Canakinumab, an IL-1 blocking drug, decreases the frequency and severity of the attacks and decreases the proteinuria level in colchicine resistant/intolerant familial Mediterranean fever (FMF) patients. However, it is not known whether patients with impaired or preserved renal functions respond differently to IL-1 blocking therapies in terms of proteinuria reduction and progression of kidney dysfunction which was the aim of this study.
Materials and methodsAdult FMF subjects with biopsy proven amyloidosis who had 24-h urine protein excretion>150mg/day before initiation of canakinumab were divided into two groups as patients with preserved renal function (GFR≥60mL/min) and patients with impaired renal function (GFR<60mL/min). The response in proteinuria and renal functions are compared between two groups in this cross-sectional study.
ResultsA total of 18 patients (11 with preserved and 7 with impaired renal function) were included in this study. Although proteinuria levels of both groups were similar at the baseline and at six months after initiation of canakinumab, proteinuria at 12 months was significantly lower for patients with preserved renal function compared to patients with impaired renal function (2462±1760mg/day vs. 7065±3035mg/day respectively, p=0.02). All of the patients with preserved renal function had more than 50% decrease in proteinuria at 12 months compared to baseline values, while none of the patients with impaired renal function had more than 50% decrease in proteinuria.
ConclusionsCanakinumab, an IL-1 blocking agent, is not effective in decreasing proteinuria in FMF patients with already impaired renal functions and should be started early in the course of disease to prevent renal impairment.
El canakinumab, un fármaco bloqueante de la IL-1, disminuye la frecuencia y la gravedad de los ataques y reduce el nivel de proteinuria en pacientes con fiebre mediterránea familiar (FMF) resistentes o intolerantes a la colchicina. Sin embargo, se desconoce si los pacientes con función renal deteriorada o preservada responden de forma diferente a los tratamientos de bloqueo de la IL-1 en cuanto a la reducción de la proteinuria y la progresión de la disfunción renal, que era el objetivo de este estudio.
Materiales y métodosLos sujetos adultos con FMF y amiloidosis demostrada por biopsia que tenían una excreción de proteínas en orina de 24 h > 150 mg/día antes de iniciar el tratamiento con canakinumab, se dividieron en dos grupos: pacientes con función renal preservada (TFG ≥ 60 mL/min) y pacientes con función renal deteriorada (TFG < 60 mL/min). En este estudio transversal se comparan la respuesta en la proteinuria y las funciones renales entre dos grupos.
ResultadosEn este estudio se incluyeron 18 pacientes (11 con función renal preservada y siete con función renal deteriorada). Aunque los niveles de proteinuria de ambos grupos fueron similares al inicio y a los seis meses de iniciar el tratamiento con canakinumab, la proteinuria a los 12 meses fue significativamente menor en los pacientes con función renal preservada, en comparación con los pacientes con función renal deteriorada (2.462 ± 1.760 mg/día vs. 7.065 ± 3.035 mg/día, respectivamente, p = 0,02). Todos los pacientes con función renal preservada presentaron una disminución de la proteinuria superior al 50% a los 12 meses, en comparación con los valores iniciales, mientras que ninguno de los pacientes con función renal deteriorada presentó una disminución de la proteinuria de más del 50%.
ConclusionesEl canakinumab, un fármaco bloqueante de la IL-1, no es eficaz en la disminución de la proteinuria en pacientes con FMF que ya tienen la función renal deteriorada, y el tratamiento debe iniciarse en una fase temprana de la evolución de la enfermedad para prevenir una insuficiencia renal.
Familial Mediterranean fever (FMF) is the most common monogenic autoinflammatory disease characterized by recurrent episodes of fever, abdominal pain, serositis and synovitis. It is most prevalent among Arabs, Armenians, Sephardic Jews and Turks. The disease is caused by mutations in MEFV gene located in the short arm of chromosome 16 that encodes a protein called pyrin. Pyrin is involved in the conversion of pro-IL-1β to mature IL-1β which is a key mediator in immune response. Mutations in MEFV gene results in absence of pyrin action leading to overexpression of IL-1β and causes an inflammatory response.1 If not properly treated, patients with FMF are under the risk of development of AA type amyloidosis and end stage renal disease (ESRD).
