,17,19; TALH: thick ascending loop of Henle; sEH: epoxide hydrolase.
There is evidence indicating that some metabolites of AA produced by cytochromes P450 (CYP), such as 20-hydroxyeicosatetraenoic acid (20-HETE) and some epoxyeicosatrienoic acids (EETs), play an important role in blood pressure regulation (Fig. 1). In this sense, if there are alterations in the synthesis of these lipids could contribute to the development of HT and kidney damage.15 On the other hand, the enzyme soluble epoxide hydroxylase (sEH) converts EETs into dihydroxyeicosatrienoic acids (DHETE; Fig. 1), which to participate in attenuating the development of HT and kidney damage in different animal models.15
The role of 20-HETE in the development of HT remains unresolved since 20-HETE has both antihypertensive and prohypertensive properties because it not only increases renal and peripheral vascular tone, but also inhibits sodium transport.17 20-HETE is a potent peripheric vasoconstrictor and it inhibits sodium transport in renal tubule, and therefore, it affects the feedback from the macula densa. It inhibits the NaK pump in the proximal tubule and the NaKCl cotransporter in the TALH, reducing dopamine-mediated natriuresis by 65%.18 In contrast, EETs are vasodilators and natriuretic substances that lower blood pressure.17 Similarly, EETs inhibit sodium reabsorption at the level of the proximal tubule and collecting tubule (Fig. 1). Therefore, it has actions that could be related to the genesis of essential HT, with the possibility of needing a greater number of drugs for its control, mostly diuretics that counteract Na excess and, in turn, protect against damage of the target organ that causes HT, and therefore related to protection against cardiovascular and renal risk.15
In the CYP pathway, AA is converted to EETs and HETEs by CYP epoxygenases and CYP ω-hydroxylases, respectively (Fig. 1). All EETs are later metabolized by sEH, coding by EPXH2 gene, giving rise to the formation of less active compounds such as DHETs.14,19,20 HETEs are produced mainly by the enzymes CYP4A11 and CYP4F2, while CYP2C8, CYP2C9, and CYP2J2 are the primary epoxygenases that produce EETs in humans, as well as sEH metabolizes EETs to the inactive DHETs (Fig. 1).
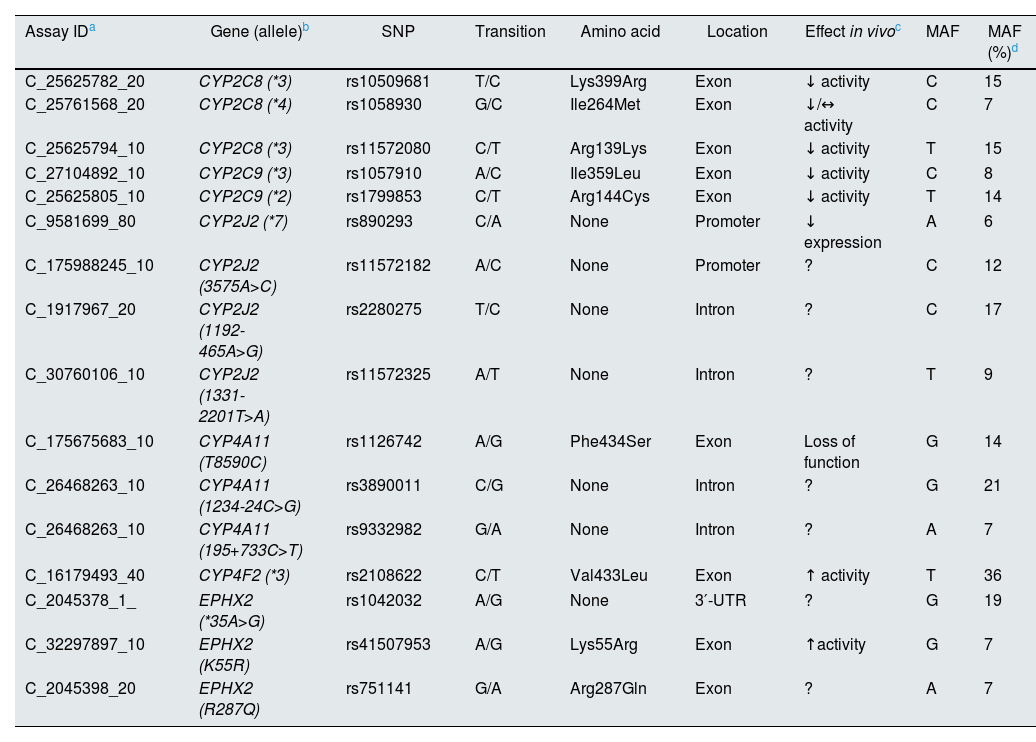
Different polymorphisms of genes encoding for the CYPs and the enzyme sEH involved in the metabolism of AA have related to enzymes with altered functional activity, and that could act for or against renal and cardiovascular risk. Table 1 shows some of these polymorphisms on which there is evidence of their alteration in in vivo activity and present in a relatively frequent percentage (>5%) in the European and Spanish populations, although, in previous studies, the results of the association of these polymorphisms with HT or renal pathology are contradictory.21–26
Polymorphisms of the genes encoding the enzymes involved in the formation of eicosanoids.
| Assay IDa | Gene (allele)b | SNP | Transition | Amino acid | Location | Effect in vivoc | MAF | MAF (%)d |
|---|---|---|---|---|---|---|---|---|
| C_25625782_20 | CYP2C8 (*3) | rs10509681 | T/C | Lys399Arg | Exon | ↓ activity | C | 15 |
| C_25761568_20 | CYP2C8 (*4) | rs1058930 | G/C | Ile264Met | Exon | ↓/↔ activity | C | 7 |
| C_25625794_10 | CYP2C8 (*3) | rs11572080 | C/T | Arg139Lys | Exon | ↓ activity | T | 15 |
| C_27104892_10 | CYP2C9 (*3) | rs1057910 | A/C | Ile359Leu | Exon | ↓ activity | C | 8 |
| C_25625805_10 | CYP2C9 (*2) | rs1799853 | C/T | Arg144Cys | Exon | ↓ activity | T | 14 |
| C_9581699_80 | CYP2J2 (*7) | rs890293 | C/A | None | Promoter | ↓ expression | A | 6 |
| C_175988245_10 | CYP2J2 (3575A>C) | rs11572182 | A/C | None | Promoter | ? | C | 12 |
| C_1917967_20 | CYP2J2 (1192-465A>G) | rs2280275 | T/C | None | Intron | ? | C | 17 |
| C_30760106_10 | CYP2J2 (1331-2201T>A) | rs11572325 | A/T | None | Intron | ? | T | 9 |
| C_175675683_10 | CYP4A11 (T8590C) | rs1126742 | A/G | Phe434Ser | Exon | Loss of function | G | 14 |
| C_26468263_10 | CYP4A11 (1234-24C>G) | rs3890011 | C/G | None | Intron | ? | G | 21 |
| C_26468263_10 | CYP4A11 (195+733C>T) | rs9332982 | G/A | None | Intron | ? | A | 7 |
| C_16179493_40 | CYP4F2 (*3) | rs2108622 | C/T | Val433Leu | Exon | ↑ activity | T | 36 |
| C_2045378_1_ | EPHX2 (*35A>G) | rs1042032 | A/G | None | 3′-UTR | ? | G | 19 |
| C_32297897_10 | EPHX2 (K55R) | rs41507953 | A/G | Lys55Arg | Exon | ↑activity | G | 7 |
| C_2045398_20 | EPHX2 (R287Q) | rs751141 | G/A | Arg287Gln | Exon | ? | A | 7 |
MAF: minor allele frequency.
