Over the past decade, obesity has become a risk factor for developing chronic kidney disease. Proteinuria is known to be an independent determinant of the progression of chronic kidney disease, and adipose tissue is a recognized source of components of the renin–angiotensin–aldosterone system (RAAS). Recent studies have shown that plasma aldosterone levels are disproportionately higher in patients with obesity. Drugs that block the RAAS are unable to inhibit aldosterone in the long term. The aim of our study was to analyze the renoprotective effect of an aldosterone antagonist in combination with RAAS blockers in patients with obesity and proteinuric nephropathy.
Material and methodsThis study is a substudy of previously published study on the renoprotective effect of mineralocorticoid receptor blockers in patients with proteinuric nephropathies. Patients with proteinuria levels >1g/24h who were taking spironolactone and were being treated with other RAAS blockers were divided according to body mass index (BMI) into an obesity group (BMI ≥30kg/m2) and a control group.
ResultsSeventy-one patients were included in the study, with a mean age of 56.7±15.1 years. More than 50% of the patients in both groups had diabetes. Thirty-two patients were included in the obesity group and 39 were included in the control group. There were no significant differences in renal function, proteinuria, blood pressure, serum potassium levels and the percentage of RAAS blockers in both groups. After a follow-up of 28.9 (14–84) months, there was a 59.4% reduction in proteinuria in the obesity group (2.8±2.1 vs. 1.3±1.6g/24h, p<.05). The reduction in proteinuria was greater than 50% in 22 (68.8%) cases, and the mean blood pressure showed a significant decrease (from 100.6±9 to 92.1±7.4mm Hg, p<.05). The control group showed a 69.6% reduction in proteinuria (1.9±1.4 to 0.8±0.5, p<0.05). The reduction of proteinuria was higher than 50% in 22 (68.8%) cases in obese patients and in 33 (84.6%) cases in non-obese group. Renal function remained stable in both groups during the follow-up. Nine patients (28.1%) in the obesity group experienced gynecomastia. The incidence of hyperkalemia was similar for the 2 groups (6.3%).
ConclusionAldosterone antagonist treatment in obese patients with proteinuric nephropathies induces a drastic and sustained reduction in proteinuria but not more than the non-obese group. There was a trend toward slowing progression of renal failure with few adverse events.
Durante la última década, la obesidad se ha convertido en un factor de riesgo para el desarrollo de la enfermedad renal crónica. La proteinuria está considerada un factor independiente de la progresión de la enfermedad renal crónica y el tejido adiposo se reconoce como una fuente de los componentes del sistema renina-angiotensina-aldosterona (SRAA). Estudios recientes han demostrado que los niveles de aldosterona plasmática son desproporcionadamente mayores en pacientes con obesidad. Los fármacos que bloquean el SRAA son incapaces de inhibir la aldosterona a largo plazo. El objetivo de nuestro estudio fue analizar el efecto protector a nivel renal de un antagonista de la aldosterona en combinación con bloqueadores del SRAA en pacientes con obesidad y nefropatía con proteinuria.
Material y métodosEste estudio es un subestudio del estudio publicado previamente sobre el efecto protector a nivel renal de los bloqueadores del receptor de mineralocorticoides en pacientes con nefropatías con proteinuria. Se dividió a los pacientes con niveles de proteinuria >1 g/24 h que estaban tomando espironolactona y se los trataba con otros bloqueadores del SRAA según el índice de masa corporal (IMC) en un grupo de obesidad (IMC ≥30 kg/m2) y un grupo de control.
