Chronic kidney disease (CKD) is a prevalent health condition associated with numerous complications, including olfactory and taste dysfunction, and malnutrition. This systematic review focused on dysgeusia, smell disorders and malnutrition in CKD patients. The search included scientific databases such as PubMed, Cochrane Library, CINAHL, Scopus, and Web of Science, as well as sources of grey literature. Quality assessment and risk of bias were evaluated using JBI guidelines, while the certainty of evidence was assessed with the Oxford Centre methods. Seven studies were included: two focusing on anosmia and five on dysgeusia. Anosmia was associated with poorer nutritional status, and interventions such as intranasal theophylline showed promise in enhancing olfactory function. Dysgeusia studies highlighted the potential role of zinc deficiency in malnutrition among dialysis patients, with zinc supplementation showing mixed results to improve taste dysfunction. Taste alterations were correlated with upper gastrointestinal symptoms and malnutrition in CKD patients. The analysis of the interconnection between anosmia, dysgeusia, and malnutrition emerges as a crucial starting point for improving nutritional outcomes in chronic kidney disease (CKD) patients, emphasizing the need for accurate assessment and targeted therapeutic interventions to ensure better nutritional health and improved quality of life for these patients.
La enfermedad renal crónica (ERC) es una condición de salud prevalente asociada a numerosas complicaciones, incluyendo disfunción olfativa y gustativa, así como malnutrición. Esta revisión sistemática se centró en la disgeusia, los trastornos olfativos y la malnutrición en los pacientes con ERC. La búsqueda incluyó bases de datos científicas tales como PubMed, Cochrane Library, CINAHL, Scopus y Web of Science, así como fuentes de la literatura gris. La evaluación de la calidad y el riesgo de sesgo se analizaron utilizando las guías JBI, mientras que la certidumbre de la evidencia se evaluó mediante los métodos de Oxford Centre. Se incluyeron siete estudios: dos de ellos centrados en la anosmia y cinco en la disgeusia. La anosmia estuvo asociada a un peor estatus nutricional, y las intervenciones tales como teofilina intranasal resultaron prometedoras para mejorar la función olfativa. Los estudios sobre disgeusia destacaron el rol potencial de la deficiencia de zinc en la malnutrición entre los pacientes de diálisis, y la aportación de suplementos de zinc mostró resultados mixtos para mejorar la disfunción gustativa. Las alteraciones del gusto se correlacionaron con los síntomas del tracto gastrointestinal superior y la malnutrición en los pacientes de ERC. El análisis de la interconexión entre anosmia, disgeusia y malnutrición surge como punto de inicio fundamental para mejorar los resultados nutricionales en los pacientes con ERC, subrayando la necesidad de realizar evaluaciones precisas e intervenciones terapéuticas focalizadas para garantizar una mejor salud nutricional y mejorar la calidad de vida de dichos pacientes.
Chronic kidney disease (CKD) represents a major global health challenge, characterized by the progressive deterioration of renal function over time.1,2 The latest data from the National Health and Nutrition Examination Survey (NHANES III) highlight the escalating prevalence of CKD within the general population,3 with current estimates indicating that between 9.1% and 13.4% of individuals worldwide are affected.4 This trend is alarming, as the World Health Organization (WHO) recently ranked CKD as the 10th leading cause of death globally in 2020, and projections suggest it could rise to become the fifth by 2040.5,6
Patients with CKD face a myriad of health complications, including but not limited to anaemia, electrolyte imbalances, bone metabolism disorders, and severe malnutrition.7,8 Notably, a significant portion also suffers from alterations in taste and smell, which further complicates their condition.8,9 Studies, including those from NHANES III, report a prevalence of anosmia (or olfactory dysfunction) at 14%, and dysgeusia (or gustatory dysfunction) at 18% in the general population, highlighting the need for special attention in CKD patients.10,11 Indeed, these taste and smell disorders, characterized by symptoms such as dry mouth, tongue coating, and mucosal inflammation, drastically diminish patients’ quality of life.12
Various factors, including oral infections, diabetes mellitus, and zinc deficiency, have been identified as contributors to these sensory impairments,13 though the precise mechanisms remain elusive. For example, zinc deficiency might decrease taste perception, although few studies have revealed a positive relationship between taste alteration and zinc plasma levels.14 Similarly, research suggests that smell alterations may be caused by the accumulation of toxic substances or electrolytic imbalances,15 but the causes remain unclear.