There is evidence that 20-HETE and EET have renoprotective actions in HT and could prevent the development of CKD14,17,20, however there are polymorphisms that modify the activity of the EET and 20-HETE would facilitate the appearance of HT, increasing the risk of cardiovascular events and the development of CKD. Therefore, the main aim of the study was to evaluate whether the genetic polymorphisms of CYP2C8, CYP2C9, CYP2J2, CYP4F2, CYP4F11 and EPHX2, responsible for the formation of HETEs, EETs and DHETEs, are related to the progression of impaired renal function in a group of patients with HT. In addition, relationship between gene polymorphisms, involved in the formation of epoxy- and hydroxy-eicosanoids, and dyslipidemia and risk of cardiovascular events was also evaluated.
Materials and methodsStudy populationsAny patient with HT diagnosis (systolic and/or a diastolic blood pressure values ≥140 and ≥90mm Hg, respectively) referred to the Service of Nephrology from “Virgen del Puerto” Hospital (Plasencia, Spain) who was ≥18 years old was eligible for recruitment. A group of 151 HT patients (62.7% males; 68.0±13.6 years) were finally enrolled in the study.
Additionally, a group of 87 normotensive (NT; systolic and/or a diastolic blood pressure reading<140 and <90mm Hg, respectively) subjects (74.7% females; 28.1±11.3 years) ≥18 years old, mainly students and staff from the University of Extremadura (Plasencia, Spain), were involved in the study as control group. Signed informed consent was obtained from all subjects involved in the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of Cáceres (Extremadura Health Service; reference: MASR/2016), and by the Bioethics and Biosecurity Committee (University of Extremadura; reference: 64/2016).
In the group of HT patients, CKD was established according to the KDIGO guideline.2 CKD was considered in the presence of albuminuria, albumin/creatinine ratio (A/C)≥30mg/g or an estimated glomerular filtration ratio (eGFR) using the equation CKD-Epidemiology Collaboration2 (CKD-EPI)<60ml/min/1.73m2.
HT patients were classified as G1 stage when eGFR was normal or elevated (≥90ml/min/1.73m2), G2 or slightly decreased eGFR (60–89ml/min/1.73m2), G3a or mild to moderately decreased eGFR (45–59ml/min/1.73m2), G3b or moderately to severely decreased eGFR (30–44ml/min/1.73m2), G4 or severely decreased eGFR (15–29ml/min/1.73m2) and G5 or renal failure (<15ml/min/1.73m2).
For HT patients who consented to participate in the study on a first visit, an interview was conducted in which their demographical and clinical data (history of diseases, concomitant diseases, drug treatment) were collected, and a general biochemistry analysis was performed, the eGFR was calculated, and a blood sample was taken from each participant for DNA extraction after the first visit. Later, according to the clinical requirements of each patient, they were cited 6, 12, 18 and 24 months. At each visit, a general biochemistry analysis was performed and the eGFR was calculated. In addition, cardiovascular events that occurred during the 24-month were also recorded.
Genetic analysisDNA was isolated and purified from blood samples using QIAmp DNA extraction kit (Qiagen, Hilden, Germany). Genotyping for different CYPs and EPHX2 variant alleles was performed using a fluorescence-based allele-specific TaqMan (Thermo Fisher Scientific, Waltham, MA, USA) allelic discrimination assay (Table 1). PCR amplification for all single-nucleotide polymorphisms was performed in a PCR mixture consisting of 5μL of 2× Ex Taq Premix, 0.2μL of 50× Rox Reference Dye, 40× SNP Genotyping Assay Mix (Takara Bio Inc., Shiga, Japan) and 1μL of DNA (0.25ng/μL). Nuclease-free water was added to a final volume of 10μL. Amplification was carried out in an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The PCR conditions were an initial denaturation at 95°C for 30s, 40 cycles of 95°C for 5s and 60°C for 31s.
Statistical analysisTo assess for impaired kidney function, HT patients were categorized as progressors and non-progressors, defining progression as the decrease of eGFR≥10ml/min/1.73m2 in 24 months (≥5ml/min/1.73m2 per year).27
Logistic regression analysis (adjusted by age, gender, DM and dyslipidemia) was performed to assess the potential association between genotypes and impaired kidney function for 24 months in multiple inheritance models (dominant, recessive and additive). Odds ratios (OR) with corresponding 95% confidence intervals (95% CI), and the Hardy–Weinberg equilibrium (HWE) were determined using SNPStats software (www.snpstats.net).
Continuous variables were compared into different subgroups (eGFR stages, progression or genotypes) using Kruskal–Wallis test, Student's t-test or Mann–Whitney U test by SPSS statistical software (IBM SPSS Statistics 26; Chicago, IL, USA).
Fisher's exact tests were used to compare differences into variant allele frequencies between different groups (IBM SPSS Statistics 26; Chicago, IL, USA), and linkage disequilibrium (LD) statistics were obtained using Haploview software (www.broad.mit.edu/mpg/haploview). P-values lower than 0.05 were regarded as statistically significant.