ResultadosSe incluyó a 71 pacientes en el estudio, con una media de edad de 56,7±15,1 años. Más del 50% de los pacientes en ambos grupos tenía diabetes. Se incluyó a 32 pacientes en el grupo de obesidad y a 39 en el grupo de control. No hubo diferencias significativas en la función renal, proteinuria, presión arterial, niveles de potasio sérico y el porcentaje de bloqueadores del SRAA en ambos grupos. Tras un seguimiento de 28,9 meses (14-84), hubo una reducción del 59,4% de la proteinuria en el grupo de obesidad (2,8±2,1 frente a 1,3±1,6 g/24 h, p<0,05). La reducción de la proteinuria fue superior al 50% en 22 casos (68,8%) y la presión arterial media experimentó una disminución significativa (de 100,6±9 a 92,1±7,4 mm Hg, p<0,05). El grupo de control experimentó una reducción del 69,6% de la proteinuria (de 1,9±1,4 a 0,8±0,5, p<0,05). La reducción de la proteinuria fue superior al 50% en 22 casos (68,8%) en pacientes obesos y en 33 casos (84,6%) en el grupo de no obesos. La función renal de ambos grupos permaneció estable durante el seguimiento. En 9 pacientes (28,1%) del grupo de obesidad se observó ginecomastia. La incidencia de hiperpotasemia fue similar en los 2 grupos (6,3%).
ConclusiónEl tratamiento con un antagonista de la aldosterona en pacientes obesos con nefropatías con proteinuria induce una reducción drástica y sostenida de la proteinuria, pero no superior a la del grupo de no obesos. La tendencia fue frenar la progresión de la insuficiencia renal con pocos eventos adversos.
Obesity is a known cause of proteinuria and progressive renal damage.1–3 Recent studies have shown that glomerulopathy associated with obesity is an increasingly diagnosed condition and has a greater incidence.3,4 Furthermore, obesity has been shown to participate in the progression of various kidney diseases.5–7 Higher levels of proteinuria are a significant risk factor in the progression of kidney disease in patients with diabetic and nondiabetic nephropathy. Any therapeutic measure that reduces proteinuria will have a positive renoprotective effect on the long-term outcome of renal function.8,9 The most effective antiproteinuric measures among these therapies are a renin–angiotensin–aldosterone system (RAAS) blockade in its various modalities, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor antagonists (ARAs) and, more recently, aldosterone antagonists (AAs).10,11 Considering the above, as well as the role of obesity in the progression of kidney diseases and the epidemic proportions that obesity has reached in developed societies, the response of patients with obesity and proteinuric nephropathy to the various strategies that block RAAS is a topic of extraordinary clinical importance, considering that RAAS activity is greater in obesity.12,13 Although the initials data on the antiproteinuric response of ACEIs or ARAs in obese patients were contradictory, recently the post hoc analysis of the Ramipril Efficacy in Nephropathy (REIN) trial indicated that the risk reduction for renal disease progression to end-stage-renal-diseases (ESRD) and the antiproteinuric effect by ramipril was more pronounced in obese population.14 Current studies suggest that patients with obesity have increased aldosterone synthesis, which could play an important role in the various complications associated with obesity, including renal damage.15,16 Experimental studies conducted with obese animals have shown that the use of AAs drastically reduces renal lesion progression.17,18 This research suggests that patients with obesity and proteinuric nephropathy could have a more favorable antiproteinuric response to AAs than to traditional RAAS blockage with ACEIs or ARAs. However, there is little information in the literature on the role of AAs in the proteinuria of patients with obesity.19,20
There is little information regarding the antiproteinuric effect of AA alone or combined with RAAS blockade long term obese patients with proteinuric nephropathies. In the scientific community there is growing concern about the new epidemic of the XXI century, the obesity. The aim of this study was analyze how obese patients responded to treatment with AA compared with non-obese patients.
In 2004, a clinical protocol was started based on the addition of spironolactone to patients with proteinuric nephropathy who maintained proteinuria levels >1g/day, despite treatment with ACEIs or ARAs.21 This study is a substudy that analyzes (a) the antiproteinuric effect over time of AAs on patients with obesity and (b) whether treatment with spironolactone slows the progression of renal failure in this patient group.