Colchicine treatment decreases the frequency and severity of the attacks and prevents the development of amyloidosis in most of the patients. However, 5–10% of patients are either resistant to colchicine treatment or cannot tolerate the drug because of side effects.2,3 For these patients, IL-1 blocking therapies such as anakinra and canakinumab emerged as highly effective alternatives. These drugs decrease the frequency and severity of the attacks and suppress the inflammatory activity in FMF patients including patients under dialysis treatment and renal transplant recipients.4–7
Case reports and small-scale studies with IL-1 blocking therapies also reported decreased proteinuria levels in FMF patients with high baseline proteinuria. However, it is not known whether patients with impaired or preserved renal functions respond differently to IL-1 blocking therapies in terms of proteinuria reduction and progression of kidney dysfunction. We hypothesized that canakinumab, an IL-1 blocking agent, can be more effective in decreasing proteinuria in patients with preserved renal functions and may prevent renal function deterioration.
The aim of this study was to determine if amount of decrease in proteinuria after canakinumab treatment differ in FMF patients with biopsy proven amyloidosis who have normal or impaired renal functions at the time of initiation of canakinumab and to evaluate the effect of canakinumab treatment on renal disease progression in chronic kidney disease patients with amyloidosis.
Material and methodsIn this cross-sectional study, we reviewed the medical files of all FMF patients followed in Hacettepe University Medical Faculty Nephrology Department that ever used canakinumab treatment. Among these patients, all adult subjects with biopsy proven amyloidosis who had 24-h urine protein excretion>150mg/day before initiation of canakinumab were included in the study. Patients that had been using canakinumab for <6 months and patients that were under any type of renal replacement therapy at the time of initiation of canakinumab were excluded. Flow chart for study participants is presented in Fig. 1. Canakinumab had been used with off-label permission from Turkish Health Ministry in all patients. The study was approved by local ethics committee of Hacettepe University Medical Faculty and was conducted in accordance with Declaration of Helsinki. All participants gave written informed consents.
Patients were divided into two groups based on glomerular filtration rate (GFR) at the time of canakinumab initiation as patients with preserved renal function (GFR≥60mL/min) and patients with impaired renal function (GFR<60mL/min). A constant dose of 150mg every four weeks subcutaneously had been administered to all patients. Demographic and clinical data including age, gender, type of MEFV gene mutation, biopsy site that revealed amyloidosis, extent of amyloid deposition, percentage of sclerotic glomeruli and degree of interstitial fibrosis/tubular atrophy in renal biopsy specimens, indication of canakinumab, duration of canakinumab use, data about the side effects and discontinuation of canakinumab were recorded for all patients and the patients are followed to the present day. Attack frequency, colchicine dose, erythrocyte sedimentation rate, c-reactive protein, fibrinogen, serum creatinine, glomerular filtration rate and proteinuria levels before and during canakinumab treatment were compared between the two groups.
Statistical Package for Social Sciences (SPSS) version 20 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables were presented as mean±standard deviation or median [min–max] and categorical variables were presented as number and percentages. Wilcoxon signed rank and Kruskal–Wallis tests were used to compare repeated measurements. Intergroup comparisons were made by Student's T and Mann–Whitney U tests. p values<0.05 were considered statistically significant.
ResultsA total of 18 patients (13 males, 5 females) were included in this study. The mean age of the patients was 35.8±10.2 years. Amyloidosis had been demonstrated by renal biopsy in twelve patients and by rectal biopsy in the remaining six patients. MEFV gene mutations of the patients were as follows: M694V/M694V in eleven patients, M694V/M680I in five patients and M694V/V726A in two patients. All patients were under colchicine treatment and eight patients were attack free before the initiation of canakinumab.