Olfactory and taste impairments significantly predispose CKD patients to malnutrition, affecting their quality of life16 and increasing morbidity and mortality in this population.17–19 Malnutrition is defined as “faulty nutrition resulting from inadequate or unbalanced intake of nutrients or impaired digestion or utilization of foods”.20 The prevalence of malnutrition in CKD patients is significant, ranging from 18% to 75%,21 with prevalence rates varying between 20 and 80% in pre-dialysis stage.22–24 Moreover, the prevalence of malnutrition increases with increasing age and worsening kidney function, suggesting that nutritional status should be assessed early and regularly in these patients.25 The pathogenesis of malnutrition in CKD patients is multifactorial,26 and a study has reported an association between taste changes, gastrointestinal symptoms, and malnutrition in CKD patients.27
Recognizing the profound effects of dysgeusia, anosmia, and malnutrition on this patient population is fundamental for developing targeted therapeutic interventions aimed at enhancing their nutritional status and overall quality of life. This paper seeks to explore these interconnections and the potential pathways for intervention, with the objective of mitigating the adverse outcomes associated with CKD.
AimThe aim of this systematic review is to evaluate the relationship between anosmia and dysgeusia and nutritional status in CKD patients, as well as to identify clinical interventions currently available to address these clinical conditions in this population.
MethodsReview methodologyThis systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines and following the PRISMA checklist,28 reported in Supplementary File 1.
Systematic review protocol registrationThe protocol of this systematic review was registered in the International prospective register of systematic reviews (PROSPERO) of the National Institute of Health Research (https://www.crd.york.ac.uk/prospero/) with protocol registration number: CRD42023469530.
Research questionFor the present study, the research query was formulated utilizing the PICO model.29 The PICO model serves as a methodology employed by scholars to refine a research subject. It revolves around four core elements: patient or problem (P), intervention or indicator (I), comparison (C), and outcome (O). This review considered three components of the PICO methodology, adopting a PIO. The following aspects were therefore considered based on the approach: P: patients with CKD; I: identification of primary research studies describing olfactory and taste dysfunctions and malnutrition; O: evaluate the correlation between olfactory and taste dysfunctions and malnutrition and the impact of healthcare interventions in reducing these clinical conditions.
Search strategyBetween September 2023 and June 2024, a comprehensive and systematic bibliographic search was conducted in scientific databases, including PubMed, Cochrane Library, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Scopus and Web of Science. To achieve a targeted and precise search, we employed a combination of specific keywords and Mesh Terms (Medical Subject Headings), using the keywords “chronic kidney disease”, “olfactory dysfunctions”, “taste dysfunctions”, “Therapy”, “Treatment”, “Management”, “Malnutrition”, and their variations, opportunely combined by Boolean operators. Additionally, a manual search was conducted in Google Scholar to retrieve additional records in grey literature; full search algorithms are available in the data available statement (supplementary file 1). In the initial screening phase, two expert reviewers (SM and MS) independently assessed all titles and abstracts extracted from the electronic database searches. Utilizing the EndNote 20 software (https://endnote.com/), duplicates and non-relevant records were systematically eliminated. In instances of discordance, a third expert reviewer (MP) was consulted to facilitate consensus. For those studies deemed potentially relevant in the initial screening, full-text articles were obtained. Each of these was subjected to a rigorous independent assessment by the reviewers (SM and MS) in alignment with predetermined eligibility criteria. In situations where consensus was challenging, dialogues were initiated between the primary reviewers. If no agreement was reached, the adjudication of the third reviewer (MP), previously uninvolved, was sought to ensure an unbiased decision-making process.
Inclusion criteriaIn this study, we defined inclusion and exclusion criteria to align with the review's scope and methodology, ensuring the selection of pertinent and recent articles.
Inclusion criteria: 1. Primary study: Any type of experimental or observational study design, including qualitative and mixed-method studies; 2. Related to identify or treat anosmia or dysgeusia; 3. Participants: CKD patients.
Exclusion criteria: 1. Studies that do not meet the inclusion criteria; 2. Studies that focus on individuals without CKD; 3. Olfactory or taste dysfunctions caused by COVID-19 or diseases other than CKD.