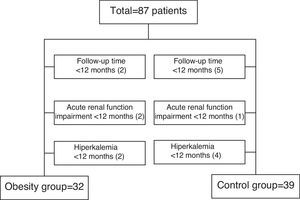
Material and methodsPatientsIn January 2004, we began a prospective cohort study based on the addition of spironolactone to patients who had persistent proteinuria levels >1g/24h, despite the maximum tolerated dosages of ACEIs, ARAs or their combination for more than 6 months regardless of the etiology of renal disease. There were no restrictions based on age or renal function. We excluded patients with the same criteria as in the previous study.21 This substudy excluded those patients with follow-up less than 12 months for various reasons. In the obese group were excluded 6 patients, 2 for follow-up less than 3 months, 2 patients had developed a deterioration of renal function in the first month after AA treatment and 2 patients for hyperkalemia uncontrolled in the first month after AA treatment. In the control group 10 patients were excluded, 5 for follow-up less than 3 months, 1 patient had developed an acute deterioration of renal function and 4 patients developed hyperkalemia after AA treatment (Fig. 1).
Seventy-one patients were included in this protocol. We established 2 patient groups according to body mass index (BMI): an obesity group for patients with a BMI≥30kg/m2 and a control group for patients with a BMI<30kg/m2. The study was approved by the Hospital's Ethics Committee.
Therapeutic interventionSpironolactone was added at a dosage of 25mg/day to the baseline therapy of all patients. During the follow-up, the spironolactone dosage was adjusted according to measurements of blood pressure (BP) or serum potassium levels. For a number of the patients who had an insufficient antiproteinuric response (<30% of baseline values) but had good tolerance, the dosage was increased to 50mg/day. For the patients who experienced adverse effects other than hyperpotassemia (mainly gynecomastia), spironolactone was replaced with eplerenone at a dosage of 25mg/day. The baseline dosages of ACEIs, ARAs or both were not modified at the start of the study. The dosages were subsequently modified for a number of patients based on measurements of BP and serum potassium levels. For a number of the patients with antiproteinuric responses >30% of baseline values and a difficult management of serum potassium levels, the ACEI and/or ARA-2 dosages were progressively lowered or, in a number of cases, had to be discontinued. For proper control of serum potassium levels, we recommended the same measures as in the previous study.21 The BP objective was established at levels below 130/80mm Hg.
Follow-up and data collectionAll patients were treated in outpatient clinics after 1 month of treatment with spironolactone. These data were collected as in the previous study.21 The mean follow-up was 28.9±14 (14–84) months.
Study objectivesThe primary study objective was to compare the reduction in proteinuria in the obesity group at the end of the follow-up compared with the control group. We analyzed the number of patients who achieved a >50% reduction in proteinuria from baseline values during their follow-up. The secondary objectives included comparing the change in the glomerular filtration rate (GFR) during the 12-month period prior to spironolactone treatment compared with the period between baseline and the end of the follow-up and the period between the first month following treatment and the end of follow-up. The change in GFR was measured in mL/min/year. The response to AA treatment was analyzed separately for the obesity and control groups. We also analyzed the tolerance to spironolactone and its adverse effects.
DefinitionsThe follow-up period was calculated with the same criteria as in the previous study.21 Renal function was measured by GFR using the simplified 4-variable Modification of Diet in Renal Disease formula (MDRD-4). BMI was measured as weight in kilograms divided by the height squared in meters. Mean arterial pressure (MAP) was calculated as the sum of the diastolic blood pressure and one third of the pulse pressure. To calculate the improvement in the loss of GFR, we established a cutoff for the mean value of the GFR slope in the first 12 months prior to treatment (−3mL/min/year), considering that the patients who managed to reduce this loss of GFR from the start of treatment to the end of follow-up were categorized as patients who achieved an improvement in renal function.
Statistical analysisThe data are expressed as mean±standard deviation (SD) or median and interquartile range for continuous normal and non-normal variables, respectively. The continuous variables with normal distribution are expressed as mean and SD, while the noncontinuous variables are expressed as medians, 25 and 75 percentiles and interquartile range. Categorical variables are expressed as frequencies and percentages. The Spearman correlation test, paired t tests and Wilcoxon test were employed for the analysis of continuous variables when indicated. Differences between the qualitative variables were compared using the chi-squared test. For all tests, values of p<.05 were considered statistically significant. The data were assessed with the SPSS program, version 15.0 for Windows (SPSS, Chicago, IL, USA).