There were eleven patients with preserved renal function (GFR≥60mL/min) and seven patients with impaired renal function (GFR<60mL/min). Demographic characteristics, clinical features and baseline laboratory values (except for renal functions and the dose of colchicine) were not significantly different between the two groups at the time of the initiation of canakinumab treatment (Table 1).
Demographic characteristics, clinical features and baseline laboratory values of two groups at the time of the initiation of canakinumab treatment.
| Patients with GFR≥60mL/min | Patients with GFR<60mL/min | P | |
|---|---|---|---|
| (n=11) | (n=7) | ||
| Age (years) | 33.6±10.1 | 39.1±10.1 | NS |
| Sex (n, %) | NS | ||
| Male | 8 (72.7%) | 5 (71.4%) | |
| Female | 3 (27.3%) | 2 (28.6%) | |
| Site of biopsy (n, %) | NS | ||
| Renal | 7 (63.6%) | 5 (71.4%) | |
| Rectal | 4 (36.4%) | 2 (28.6%) | |
| MEFV mutation (n, %) | NS | ||
| M694V/M694V | 7 (63.6%) | 4 (57.1%) | |
| M694V/M680I | 3 (27.3%) | 2 (28.6%) | |
| M694V/V726A | 1 (9.1%) | 1 (14.3%) | |
| Colchicine dose before canakinumab (tablets) | 3.2±0.8 | 2.3±0.5 | 0.01 |
| Attacks before canakinumab (n, %) | NS | ||
| Yes | 7 (63.6%) | 3 (42.9%) | |
| No | 4 (36.4%) | 4 (57.1%) | |
| GFR (mL/min) | 119.5±25.9 | 33.3±11.8 | <0.0001 |
| Creatinine (mg/dL) | 0.72±0.27 | 2.45±0.88 | <0.0001 |
| Proteinuria (mg/day) | 7132±5921 | 8358±5975 | NS |
| Albumin (g/dL) | 3.08±0.79 | 3.16±1.20 | NS |
| Sedimentation (mm/h) | 44.2±20.4 | 49.8±42.6 | NS |
| CRP (mg/dL) | 1.93±2.17 | 1.08±0.96 | NS |
| Fibrinogen | 593.0±165.6 | 541.7±260.6 | NS |
CRP: C-reactive protein, GFR: glomerular filtration rate, NS: not significant.
Mean treatment duration with canakinumab was 17.1±10.7 months. There was no statistically significant difference in treatment duration between the two groups (15.2±9.4 months for patients with preserved renal function vs. 20.0±12.7 months for patients with impaired renal function, p=0.367). No FMF attacks were observed under canakinumab treatment in the patients. Mean colchicine dose decreased from 2.8±0.8 tablets/day to 2.1±0.8 tablets/day after initiation of canakinumab (p=0.003). Sedimentation, CRP and fibrinogen at 12 months and at the end of follow up period were within normal ranges for all patients.
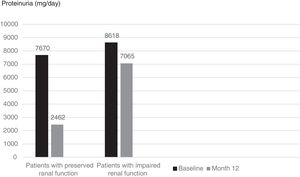
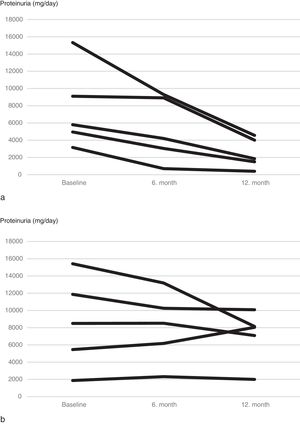
Although mean proteinuria level at six months after initiation of canakinumab was lower in patients with preserved renal function compared to patients with impaired renal function, the difference did not reach statistical significance (4392±4259mg/day vs. 6837±4282mg/day respectively, p=0.257). However, proteinuria at 12 months after initiation of canakinumab was significantly lower for patients with preserved renal function compared to patients with impaired renal function (2462±1760mg/day vs. 7065±3035mg/day respectively, p=0.02) (Fig. 2). All of the patients with preserved renal function had more than 50% decrease in proteinuria at 12 months compared to baseline values, while none of the patients with impaired renal function had more than 50% decrease in proteinuria (Fig. 3).