Evaluation of risk of bias and methodological quality of studiesThe risk of bias and the methodological quality of the included articles were initially evaluated by two reviewers (GF and SMP). Conflicts were resolved by the involvement of a third review author (SM). To rigorously assess the methodological quality and relevance of the selected studies, we employed the JBI Critical Appraisal Tools.30 These tools, recognized for their precision in evaluating various research designs, provided a structured framework to discern the reliability and applicability of each study. By utilizing these instruments, we ensured that only the most robust and pertinent studies were incorporated into our systematic review.31 High-quality studies were identified on the basis of a previous meta-analysis32 in which studies with a JBI score higher than 70% were classified as having a high quality, those with a score between 69.9% and 50% as having a medium quality, and those with a score less than 50% as having a low quality. The result of this evaluation is reported in Supplementary File 1.
Assessment of evidence certaintyIn this SR, the robustness of the evidence was ascertained using the framework established by the Oxford Centre for Evidence-Based Medicine (OCEBM) in 2011.33 The OCEBM's framework categorizes research into five distinct levels of evidence, contingent upon the study design and the quality of the research conducted. High-level studies, encompassing systematic reviews of randomized controlled trials and good quality individual trials, were assigned to the first evidence level. In contrast, research predominantly grounded in expert consensus or lacking empirical support was lowered to the fifth level. Intermediate-stage research, which includes but is not limited to less rigorous randomized controlled trials, cohort studies, and methodologies such as case series or case-control investigations, was allocated to the second, third, and fourth levels, respectively. Certain studies underwent a recalibration of their evidence level, either being elevated or diminished, influenced by parameters like methodological rigour, precision of findings, and the relevance of the results to the topic at hand.
Data extractionData from selected articles was extracted and reported in tables as follows: authors, year of publication, country, study design, populations, type of intervention and assessment of quality/bias.