ResultsPatient characteristicsSeventy-one patients were included in the protocol with spironolactone. Their demographic, clinical and laboratory test characteristics at the start of the study are shown in Table 1. The number of patients who were treated with antihypertensive drugs other than RAAS blockers at the start and end of the follow-up was as follows: calcium-antagonists, 8 (11%) and 6 (8%), respectively; beta-blockers, 6 (8%) and 6 (8%); and alpha blockers, 5 (7%) and 4 (6%). Before starting treatment with an AA, 15 (21%) patients were treated with an ACEI, 37 (52%) were treated with an ARA and 19 (27%) were treated with a combination of an ACEI and ARA. Forty-five patients were treated with ARA+AA, 20 patients were treated with ACEI+AA and 4 were treated with ACEI+ARA+AA at the end of the follow-up, while 2 patients were treated with an AA alone. In Table 1, we can observe the main differences between the 2 study groups.
Demographic, clinical and analytical characteristics at baseline of the overall patient group treated with AA (n=71), the obesity group (N=32) and control group (N=39).
| Variable | Total group (n=71) | Patients with obesity (n=32) | Patients with no obesity (n=39) | P value |
|---|---|---|---|---|
| Age, years | 56.7±15.1 (22–79) | 61.8±11.3 (34–79) | 52.5±16.6 (22–79) | 0.01 |
| Sex, M/F | 46/25 | 21/11 | 25/14 | 0.89 |
| SCr, mg/dL | 1.4±0.6 (0.4–3.4) | 1.4±0.5 (0.5–2.6) | 1.3±0.7 (0.4–3.4) | 0.64 |
| eGFR, ml/min/1.73m2 | 64.3±36.3 (19.2–212) | 56.9±27 (24.9–138.2) | 70.5±41.8 (19.1–212) | 0.11 |
| Proteinuria, g/24h | 3±2.1 (1–10.4) | 2.8±2.1 (1–10.3) | 3.2±2.1 (1–10.4) | 0.41 |
| MAP, mm Hg | 99.6±10.9 (68.3–125.3) | 100.6±9 (88.3–120) | 98.8±12.2 (68.3–125.3) | 0.49 |
| Serum potassium, mEq/L | 4.6±0.5 (3.5–5.7) | 4.7±0.3 (3.7–5.3) | 4.5±0.5 (3.8–5.7) | 0.18 |
| BMI, kg/m2 | 29.4±4.5 (21.6–42.5) | 33.3±3.2 (30–42.4) | 26.2±2.4 (21.6–29.9) | 0.00 |
| Treatment (%) | ||||
| • ACEI | 15 (21.1) | 8 (25) | 7 (17.9) | 0.46 |
| • ARA | 37 (52.1) | 15 (46.9) | 22 (56.4) | 0.42 |
| • ACEI+ARA | 19 (26.8) | 9 (28.1) | 10 (25.6) | 0.81 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARA, angiotensin-receptor antagonist; BMI, body mass index; eGFR, estimated glomerular filtration rate; F, female; M, male; MAP, mean arterial pressure; SCr, serum creatinine. Numbers in brackets correspond to ranges, except for the “Treatment” variable, where they correspond to percentages.
Note: To convert to SI units, for serum creatinine (μmol/L), multiply by 88.4; for glomerular filtration rate (mL/min/1.73m2, multiply by 0.0167; for potassium (mmol/L).
