Recently it has been published in this journal a consensus document about Chronic Kidney Disease (CKD) management including prevention and treatment strategies of CKD itself and associated comorbidities.1
In a previous publication2 we reported the existence of a statistically significant correlation between event rates in the placebo (PBO) groups and cardiovascular death (CVD) relative risk reduction in SGLT2i treated groups in the EMPA-REG-OUTCOME, CANVAS and DECLARE-TIMI-58 trials, when subpopulations with and without previous atherosclerotic events were taken separately. Also, in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF),3 the outcome All-Cause Mortality (ACM) was assigned to 276 (11.6%) subjects in the Dapagliflozin-treated group, while it was assigned to 329 (13.9%) subjects in the PBO group, namely an absolute Risk Reduction (ARR) of 2.3% (Table 1).
Events-rate per 1000 person-years in PBO groups, ACM in dapagliflozin-treated groups and ARR in ACM.
| DAPA-HF | McMurrayCKD without CV disease | McMurrayCKD with CV disease | McMurrayCKD without HF | McMurrayCKD with HF | WiviottT2D with MCVRF | WiviottT2D with ACVD | |
|---|---|---|---|---|---|---|---|
| Events-Rate in PBO groups | 13.9 | 3.9 | 11.7 | 5.5 | 17.2 | 2.3 | 3.4 |
| ACM in DAPA groups (%) | 11.6 | 2.5 | 8.4 | 4.0 | 10.2 | 2.2 | 2.9 |
| ARR in ACM (%) | 2.3 | 1.4 | 3.3 | 1.5 | 7.0 | 0.1 | 0.5 |
PBO: placebo; ACM: all-cause mortality; DAPA: dapagliflozin; CKD: chronic kidney disease; HF: heart failure; T2D: type 2 diabetes; MCVRF: multiple cardiovascular risk factors; ACVD: atherosclerotic cardiovascular disease; ARR: absolute risk reduction; ACM: all-cause mortality.
Thus, since significant cardiovascular (CV) and renal protection in patients treated with Dapagliflozin has been observed in these large clinical trials, we decided to find out if there is a similar correlation to that reported in our previous letter, but now in a broad spectrum of patients in different clinical situations and affected at baseline by different degrees of risk, expressed by the number of deaths per 1000 Patients-year in the PBO groups. For this purpose, we selected several population groups in which dapagliflozin was widely tested, such as patients with type 2 diabetes (T2D), CV disease, heart failure (HF) and CKD.
In a study performed by McMurray et al.4 in Patients with CKD without and with CV disease, within the population without CV disease at baseline 33 (2.5%) and 53 (3.9%) deaths occurred in the Dapagliflozin-treated and PBO groups respectively (ARR 1.4%); moreover, within the population with CV disease at baseline 68 (8.4%) and 93 (11.7%) deaths occurred in the Dapagliflozin-treated and PBO groups respectively (ARR 3.3%) (Table 1).
Also, in other study performed by McMurray et al.5 in Patients with CKD without and with HF, within the population without HF at baseline 77 (4.0%) and 106 (5.5%) deaths occurred in the Dapagliflozin-treated and PBO groups respectively (ARR 1.5%). On the other hand, in the population with HF 24 deaths (10.2%) occurred in the Dapagliflozin group and 40 deaths (17.2%) occurred in the PBO group; (ARR 7.0%) (Table 1).
In a study performed by Wiviott et al.6 in patients with T2D, within the population with multiple CV risk factors there were 111 (2.2%) and 118 (2.3%) deaths in the Dapagliflozin-treated and PBO groups respectively (ARR 0.1%); furthermore, within the population with atherosclerotic CV disease at baseline 100 (2.9%) and 120 (3.4%) deaths occurred in the Dapagliflozin-treated and PBO groups respectively (ARR 0.5%) (Table 1).
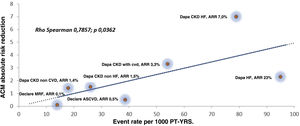
Using a similar statistical assessment to that used in our previous letter, we analyzed data published in the aforementioned studies and we found a significant correlation between event rates in the PBO groups and absolute risk reduction of ACM in the Dapagliflozin-treated groups; Rho Spearman 0.7857; p 0.0362 (Fig. 1).
We think that our finding is of special interest because it strongly suggests that Dapagliflozin treatment leads to a reduction in ACM in a broad spectrum of patients in different clinical situations affected by different degrees of CV and renal risk, from those with a low probability of having a fatal event, to those with high risk of death due to the presence of cardiac and/or kidney advanced disease, both in T2D and non-T2D patients. In conclusion, we believe that it is reasonable to affirm that the protection provided by dapagliflozin is more pronounced if the intrinsic risk of the patient is higher, and since this benefit begins in early stages of CV disease, the early treatment with Dapagliflozin may be an adequate option to take in account in the clinical setting.
Conflict of interestAntonio Gippini has received fees for presentations and advisory boards from Amgen, AstraZeneca, Boehringer-Ingelheim, Esteve, Ferrer, Janssen, Lilly, Mundipharma, Mylan, Novartis and NovoNordisk.
Alberto Prado works in Cardiovascular Renal and Metabolism (CVRM) Medical Department of AstraZeneca, Spain.