Data synthesisThe articles incorporated into this review were systematically categorized based on the type of interventions (prevention, education, clinical treatment). For each of these types of intervention classifications, the study methodologies and primary results were first reported through a narrative synthesis and subsequently in specific tables and graphs.
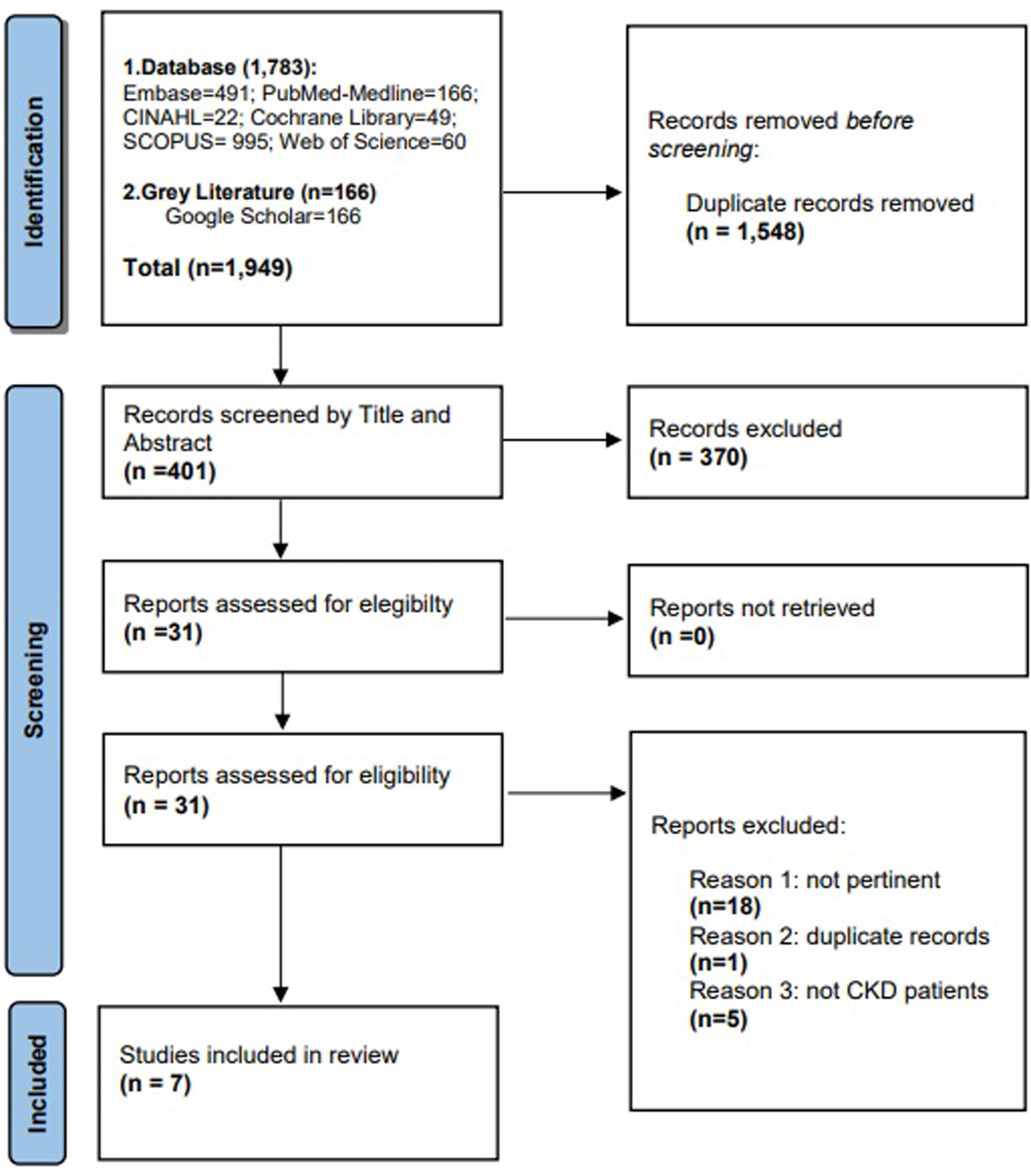
ResultInclusion and evaluation processA total of 1949 records were identified, with 1783 articles were through electronic database searches (49 Cochrane Library, 166 PubMed, 22 CINAHL, 995 Scopus and 60 Web of Science, 491 Embase) and 166 articles were identified through ‘grey literature’ (Google Scholar). After removing 1548 duplicate articles, all titles were screened, and 401 articles were retained and evaluated for eligibility by reading the abstract. Of these, 370 studies were judged not relevant for various reasons, and the remaining 31 full text were evaluated; 24 studies were subsequently excluded as they did not meet the selection criteria for our research. The screening process ultimately included only seven studies. Two studies related to anosmia and five studies with dysgeusia, as represented in Fig. 1.
Characteristics of studies, population, and interventionsThe studies were conducted in various countries, including the United States (n=4),8,34–36 the United Kingdom (n=1),37 Australia (n=1),38 and Taiwan (n=1).39 In total, seven studies were included in this systematic review in line with our objectives. Among these, five studies8,36–39 assessed dysgeusia, while two studies34,35 focused on anosmia in CKD patients. Our analysis, based on the reviewed studies, involved a sample of 219 patients, with 67 included in the anosmia analysis (range 31–36) and 152 included in the dysgeusia analysis (range 7–145). The risk of bias, assessed using the framework proposed by JBI, demonstrated high quality of the included studies (range 75–100%), with an average score of 87.63%. Specifically, all studies exhibited high quality8,34–39; none of the selected studies were of medium or low quality.
Anosmia and malnutrition in chronic kidney diseaseTwo studies34,35 investigated anosmia in CKD and end-stage renal disease (ESRD) patients, using the subjective global assessment (SGA) score to comprehensively evaluate nutritional status. The SGA score, ranging from 7 (indicating normal nutritional status) to 1–2 (indicating severe malnutrition status), provided an insightful assessment of nutritional levels. Additionally, the University of Pennsylvania Smell Identification Test (UPSIT) was employed to gauge olfactory function. The characteristics of these studies are reported in Table 1.