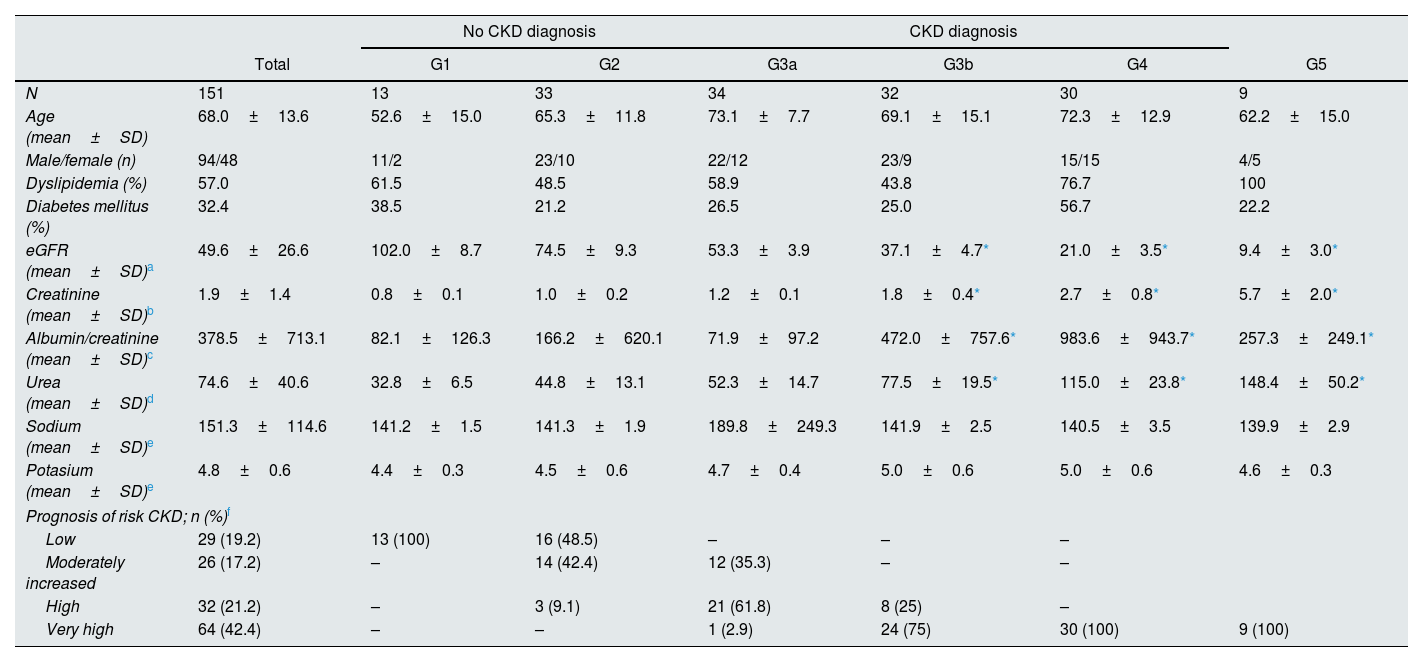
ResultsThe descriptive and clinical characteristics of 151 HT patients at the start of study are summarized in Table 2. According to CKD diagnostic criteria (KDIGO 2012), 105 patients (69.5%) stated diagnosis of CKD and, it should be noted that 57% and 32.4% of the HT patients presented concomitant dyslipidemia and DM, respectively. No significant differences were found between the sodium and potassium levels between the different stages, although differences were found in the urea concentrations between different eGFR stages (P<0.0001), mainly between the G3b, G4 and G5 stages and G1 and G2. These significant differences were also found in creatinine concentrations and in eGFR (P<0.001), as expected (Table 2).
Initial clinical characteristics of the hypertension patients included in the study according to eGFR stage (n=151).
| No CKD diagnosis | CKD diagnosis | ||||||
|---|---|---|---|---|---|---|---|
| Total | G1 | G2 | G3a | G3b | G4 | G5 | |
| N | 151 | 13 | 33 | 34 | 32 | 30 | 9 |
| Age (mean±SD) | 68.0±13.6 | 52.6±15.0 | 65.3±11.8 | 73.1±7.7 | 69.1±15.1 | 72.3±12.9 | 62.2±15.0 |
| Male/female (n) | 94/48 | 11/2 | 23/10 | 22/12 | 23/9 | 15/15 | 4/5 |
| Dyslipidemia (%) | 57.0 | 61.5 | 48.5 | 58.9 | 43.8 | 76.7 | 100 |
| Diabetes mellitus (%) | 32.4 | 38.5 | 21.2 | 26.5 | 25.0 | 56.7 | 22.2 |
| eGFR (mean±SD)a | 49.6±26.6 | 102.0±8.7 | 74.5±9.3 | 53.3±3.9 | 37.1±4.7* | 21.0±3.5* | 9.4±3.0* |
| Creatinine (mean±SD)b | 1.9±1.4 | 0.8±0.1 | 1.0±0.2 | 1.2±0.1 | 1.8±0.4* | 2.7±0.8* | 5.7±2.0* |
| Albumin/creatinine (mean±SD)c | 378.5±713.1 | 82.1±126.3 | 166.2±620.1 | 71.9±97.2 | 472.0±757.6* | 983.6±943.7* | 257.3±249.1* |
| Urea (mean±SD)d | 74.6±40.6 | 32.8±6.5 | 44.8±13.1 | 52.3±14.7 | 77.5±19.5* | 115.0±23.8* | 148.4±50.2* |
| Sodium (mean±SD)e | 151.3±114.6 | 141.2±1.5 | 141.3±1.9 | 189.8±249.3 | 141.9±2.5 | 140.5±3.5 | 139.9±2.9 |
| Potasium (mean±SD)e | 4.8±0.6 | 4.4±0.3 | 4.5±0.6 | 4.7±0.4 | 5.0±0.6 | 5.0±0.6 | 4.6±0.3 |
| Prognosis of risk CKD; n (%)f | |||||||
| Low | 29 (19.2) | 13 (100) | 16 (48.5) | – | – | – | |
| Moderately increased | 26 (17.2) | – | 14 (42.4) | 12 (35.3) | – | – | |
| High | 32 (21.2) | – | 3 (9.1) | 21 (61.8) | 8 (25) | – | |
| Very high | 64 (42.4) | – | – | 1 (2.9) | 24 (75) | 30 (100) | 9 (100) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; No CKD diagnose: eGFR>60ml/min/1.73m2; No CKD diagnose: eGFR<60ml/min/1.73m2.
HT patients were classified according to the prognostic risk of CKD progression according to the eGFR and albuminuria categories2 that they presented at the beginning of the study (Table 2). It can be noted that 100% of the HT patients classified as G1 showed low or no risk, while 100% of those classified as G4 and G5 showed a very high risk (Table 2).