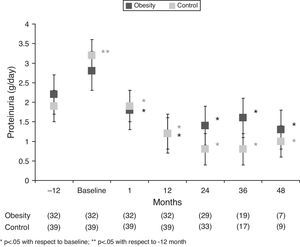
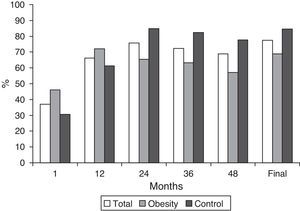
In the obesity group, we observed a significant reduction in proteinuria from the first month of treatment with spironolactone (2.8±2.1 to 1.8±1.8g/day, p<.05, which represents a 46.3% reduction [range 26.9–58.4] from baseline values). The mean reduction in proteinuria was maintained in 65% of the patients (range 48.7–82.8) at 12 months, with no tendency toward reduction during the follow-up (Tables 2 and 3 and Figs. 2 and 3). At the end of the follow-up, the proteinuria levels were 1.3±1.6g/day (p<.0001 compared with baseline proteinuria), which represents a 59% reduction (range 43–73%) when compared with baseline values. A shown in Fig. 3, most patients of obesity group achieved reductions of more than 50% in proteinuria from baseline values, and this reduction was maintained over the course of the follow-up (72% of patients at month 12, 65.5% at month 24, 63.2% at month 36, 57.1% at month 48 and 68.8% of at the end of follow-up). There was no correlation between the changes in GFR and the changes in proteinuria (r=0.27, p=.12) or between the changes in blood pressure and the reduction in proteinuria (r=0.24, p=.19). In the control group, the mean reduction in proteinuria was maintained in 63% of the patients (range 25.1–78.9) at 12 months, similar to the obesity group. This reduction was maintained throughout the follow-up and was even higher than the obesity group 69% (range 57–83) vs 59% (range 43–73%), p<0.05 at the end follow-up (Table 2).
Evolution of the main clinical and analytical factors before and after treatment with AA in the obesity group with ACEI (n=32) and the control group (n=39) at baseline.
| Variable | −12m | Baseline | +1m (n=32/39) | +12m (n=32/39) | +24m (n=29/33) | +36m (n=19/17) | +48m (n=7/9) | Last visit |
|---|---|---|---|---|---|---|---|---|
| SCr, mg/dL | ||||||||
| Obesity | 1.3±0.4 | 1.4±0.5** | 1.5±0.4 | 1.5±0.4* | 1.5±0.5* | 1.5±0.6* | 1.4±0.6 | 1.5±0.5* |
| Control | 1.3±0.6 | 1.3±0.6 | 1.5±0.7* | 1.6±0.8* | 1.5±0.7* | 1.4±0.7* | 1.4±0.7 | 1.5±0.7* |
| eGFR, mL/min/1.73m2 | ||||||||
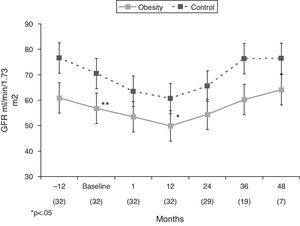
| Obesity | 60.9±28.3 | 56.8±27** | 53.5±26.9 | 49.9±20.8* | 54.4±32.1 | 60.3±36.9 | 64.1±38.5 | 54.1±30.9 |
| Control | 76.6±51.7 | 70.5±41.8 | 63.5±36.7* | 60.7±43.5* | 65.6±48* | 76.3±57.3 | 76.4±39.9 | 65.4±45.9* |
| Serum Potassium, mEq/L | ||||||||
| Obesity | 4.6±0.4 | 4.7±0.3 | 5±0.4* | 5±0.5* | 4.9±0.4* | 4.8±0.5 | 4.7±0.4 | 4.9±0.5* |
| Control | 4.6±0.5 | 4.5±0.4 | 4.9±0.6* | 4.9±0.5* | 4.8±0.5* | 4.7±0.5 | 4.7±0.5 | 4.8±0.5* |
| Proteinuria, g/24h | ||||||||
| Obesity | 2.2±2.5 | 2.8±2.1 | 1.8±1.8* | 1.2±1.7* | 1.4±1.7* | 1.6±2.1* | 1.3±1.1* | 1.3±1.6* |
| Control | 1.9±1.4 | 3.2±2.1** | 1.9±1.2* | 1.2±0.9* | 0.8±0.6* | 0.8±0.6* | 1±0.6* | 0.8±0.5* |