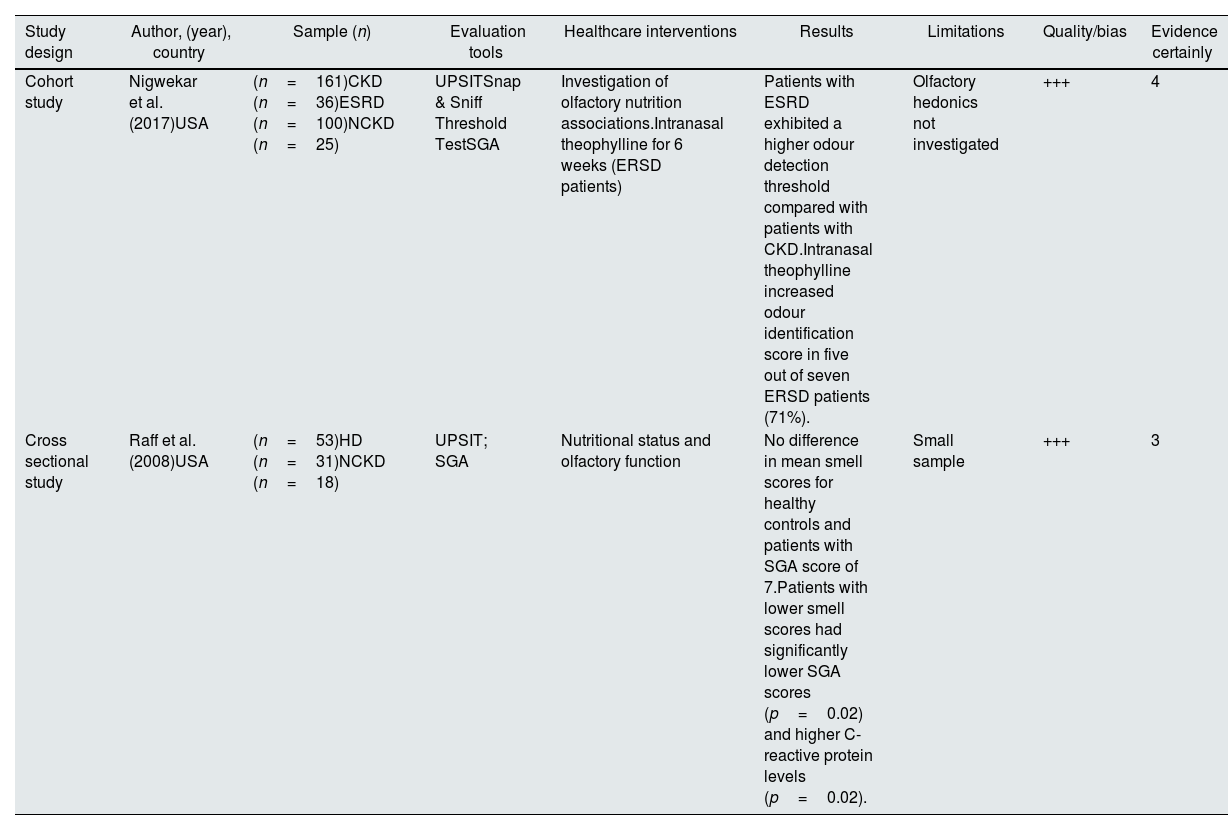
Characteristics of studies concerning anosmia and chronic kidney disease.
| Study design | Author, (year), country | Sample (n) | Evaluation tools | Healthcare interventions | Results | Limitations | Quality/bias | Evidence certainly |
|---|---|---|---|---|---|---|---|---|
| Cohort study | Nigwekar et al. (2017)USA | (n=161)CKD (n=36)ESRD (n=100)NCKD (n=25) | UPSITSnap & Sniff Threshold TestSGA | Investigation of olfactory nutrition associations.Intranasal theophylline for 6 weeks (ERSD patients) | Patients with ESRD exhibited a higher odour detection threshold compared with patients with CKD.Intranasal theophylline increased odour identification score in five out of seven ERSD patients (71%). | Olfactory hedonics not investigated | +++ | 4 |
| Cross sectional study | Raff et al. (2008)USA | (n=53)HD (n=31)NCKD (n=18) | UPSIT; SGA | Nutritional status and olfactory function | No difference in mean smell scores for healthy controls and patients with SGA score of 7.Patients with lower smell scores had significantly lower SGA scores (p=0.02) and higher C-reactive protein levels (p=0.02). | Small sample | +++ | 3 |
CKD: chronic kidney disease; ERSD: end stage renal disease; HD: haemodialysis; NCKD: non-CKD patients; SGA: subjective global assessment; UPSIT: University of Pennsylvania Smell Identification Test; USA: United State of America. Quality was assessed according to the JBI guidelines and using the JBI quality appraisal checklist. The level of bias risk was considered: low<50% (+); moderate 50–70% (++); high>70% (+++); Certainty of evidence was assessed according to the Oxford.