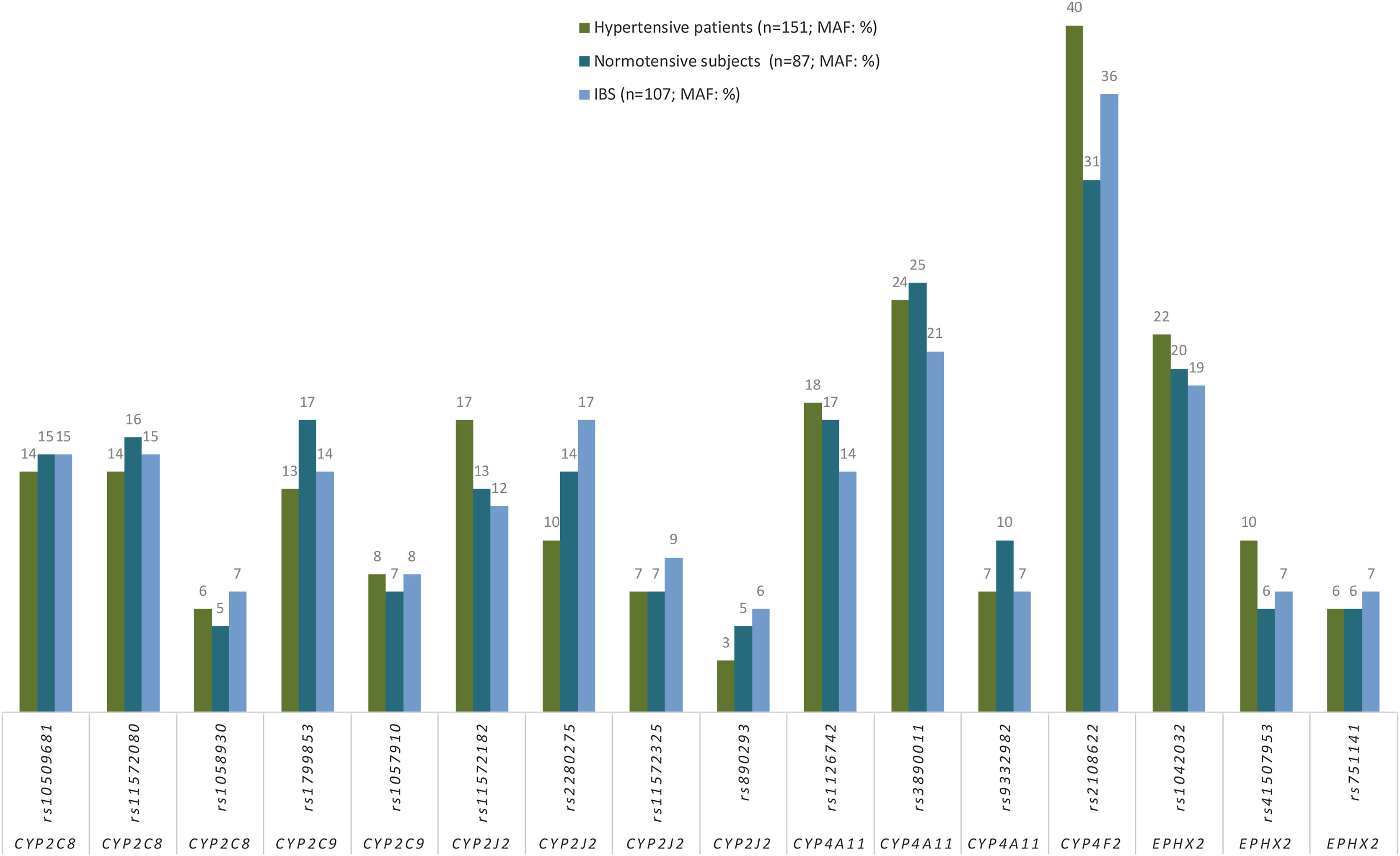
Association between hypertension and CYPs and EPHX2 polymorphismsGenotype frequencies both in HT patients (n=151) and in NT subjects (n=87) were in HWE. No significant differences in allele frequencies between HT patients (n=151) and in NT subjects for any of the SNP analyzed (Fig. 2), nor between the frequencies of the allelic variants in these two subpopulations and in the Spanish population (Fig. 2). In addition, after adjusting for age, gender, DM and dyslipidemia, there was no association with HT (data not shown), consequently, no association was found between any of the analyzed alleles and HT (Supplementary Table 1).
Frequencies (%) of minor allele frequencies (MAF) of CYPs and EPHX2 alleles in hypertensive patients (n=151), normotensive subjects (n=87) and in a Spanish population (n=107; IBS* population). *IBS: Iberian populations in Spain from 1000 Genome Project (http://www.ensembl.org/Homo_sapiens/).
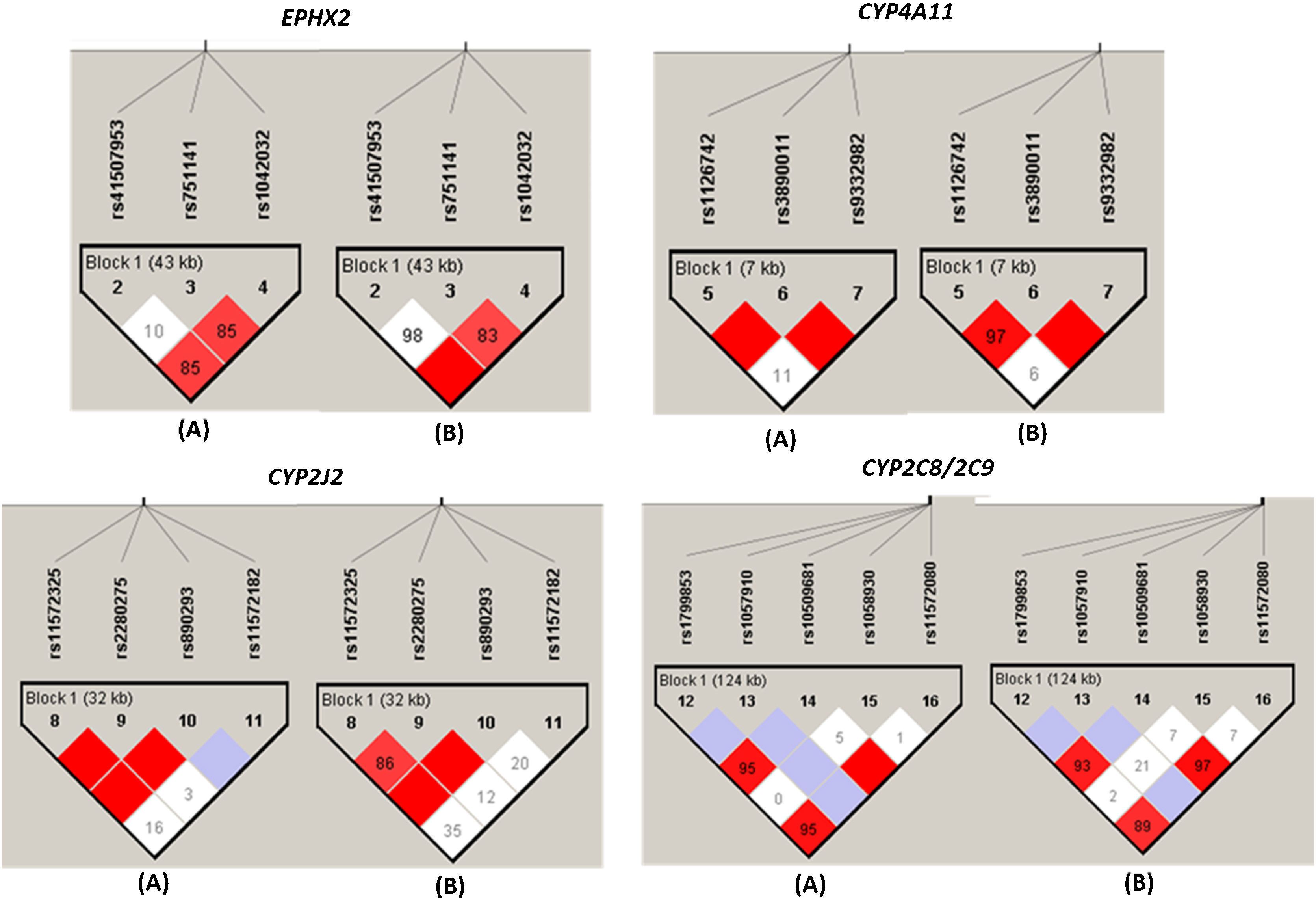
As shown in Fig. 3, the different SNPs of each EPHX2, CYP2C8/9, CYP4A11 and CYP2J2 gene loci were analyzed and all of them showed some type of LD between some of the SNPs evaluated, both in the group of HT patients and in the group of NT subjects, with the D′ values slightly higher in the group of NT subjects (Fig. 3).
Linkage disequilibrium plot of CYPs and EPHX2 variants alleles in normotensive subjects (n=87) and hypertensive patients (n=151). Linkage disequilibrium (LD) is displayed as pairwise D′ values. Shading represents the magnitude and significance of pairwise LD, with a red-to-white gradient reflecting higher-to-lower LD values. Dark red diamond without a number corresponds to D′ values of 1.0. (A) and (B) indicate the LD analysis of normotensive subjects (n=87) and hypertensive patients (n=151), respectively.