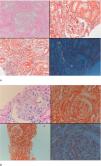
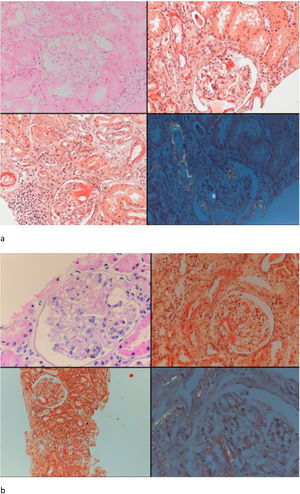
Seven patients with GFR≥60mL/min and five patients with GFR<60mL/min had undergone renal biopsy before initiation of canakinumab treatment. Except for one patient in each group, all of the patients had also tubular and/or vascular amyloid deposition in addition to glomerular deposits. Patients with low GFR had significantly higher percentage of glomerulosclerosis (50 [35–70] vs. 5 [0–50], p=0.01) and interstitial fibrosis/tubular atrophy scores (50 [30–80] vs. 10 [0–30], p=0.003) compared to patients with GFR≥60mL/min. A control renal biopsy was performed in one of the patients with preserved renal function who had a dramatic decrease in proteinuria level at 26th month of canakinumab treatment (baseline proteinuria: 3164mg/day, proteinuria at 26th months: 250mg/day). However, there was no regression of amyloidosis at control biopsy (Fig. 4).
Baseline and control renal biopsy of a patient who had a dramatic decrease in proteinuria level at 26th month of canakinumab treatment (baseline proteinuria: 3164mg/day, proteinuria at 26th months: 250mg/day). (a) Microscopic examination revealed focal amorphous eosinophilic material expanding the mesangial area and along the blood vessels. The deposits were negative with the PAS stain, argyrophobic, congophilic and showed birefringence under polarized microscopy, consistent with amyloid. (b) The patient's follow-up biopsy showed a similar pattern of deposition with focal involvement of glomeruli and vessel walls with no discernable improvement.
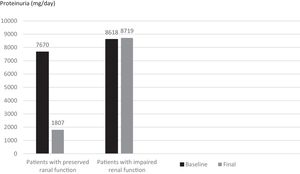
When subjects that used canakinumab longer than 12 months were analyzed, we observed that proteinuria at the end of follow-up was lower for patients with preserved renal function compared to patients with impaired renal function (1807±1932mg/day vs. 8719.6±5956.0mg/day respectively, p=0.016) (Fig. 5). All of the patients with preserved renal function had more than 50% decrease in proteinuria at the end of follow up compared to baseline values while none of the patients with impaired renal function had more than 50% decrease in proteinuria.
At the end of follow-up period there were no deterioration in GFR for patients with preserved renal function but two patients with already impaired renal function at the time of initiation of canakinumab progressed to hemodialysis (15 months and 22 months after the initiation of treatment). These patients continued to use canakinumab for attack control.
Canakinumab was perfectly tolerated in all patients. No patient developed major adverse effect that required discontinuation of canakinumab during the treatment and there was no mortality in any patient while using canakinumab treatment.
DiscussionThis study revealed that treatment with canakinumab decreases renal disease progression which was assessed by reduction of proteinuria and conservation of GFR in patients with preserved renal functions. Although there was a trend towards decreasing proteinuria at the sixth month the decrease was more significant at the end of first year of canakinumab treatment. On the other hand, GFR declined and proteinuria persisted in patients with impaired renal function despite the canakinumab treatment.