In the first study, a cohort study,34 the relationship between olfaction and nutrition was investigated, and an innovative intervention was introduced to enhance olfaction in ESRD patients. The study included 161 participants (36 CKD, 100 ESRD, and 25 controls) and revealed impaired odour identification in ESRD patients. The assessment of nutritional status using the SGA score demonstrated a reduction in odour identification scores associated with higher subjective global assessment scores, lower serum total cholesterol, and lower albumin concentrations. The subsequent pilot included seven ESRD patients self-administering 20μg intranasal theophylline daily for six weeks, resulting in improved olfactory function in 71% patients. This study underscores the need for further investigation into intranasal theophylline to enhance olfactory thresholds in ESRD patients.
The assessment of anosmia was also addressed by a second observational study,35 which enrolled 31 haemodialysis patients and 18 controls. Although no significant difference in olfactory function was observed between controls and “healthier” ESRD patients, those with lower smell scores exhibited significantly lower SGA scores and higher C-reactive protein levels. Intriguingly, neither smell scores nor nutritional status correlated with levels of the retained uraemic solution. This study supports the idea of impaired smell identification in ESRD patients, emphasizing that this impairment is not present in those with normal nutritional status.
Dysgeusia and malnutrition in chronic kidney diseaseWe included a total of five studies on dysgeusia, comprising two cross-sectional studies,8,38 one cross-over study,38 one cohort study,40 and one RCT in double blind.36 The characteristics of this studies are shown in Table 2.
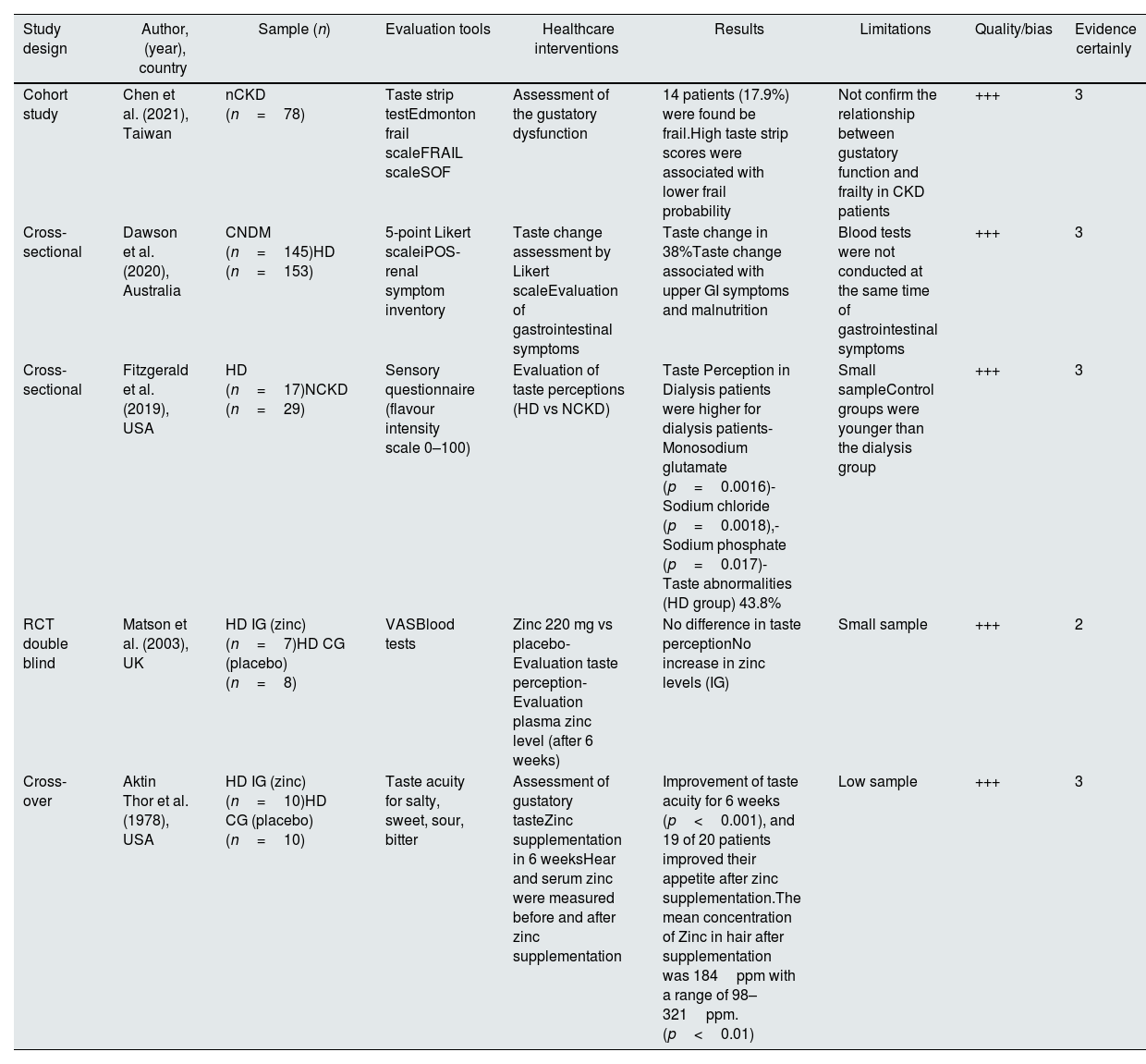
Characteristics of studies concerning dysgeusia and chronic kidney disease.
| Study design | Author, (year), country | Sample (n) | Evaluation tools | Healthcare interventions | Results | Limitations | Quality/bias | Evidence certainly |
|---|---|---|---|---|---|---|---|---|