The LDs with higher r2 values were the rs1123742 with rs3890011 variants of CYP4A11 (r2=0.80), rs11572325 with rs890293 variants of CYP2J2 (r2=0.82), and rs11572080 with rs10509681 (r2=0.97) and with rs1799853 (r2=0.87), and rs10509681 with rs1799853 (r2=0.89) of CYP2C8/2C9 locus.
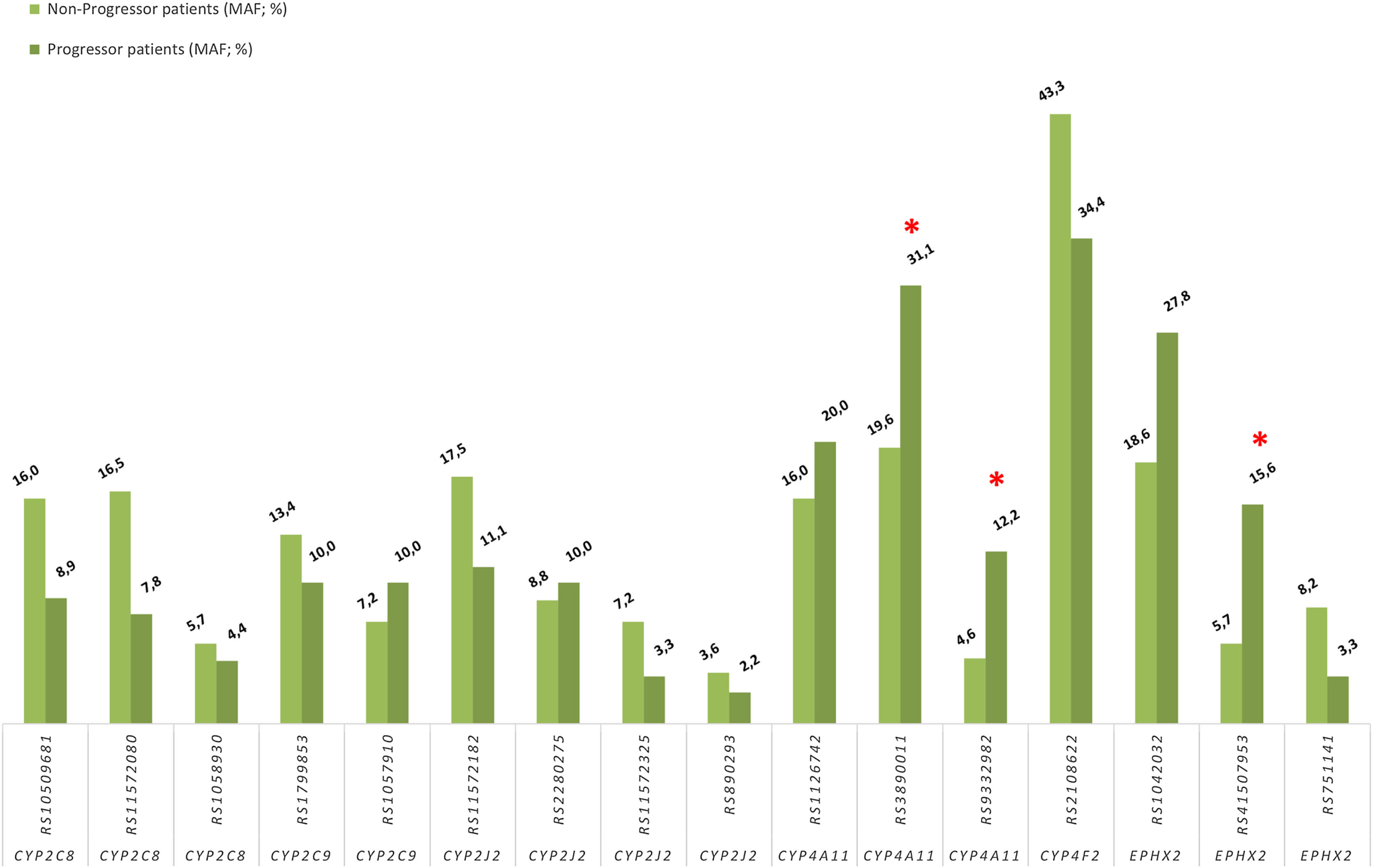
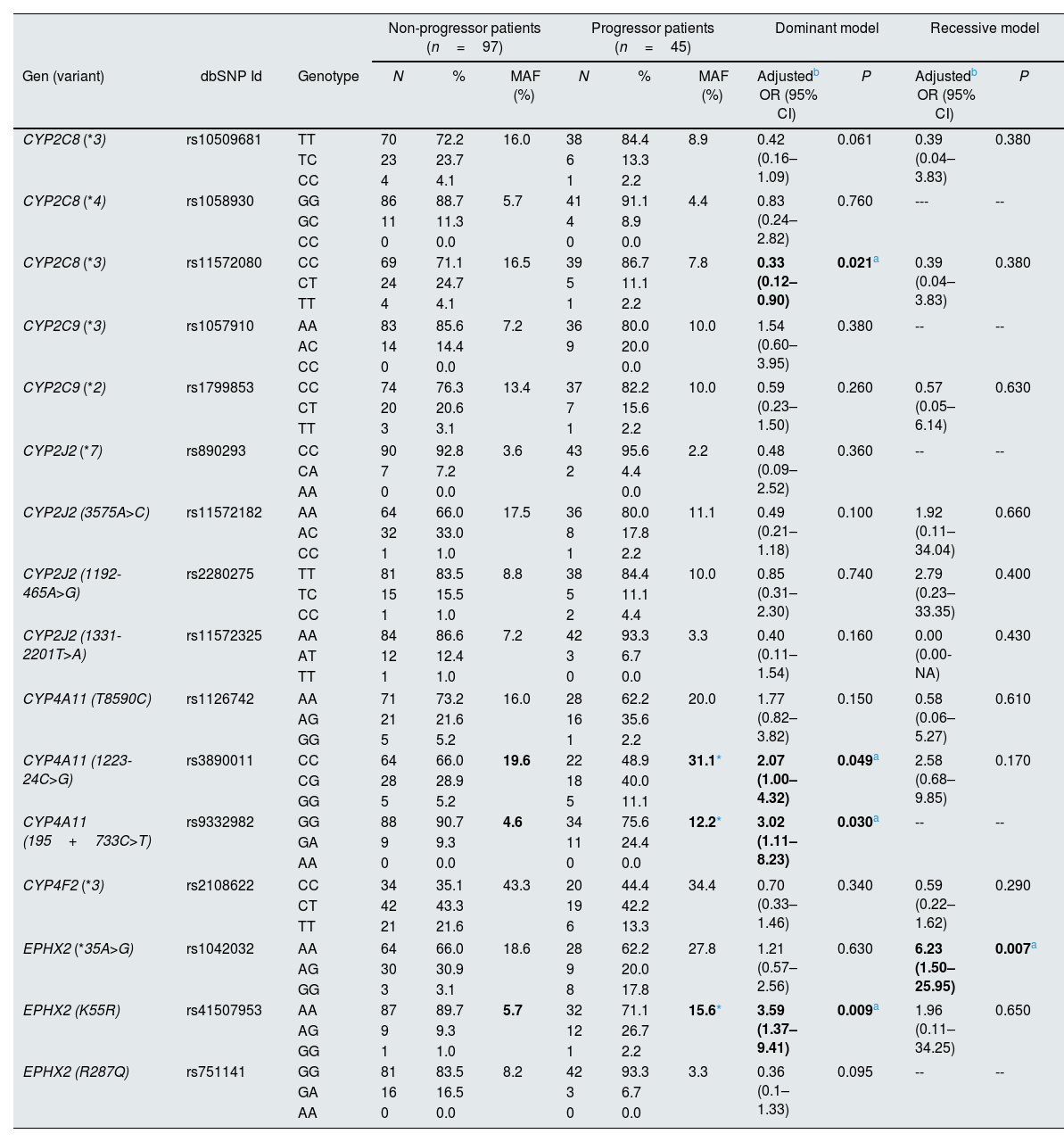
Association between progression of impaired kidney function and CYPs and EPHX2 polymorphismsTo avoid a confounding factor on progression of decline renal function, patients in stage G5 (n=9), at the beginning of the study, were not included for this analysis. Regarding the allelic frequencies, the frequencies of the SNPs of CYP4A11 rs3890011 and rs9332982, as well as EPHX2 rs41507953 were significantly higher in the progressors (n=45) than in the non-progressor (n=97) group (P=0.036, P=0.026 and P=0.012, respectively; Fig. 4).