| Reduction in proteinuria from baseline, % | ||||||||
| Obesity | 46.3 (26.9–58.4) | 65 (48.7–82.8) | 54.7 (38.4–72.5) | 60.7 (33.5–75.2) | 50.8 (33.5–64) | 59.4 (43.2–73)+ | ||
| Control | 41.8 (23.1–51.1) | 63 (25.1–78.9) | 69.6 (56.2–87.1) | 69.7 (56.2–88.2) | 69.4 (49.3–91) | 69.6 (57.4–82.8) | ||
| BMI, kg/m2 | ||||||||
| Obesity | 33.3±3.4+ | 33.3±3.2+ | 32.6±2.8+ | 32.8±3.7+ | 32.9±3.2+ | 32.4±2.8+ | 33.6±3.9+ | 33.3±3.8+ |
| Control | 26.1±2.5 | 26.2±2.4 | 26.4±2.5 | 26.4±2.8 | 26.3±3.1 | 26.6±3.4 | 26.6±4.1 | 26.3±3 |
| MAP, mm Hg | ||||||||
| Obesity | 97±8.8 | 100.6±9** | 93.2±10.8* | 91.6±7.3* | 91.9±7.5* | 91.6±6.7* | 90.2±5.4 | 92.1±7.4* |
| Control | 98.9±13.3 | 98.8±12.2 | 95.4±10.4* | 96.5±11.7 | 92.3±11.3* | 91.5±13.4* | 88.2±12.7* | 92.2±10.7* |
Abbreviations: eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; SCr, serum creatinine.
Outcome of a number of characteristics in both groups.
| Variable | Patients with obesity (n=32) | Patients with no obesity (n=39) | P value |
|---|---|---|---|
| Patients with diabetes, n (%) | 16 (50) | 14 (46.7) | 0.23 |
| Reduction in proteinuria >30%, n (%) | 27 (84.4) | 37 (94.9) | 0.14 |
| Reduction in proteinuria >50%, n (%) | 22 (68.8) | 33 (84.6) | 0.11 |
| Renal function stabilization, n (%) | 20 (62.5) | 23 (59) | 0.76 |
| Gynecomastia, n (%) | 9 (28.1) | 2 (5.1) | 0.00 |
| Change in spironolactone, n (%) | 9 (28.1) | 3 (7.7) | 0.022 |
| Hyperpotassemia, n (%) | 2 (6.3) | 2 (5.1) | 0.84 |
| Treatment, n (%) | |||
| • ACEI+AA | 10 (31.3) | 10 (25.6) | 0.60 |
| • ARA+AA | 21 (65.6) | 24 (61.5) | 0.72 |
| • ACEI+ARA+AA | 0 (0) | 4 (10.3) | 0.06 |
| • AA | 1 (3.1) | 1 (2.6) | 0.88 |
Abbreviations: AA, aldosterone antagonists; ACEI, angiotensin-converting enzyme inhibitors; ARA, angiotensin-receptor antagonists.
As shown in Tables 2 and 3 and Fig. 4, renal function deteriorated significantly during the previous period 12 months before the start of treatment with spironolactone (−0.27±0.6mL/min/1.73m2/month). After the first month of treatment with spironolactone, renal function showed a significant drop (−3.2±8.8mL/min/1.73m2 with respect to baseline values). The estimated glomerular filtration rate (eGFR) showed an acute fall in the first month of treatment, but it remained stable thereafter (+0.01±0.35mL/min/1.73m(2)/month), with a tendency toward no significant difference with respect to the eGFR slope during the 12-month pre-treatment period. There was a non significant improvement in the slope of the GFR drop between the period prior to treatment with spironolactone (−3±7.1mL/min/year) and from the start of treatment to the end of the follow-up (−1.5±3.9mL/min/year, p=.37). Twenty patients (62.5%) showed improvement in renal function during the follow-up.