Previous studies revealed that canakinumab is highly effective in controlling the attacks and suppression of inflammation in patients with colchicine resistant FMF, however effects of canakinumab treatment on the progression of amyloidosis have not been widely studied. Although some studies indicated decreased proteinuria with anti IL-1 treatment, they did not investigate the effect of baseline renal function on proteinuria reduction or kidney disease progression under this treatment. As an example, a nationwide study from Turkey in 47 colchicine resistant/intolerant FMF patients with baseline 24-h proteinuria>500mg, reported significant reduction in mean proteinuria level from 5.5g to 3.5g with anti-IL-1 treatment. Proteinuria decreased in 77% of the patients. Renal functions of the responders and non-responders before the initiation of anti-IL-1 treatments were not reported in this study.8 Another study from Germany reported decrease in proteinuria from 5.0g/day to 0.4g/day with anakinra after 24±18 months in twelve patients with amyloidosis. Although baseline creatinine level was reported as 2.4±0.6mg/dL, effect of renal function was not separately investigated in this study population which included patients with normal and decreased renal function and even two patients with ESRD.5 A recent study reported significant decrease in 24-h proteinuria from 1.6g to 0.5g in 17 colchicine resistant FMF patients treated with either anakinra or canakinumab within a median follow up of 16 (min:3 max: 58 months). The median creatinine level of the patients in this study was reported as 0.83mg/dL, ranging from 0.46 to 1.78mg/dL. Once again effect of renal function on proteinuria response is not reported in this study.9 Finally, Kucuksahin et al. reported reduction in proteinuria in some patients while increased proteinuria in some other patients after treatment with anakinra. Renal functions of responders and non-responders had not been revealed in this study.10
Several case reports also indicated decreased urinary protein levels in both adult and pediatric patients with anti-IL-1 treatment. Renal functions were normal or not disclosed in majority of the cases,11–13 only a single case report indicated complete remission of proteinuria in a patient with amyloidosis secondary to FMF after anakinra treatment who presented with 6.1 gr/day proteinuria and had a serum creatinine of 1.33mg/dL (GFR not reported).14
Our observation that baseline renal function is an important determinant of response to canakinumab treatment had also been previously reported for colchicine treatment. A study that analyses factors affecting the outcome of FMF revealed that an initial serum creatinine of≥1.5mg/dL was a risk factor for non-response to colchicine treatment.15 In another study pediatric FMF patients compliant to colchicine treatment have a high possibility of decreasing proteinuria and stable renal functions during follow-up if their renal functions are preserved when colchicine is started. Patients with already impaired renal functions progressed to renal failure despite colchicine treatment.16
The decrease in proteinuria and improvement in renal functions may possibly indicate regression of amyloid deposition. However, we could not demonstrate a significant change in extent of amyloid accumulation in control renal biopsy in a patient with complete remission of proteinuria. Similarly, Topaloglu et al reported that although proteinuria improved with anti-IL-1 treatment in three patients with amyloidosis secondary to systemic juvenile arthritis and cryopyrin associated periodic syndrome, there were no regression of amyloid deposition in control renal biopsies.17 The discrepancy between clinical improvement and lack of change in amyloid deposition is difficult to explain. It can be speculated that, with the suppressed inflammation there might be a decrease in glomerular permeability despite persistence of amyloid deposition.17 Further studies are required to better understand the mechanism of proteinuria reduction despite persistence of amyloid deposits.
Limitations of this study are retrospective design, small number of the patients in both groups and relatively short duration of follow-up. However, this study is the first report in the literature that shows the importance of baseline renal function on renal protective effect of canakinumab.
In conclusion canakinumab, an IL-1 blocking agent, is not effective in decreasing proteinuria in FMF patients with already impaired renal functions and should be started early in the course of disease to prevent renal impairment.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.