Using logistic regression, the ORs with 95% interval confidence (95% CI) for the different CYPs and EPHX2 genetic polymorphisms on the progression of impaired kidney function in HT patients (n=142), adjusted by age, gender, DM and dyslipidemia are shown in Table 3 for the dominant and recessive models. It should be noted that the CYP4A11 rs3890011, rs9332982 and EPHX2 rs41507953 polymorphisms, according to the dominant model, presented a high risk of impaired kidney function, with ORs (95% CI; P) of 2.07 (1.00–4.32; P=0.049), 3.02 (1.11–8.23; P=0.030) and 3.59 (1.37–9.41; P=0.009), respectively, and the EPHX2 rs1042032 polymorphism a greater risk according to the recessive model (OR=6.23; 95% CI=1.50–25.95; P=0.007). Instead, the CYP2C8 rs11572080 polymorphism presented a lower risk of progression than non-carriers of the T allele (OR=0.33; 95% CI=0.12–0.90; P=0.021).
Assessment of the association of polymorphisms in the epoxygenase and hydroxylase pathways of arachidonic acid metabolism with the progression of impaired kidney function in hypertension patients (n=142).
| Non-progressor patients (n=97) | Progressor patients (n=45) | Dominant model | Recessive model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen (variant) | dbSNP Id | Genotype | N | % | MAF (%) | N | % | MAF (%) | Adjustedb OR (95% CI) | P | Adjustedb OR (95% CI) | P |
| CYP2C8 (*3) | rs10509681 | TT | 70 | 72.2 | 16.0 | 38 | 84.4 | 8.9 | 0.42 (0.16–1.09) | 0.061 | 0.39 (0.04–3.83) | 0.380 |
| TC | 23 | 23.7 | 6 | 13.3 | ||||||||
| CC | 4 | 4.1 | 1 | 2.2 | ||||||||
| CYP2C8 (*4) | rs1058930 | GG | 86 | 88.7 | 5.7 | 41 | 91.1 | 4.4 | 0.83 (0.24–2.82) | 0.760 | --- | -- |
| GC | 11 | 11.3 | 4 | 8.9 | ||||||||
| CC | 0 | 0.0 | 0 | 0.0 | ||||||||
| CYP2C8 (*3) | rs11572080 | CC | 69 | 71.1 | 16.5 | 39 | 86.7 | 7.8 | 0.33 (0.12–0.90) | 0.021a | 0.39 (0.04–3.83) | 0.380 |
| CT | 24 | 24.7 | 5 | 11.1 | ||||||||
| TT | 4 | 4.1 | 1 | 2.2 | ||||||||
| CYP2C9 (*3) | rs1057910 | AA | 83 | 85.6 | 7.2 | 36 | 80.0 | 10.0 | 1.54 (0.60–3.95) | 0.380 | -- | -- |
| AC | 14 | 14.4 | 9 | 20.0 | ||||||||
| CC | 0 | 0.0 | 0.0 | |||||||||
| CYP2C9 (*2) | rs1799853 | CC | 74 | 76.3 | 13.4 | 37 | 82.2 | 10.0 | 0.59 (0.23–1.50) | 0.260 | 0.57 (0.05–6.14) | 0.630 |
| CT | 20 | 20.6 | 7 | 15.6 | ||||||||
| TT | 3 | 3.1 | 1 | 2.2 | ||||||||
| CYP2J2 (*7) | rs890293 | CC | 90 | 92.8 | 3.6 | 43 | 95.6 | 2.2 | 0.48 (0.09–2.52) | 0.360 | -- | -- |
| CA | 7 | 7.2 | 2 | 4.4 | ||||||||
| AA | 0 | 0.0 | 0.0 | |||||||||
| CYP2J2 (3575A>C) | rs11572182 | AA | 64 | 66.0 | 17.5 | 36 | 80.0 | 11.1 | 0.49 (0.21–1.18) | 0.100 | 1.92 (0.11–34.04) | 0.660 |
| AC | 32 | 33.0 | 8 | 17.8 | ||||||||
| CC | 1 | 1.0 | 1 | 2.2 | ||||||||
| CYP2J2 (1192-465A>G) | rs2280275 | TT | 81 | 83.5 | 8.8 | 38 | 84.4 | 10.0 | 0.85 (0.31–2.30) | 0.740 | 2.79 (0.23–33.35) | 0.400 |
| TC | 15 | 15.5 | 5 | 11.1 | ||||||||
| CC | 1 | 1.0 | 2 | 4.4 | ||||||||
| CYP2J2 (1331-2201T>A) | rs11572325 | AA | 84 | 86.6 | 7.2 | 42 | 93.3 | 3.3 | 0.40 (0.11–1.54) | 0.160 | 0.00 (0.00-NA) | 0.430 |
| AT | 12 | 12.4 | 3 | 6.7 | ||||||||
| TT | 1 | 1.0 | 0 | 0.0 | ||||||||
| CYP4A11 (T8590C) | rs1126742 | AA | 71 | 73.2 | 16.0 | 28 | 62.2 | 20.0 | 1.77 (0.82–3.82) | 0.150 | 0.58 (0.06–5.27) | 0.610 |
| AG | 21 | 21.6 | 16 | 35.6 | ||||||||
| GG | 5 | 5.2 | 1 | 2.2 | ||||||||
| CYP4A11 (1223-24C>G) | rs3890011 | CC | 64 | 66.0 | 19.6 | 22 | 48.9 | 31.1* | 2.07 (1.00–4.32) | 0.049a | 2.58 (0.68–9.85) | 0.170 |
| CG | 28 | 28.9 | 18 | 40.0 | ||||||||
| GG | 5 | 5.2 | 5 | 11.1 | ||||||||
| CYP4A11 (195+733C>T) | rs9332982 | GG | 88 | 90.7 | 4.6 | 34 | 75.6 | 12.2* | 3.02 (1.11–8.23) | 0.030a | -- | -- |
| GA | 9 | 9.3 | 11 | 24.4 | ||||||||
| AA | 0 | 0.0 | 0 | 0.0 | ||||||||
| CYP4F2 (*3) | rs2108622 | CC | 34 | 35.1 | 43.3 | 20 | 44.4 | 34.4 | 0.70 (0.33–1.46) | 0.340 | 0.59 (0.22–1.62) | 0.290 |
| CT | 42 | 43.3 | 19 | 42.2 | ||||||||
| TT | 21 | 21.6 | 6 | 13.3 | ||||||||
| EPHX2 (*35A>G) | rs1042032 | AA | 64 | 66.0 | 18.6 | 28 | 62.2 | 27.8 | 1.21 (0.57–2.56) | 0.630 | 6.23 (1.50–25.95) | 0.007a |
| AG | 30 | 30.9 | 9 | 20.0 | ||||||||
| GG | 3 | 3.1 | 8 | 17.8 | ||||||||
| EPHX2 (K55R) | rs41507953 | AA | 87 | 89.7 | 5.7 | 32 | 71.1 | 15.6* | 3.59 (1.37–9.41) | 0.009a | 1.96 (0.11–34.25) | 0.650 |
| AG | 9 | 9.3 | 12 | 26.7 | ||||||||
| GG | 1 | 1.0 | 1 | 2.2 | ||||||||
| EPHX2 (R287Q) | rs751141 | GG | 81 | 83.5 | 8.2 | 42 | 93.3 | 3.3 | 0.36 (0.1–1.33) | 0.095 | -- | -- |
| GA | 16 | 16.5 | 3 | 6.7 | ||||||||
| AA | 0 | 0.0 | 0 | 0.0 | ||||||||
MAF: minor allele frequency.