In the non-obesity group, there was non significant improvement between the slope of GFR drop between the period prior to treatment with spironolactone (−4.6±18.7ml/min/year) and from the start of treatment to the end of the follow-up (−2.3 observed±7.3ml/min/year, p .54). Twenty-three patients (59%) showed improvement in renal function during the follow-up.
Changes in blood pressure and serum potassium levelsBlood pressure levels was significantly reduced in both groups during the first month of treatment with spironolactone (Table 2) and remained stable during the follow-up. Serum potassium levels increased significantly after the first month of treatment but remained stable during the follow in both groups (Table 2). There was no significant influence between the changes in BP and GFR (r=0.27, p=.13).
Safety and tolerance of spironolactoneDuring the study, there were no deaths and no onset of advanced chronic renal failure or duplication of baseline serum creatinine levels. Nine patients (28%) developed gynecomastia shortly after starting treatment with spironolactone and were therefore switched to eplerenone (25mg/day). It is important to note that the incidence of gynecomastia in the control group was clearly lower (5%, p<.05) (Table 3). There were no differences in terms of renal function and proteinuria results between the patients who switched to eplerenone and those who remained with spironolactone. During the study, 2 patients (6%) discontinued the treatment with AA due to persistent hyperpotassemia (>5.5–6mEq/L), despite the adopted therapeutic measures. The number of patients who discontinued the treatment in the control group was similar (2 patients, 5%). Of the 2 patients with obesity who had to discontinue the treatment, both had diabetes and 1 had a GFR<60mL/min/1.73m2 at the start of the study. The discontinuation of AA occurred 14 and 19 months after the start of the treatment. The 2 cases that had to discontinue spironolactone were treated with ARA at the start of the study.
At the end of the follow-up, 22 patients were undergoing concomitant treatment for hyperpotassemia (12 patients with cation exchange resins and 10 patients with low doses of thiazide). The mean dosage of spironolactone and eplerenone at the end of the study was 27.7±19.6mg/24h (12.5–100) and 37.5±19.8mg/24h (12.5–75), respectively.
DiscussionOur study provides clinical information on the long-term outcome of reducing proteinuria and the changes in renal function in a cohort of patients with obesity and with various types of kidney diseases who were treated with spironolactone due to persistent proteinuria >1g/d, despite treatment with ACEIs, ARAs or their combination. As shown in Table 2 and Fig. 2, renal function showed a progressive reduction in the 12-month period prior to treatment with spironolactone. Although we observed an abrupt drop in GFR (−3.2±8.8mL/min/1.73m2) after the first month of treatment, which could lead us to reconsider discontinuing the use of the drug, renal function improved after the first month of treatment until the end of the follow-up. The comparison of GFR slopes during the pretreatment period (12 months prior) and from the first month of treatment to the end of the follow-up showed a tendency that did not achieve statistical significance. An important aspect of our study is the extended follow-up time, which enables us to confidently assert the beneficial effect spironolactone has on the reduction of proteinuria and the improvement and/or stabilization of renal function.
Experimental and clinical studies have shown that RAAS activity is increased in obesity and that adipose tissue, especially visceral, synthesizes all RAAS components.13,22 Moreover, patients with obesity have high plasma aldosterone levels,17,23 and recent studies have shown that visceral adipocytes can secrete various factors that increase aldosterone production by adrenal glands, through pathways other than the classical renin-angiotensin pathways.24 Oxidized fatty acids, typically found in high levels in patients with obesity, can also increase aldosterone synthesis.25 This collection of data could suggest a more favorable effect of AAs in patients with obesity, due to the hyperaldosteronism associated with obesity. However, our study did not find any difference in the proteinuric effect, control of the blood pressure or slowing of the decline in GFR between obese and non-obese patients.
Our study shows that the antiproteinuric effect in patients with obesity persists without change during the follow-up, a fairly important fact given that it could only be evaluated over short periods of time.19,20 The antiproteinuric effect was notably homogeneous; most of the patients showed sustained proteinuria reductions greater than 50%, even 48 months after the introduction of AA.