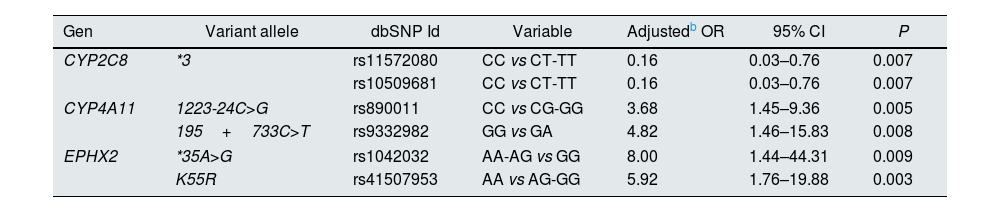
Subsequently, the same analysis was also performed just in patients diagnosed with CKD (stages 3a, 3b and 4) at the beginning of the study (n=96). It was observed that adjusted ORs (age, gender, DM and dyslipidemia) were significant for the same polymorphisms as in the group of 142 HT patients but with higher ORs for all polymorphisms (Table 4).
Assessment of the association of polymorphisms in the epoxygenase and hydroxylase pathways of arachidonic acid metabolism with the progression of impaired kidney function in CKD hypertension patients (n=96).a
| Gen | Variant allele | dbSNP Id | Variable | Adjustedb OR | 95% CI | P |
|---|---|---|---|---|---|---|
| CYP2C8 | *3 | rs11572080 | CC vs CT-TT | 0.16 | 0.03–0.76 | 0.007 |
| rs10509681 | CC vs CT-TT | 0.16 | 0.03–0.76 | 0.007 | ||
| CYP4A11 | 1223-24C>G | rs890011 | CC vs CG-GG | 3.68 | 1.45–9.36 | 0.005 |
| 195+733C>T | rs9332982 | GG vs GA | 4.82 | 1.46–15.83 | 0.008 | |
| EPHX2 | *35A>G | rs1042032 | AA-AG vs GG | 8.00 | 1.44–44.31 | 0.009 |
| K55R | rs41507953 | AA vs AG-GG | 5.92 | 1.76–19.88 | 0.003 | |
The patients with diagnosis of dyslipidemia (n=90) presented higher frequencies of EPHX2 K55R (rs41507953) and *35A>G (rs1042032) variants than patients without dyslipidemia, 4% vs. 14% (P=0.005) and 16 vs. 27% (P=0.02), respectively. The ORs (95% CI; adjusted for age and gender) calculated for the presence of dyslipidemia were 4.08 (1.43–11.68; P=0.004) and 8.20 (1.01–66.37; P=0.04) to genotype EPHX2 (K55R) A/G-GG and EPHX2 *35 G/G carriers, respectively, while the OR (95% CI, adjusted for age and gender) for the EPHX2 (K55R) G – EPHX2 *35 G haplotype was 3.95 (1.40–11.10; P=0.01).
In contrast, none of the genetic variables analyzed was associated with CV events after two years of follow-up to HT patients that presented at least one CV event (n=40).
DiscussionSince HT is one of the main risk factors for CKD, all participants in the study were HT patients, who were users of a hospital nephrology service. Up to our knowledge, this is the first study in humans in which the relationship between the progression of impaired kidney function and different genetic polymorphisms involved in the metabolism of AA, such CYP2 and CYP4 families, as well as EPHX2 gene, has been evaluated. Different previous studies have been published including some AA-derived eicosanoids, such as 20-HETE related to increased progression of kidney injury or CKD,28,29 though these studies did not evaluate the polymorphisms of the genes encoding for the enzymes that synthesize these AA-derived eicosanoids.
Hypertension and CYPs and EPHX2 polymorphismsIn the present study, it was initially evaluated the relationship between genetic polymorphisms of P450-derived eicosanoid genes and arterial HT, comparing a group of HT patients with a group of NT subjects. According to the results, not-significant differences were observed in the presence of some polymorphisms (Fig. 2). Furthermore, the frequencies found in both subgroups (HT patients and NT subjects) did not show significant differences with a Spanish control population (Fig. 2). These results do not agree with previous studies where the presence of the rs1126742 and rs9332982 polymorphisms of the CYP4A11 gene were associated with the development of essential HT in men30,31 and women,32 respectively, as well as with the Val433Leu variant (rs2108622) of the CYP4F2 gene in women.33 However, there are also studies in which no evidence was found of association between the risk of HT and the presence of these CYP4A1133,34 or CYP4F235 polymorphisms. Furthermore, the lack of association of CYP2C9, 2C8, 2J2 and EPHX2 polymorphisms with HT is consistent with that observed in previous studies.21,36–39 Therefore, the potential influence of these polymorphisms with HT is not clearly established. It may be partly due to the different groups of HT patients studied, differences in medication, concomitant diseases, lifestyle, etc. Furthermore, 20-HETE is excreted as a glucuronide conjugate and not in the free form and, in this sense, a study reported that the levels of 20-HETE glucuronide in the plasma of patients are higher than that found in the urine and that the fractional excretion is less than 1%.28 Therefore, urinary 20-HETE is not of renal origin and could simply reflects circulating levels of 20-HETE filtered and excreted.2
CKD progression and CYPs and EPHX2 polymorphismsOne of the most remarkable results of the study has been that CYP4A11 (rs3890011 and rs9332982) and EPHX2 (rs41507953 and rs1042032) polymorphisms were associated with increased progression of CKD. This fact could be explained by the hypothetical low level of EETs, which are associated with renoprotection and oppose the progression of CKD,14,17,20 since the polymorphism rs41507953 of EPHX2 codes for an enzyme with increased activity, so patients carrying this variant transform, at the renal level, more EETs towards their inactive derivatives DHETEs.
As for the other variant of EPHX2 gene associated with the progression of renal disease (rs1042032) is found in LD with the previous one (D′=1, Fig. 3), although its functional repercussion is unknown.
Regarding CYP4A11 variants associated with the progression of renal disease in this study (rs3890011, rs9332982), theirs functional repercussion in vivo (whether they increase or decrease the enzyme activity) is unknown. It is known that these polymorphisms have been associated in some studies with the risk of HT.30–32 However, at least one of them, the variant rs3890011, is in a high LD with the rs1126742 polymorphism (D′=1, r=0.80; P<0.0001; Fig. 3) which is associated with a loss of function in vivo,34 so indeed, 20-HETE levels will be low in progressor patients’ group. These results would contradict those found in a previous study where high levels of 20-HETE were related to a higher ORs of CKD progression.29 At could be possibly explained by the fact that 20-HETE concentrations were analyzed in plasma and not in urine in this study,29 which may be important as in a previous study they showed that 20-HETE conjugate levels in plasma are higher than those in urine.28 Furthermore, there are several studies supporting the hypothesis that low levels of 20-HETE at kidney might play an important role in the development of HT and renal injury, as well as in patients with inactive CYP4A11 mutations.17 This is supported by previous studies in which the formation of 20-HETE and EETs with fibrates was observed to reduce proteinuria and renal damage,40 as well as it has been reported that the 20-HETE excretion correlates with a decrease of eGFR in African American patients.28 Consequently, the results of the present study are consistent with the hypothesis that both EETs and 20-HETE oppose the development of CKD and the agents that increase intrarenal levels of EETs and 20-HETE, may protect against renal injury.17 This may be explained because reduced levels of 20-HETE and renal EETs in Dahl S rats promote sodium retention and the development of salt-sensitive HT, together with the decreased 20-HETE level also affects myogenic and tubuloglomerular feedback responses of the afferent arteriole, elevates glomerular capillary pressure, increases glomerular permeability to albumin, and up-regulates TGF-b expression, leading to the development of proteinuria and CKD.20,41,42
It should also be noted that, of the enzymes involved in the formation of CYPs-derived eicosanoids, the most expressed in the kidney are precisely these two enzymes, CYP4A11 and sEH. According to data provided by the open access resource for human proteins “The Human Protein Atlas”,43 the RNA expression in kidney of CYP4A11 and EPHX2 is 218.8 and 166.3nTPM (normalized transcript expression values), respectively. While the RNA expressions in kidney of CYP4F2, CYP2J2, CYP2C8 and CYP2C9 are much lower than the previous ones, being 40.9, 3.0, 4.3 and 1.4nTPM, respectively.
In contrast, the protective effect of progression of CYP2C8 mutation carriers is difficult to explain according to previous evidence, since it should be the progressor group who should present this polymorphism with a higher frequency than non-progressors since this polymorphism results in an enzyme with a decreased activity, which would cause low EET levels, which are related to renoprotection. However, it is an enzyme whose expression in the kidney is very low, practically insignificant (1.4nTPM), so its influence on renal function should not be as relevant as CYP4A11 or EPHX2 polymorphisms.
Dyslipidemia or cardiovascular events and CYPs and EPHX2 polymorphismsFinally, the association between rs41507953 and rs1042032 variants of EPHX2 gene and dyslipidemia was observed. A higher frequency of these variants was observed in patients with dyslipidemia compared to patients without dyslipidemia. Previous studies in animals suggest a role for the sEH enzyme and its gene EPHX2 in lipid regulation in response to diet. A study in mice fed with high-carbohydrate and high-fat (HCHF) diets, causing increased body weight, abdominal fat, and plasma lipid abnormalities, showed sEH enzyme activity in the liver 18% higher than in mice fed a standard diet.44 However, when rats fed a HCHF diet were administered an sEH inhibitor, the metabolic abnormalities were attenuated, suggesting a direct role of sEH in the aetiology of dyslipidemia in response to diet. On the other hand, total sEH activity was found to be higher in adipose tissue of rats fed a HCHF diet.45 In this sense, it has been well documented that a substantial proportion of malnourished individuals with anorexia nervosa have high serum cholesterol46 and that some variants in the EPHX2 gene were associated with total cholesterol levels in patients with anorexia nervosa.47
Regarding the relationship between the genetic variants analyzed in this study and CV events in HT patients, it was not observed in this study, similar to previous studies.36,37,39,48
Limitations, strengths, and further studiesDespite the limitations of the present study such as the number of participants, the lack of analysis of eicosanoid levels in both urine and/or plasma, as well as the potential influence of drug substrates of these enzymes on the outcome, the present results are the first to relate different polymorphisms of genes involved in eicosanoids metabolism to the progression of CKD in a group of patients over time (24 months).
Thus, further studies should be carried out focused on CYP4A11 and EPHX2 genes in a larger number of patients, analyzing levels of cytochrome P450-derived eicosanoids, primarily EETs and HETEs, in both urine and plasma and follow up patients over several years and taking into account treatments with drugs that are substrates or inhibitors of these cytochromes, such as antidiabetic, antihypertensive or NSAIDs drugs, which are widely used in this group of patients49 which can interfere with the formation of eicosanoids, especially EETs.
Moreover, and according to the results with the EPHX2 gene, the potential role of n−3 fatty acids, which could act as competitive substrates of CYPs and modify the synthesis of EETs and HETEs, should also be considered and monitored.
ConclusionsThis is the first study in humans in which the relationship between the progression of impaired kidney function and different genetic polymorphisms involved in formation of eicosanoids, such CYP2 and CYP4 gene families, as well as EPHX2, has been evaluated. In this study has been found higher odds of CKD progression associated with CYP4A11 (rs3890011 and rs9332982) and EPHX2 (rs41507953 and rs1042032) polymorphisms, which may serve as biomarkers for improve clinical interventions aimed at avoiding or delaying, in CKD patients, progress to end-stage kidney disease needing dialysis or kidney transplant.
FundingThis work was supported by Junta de Extremadura and European Regional Development Fund (ERDF) grant (IB16138; V Plan Regional de I+D+i).
Conflict of interestThe authors declare no conflicts of interest. The results presented in this paper have not been published previously in whole or part.
The authors thank all patients who kindly participated in the study, as well as Francesco G. Casino and Carlo Basile for their support and help with the review of manuscript and, the technical and human support provided by the Facility of Bioscience Applied Techniques of SAIUEx (financed by UEX, Junta de Extremadura, MICINN, FEDER and FSE).