It is very interesting the behavior of the 25 diabetic patients in this substudy. In both groups showed a significant reduction in proteinuria (68.2%) during the follow-up (3.7±2.3 to 1.2±1.6g/24h, p<0.05), with a clear slowing of decline in GFR (6.2±14.7ml/min/year to 2.7±6.5ml/min/year, p .36) since the introduction of aldosterone. These findings show us that the early introduction of these drugs in patients with diabetic nephropathy could find a beneficial renoprotective effect.
Although serum potassium levels showed a significant increase after the introduction of spironolactone, they were easily controlled with a low-potassium diet, cation exchange resins or low-dose thiazide diuretics. During the study, only 2 patients (6%) discontinued the treatment with AA due to persistent hyperpotassemia (>5.5–6mEq/L), despite the adopted therapeutic measures. Gynecomastia is a relatively common secondary effect in patients with obesity treated with spironolactone and was observed in 28.1% of the patients, which was higher than in the control group (5.1%, p=.007). The condition was completely resolved by changing to eplerenone, another AA that does not share this complication.
Although our results show a low incidence of hyperpotassemia and other severe complications, it is important to emphasize that careful monitoring of our patients is necessary and that this policy should be recommended for all patients treated with AAs, particularly those cases with mild levels of renal failure.
Recent studies have alerted us to the risk of severe complications (renal function impairment, hyperpotassemia and hypotension) in patients treated with dual ACEI+ARA blockers.26,27 We observed no more adverse effects in our patients after the introduction of the AA in patients who were treated with ACEI+ARA when compared with the patients treated with ACEI or ARA alone. All patients treated with dual blockers at the start of the study (9 of 32) progressively withdrew the ACEI or ARA during the follow-up due to better blood pressure control and satisfactory proteinuria reduction after the introduction of the AA. In this respect, our data suggest that ACEI+AA or ARA+AA combinations could be an interesting alternative to dual ACEI+ARA blockers.
Another fundamental issue is the beneficial effect of AA on cardiometabolic syndrome and resistant arterial hypertension in patients with obesity. There is increasing scientific evidence that relates an excess of circulating aldosterone to metabolic effects and endothelial function, which contribute to the genesis of hypertension, cardiovascular disease and nephropathy.28 Therefore, the use of AAs has clear utility in cardiovascular prevention and blood pressure control. In our study, we were able to observe a clear reduction in BP (8.5%), which, coupled with the renoprotective effects, may be considered an excellent therapeutic option for this patient group.
Our study has significant limitations, such as a small number of patients, the fact that it is not a randomized and controlled study and a lack of biochemical measurements for renin-aldosterone and ions in urine, which could have verified the beneficial effects of these drugs. An important limitation of this study is that the formula used to calculate the GFR-MDRD-4 is not validated in the obese population. However, this study reproduces the standard clinical practice with significant fidelity. For this reason, more comparative studies are warranted to determine whether these antiproteinuric and renoprotective effects of AA are shared by other types of diuretics in the population with obesity, given that a number of studies have shown that thiazides can induce significant reductions in proteinuria when added to ACEIs and/or ARAs.29 Similarly, sodium restriction boosts the antiproteinuric response to ACEIs and ARAs,30 and the combination of hydrochlorothiazide with a low-sodium diet increases the response even further.31
In summary, the antiproteinuric effect and trend to slowing progression of renal failure with AA treatment in proteinuric nephropathies not changed by the condition of obesity. The renoprotective effect of aldosterone antagonists should be confirmed in larger prospective trials. The rational use of these drugs in adequate doses with close monitoring of side effects can combine their antiproteinuric effect and an adequate safety profile.
Conflict of interestThe authors declare no conflict of interest.
This study has been supported by grants from REDINREN (RD012/0021) and AITER (Asociación para la Investigación y Tratamiento de la Enfermedad Renal).