| Cohort study | Chen et al. (2021), Taiwan | nCKD (n=78) | Taste strip testEdmonton frail scaleFRAIL scaleSOF | Assessment of the gustatory dysfunction | 14 patients (17.9%) were found be frail.High taste strip scores were associated with lower frail probability | Not confirm the relationship between gustatory function and frailty in CKD patients | +++ | 3 |
| Cross-sectional | Dawson et al. (2020), Australia | CNDM (n=145)HD (n=153) | 5-point Likert scaleiPOS- renal symptom inventory | Taste change assessment by Likert scaleEvaluation of gastrointestinal symptoms | Taste change in 38%Taste change associated with upper GI symptoms and malnutrition | Blood tests were not conducted at the same time of gastrointestinal symptoms | +++ | 3 |
| Cross-sectional | Fitzgerald et al. (2019), USA | HD (n=17)NCKD (n=29) | Sensory questionnaire (flavour intensity scale 0–100) | Evaluation of taste perceptions (HD vs NCKD) | Taste Perception in Dialysis patients were higher for dialysis patients- Monosodium glutamate (p=0.0016)- Sodium chloride (p=0.0018),- Sodium phosphate (p=0.017)- Taste abnormalities (HD group) 43.8% | Small sampleControl groups were younger than the dialysis group | +++ | 3 |
| RCT double blind | Matson et al. (2003), UK | HD IG (zinc) (n=7)HD CG (placebo) (n=8) | VASBlood tests | Zinc 220 mg vs placebo- Evaluation taste perception- Evaluation plasma zinc level (after 6 weeks) | No difference in taste perceptionNo increase in zinc levels (IG) | Small sample | +++ | 2 |
| Cross-over | Aktin Thor et al. (1978), USA | HD IG (zinc) (n=10)HD CG (placebo) (n=10) | Taste acuity for salty, sweet, sour, bitter | Assessment of gustatory tasteZinc supplementation in 6 weeksHear and serum zinc were measured before and after zinc supplementation | Improvement of taste acuity for 6 weeks (p<0.001), and 19 of 20 patients improved their appetite after zinc supplementation.The mean concentration of Zinc in hair after supplementation was 184ppm with a range of 98–321ppm. (p<0.01) | Low sample | +++ | 3 |
CG: control group; CKD: chronic kidney disease; CNDM: conservative non-dialysis management; FRAIL: fatigue resistant ambulation illness loss of weight; SOF: Study of Osteoporotic Fracture; iPOS: Integrated palliative outcome scale GI: Gastro intestinal; HD: haemodialysis; IG: intervention group; NCKD: non-CKD patients; nCKD: non-dialysis CKD; VAS: visual analogic scale. Quality was assessed according to the JBI guidelines and using the JBI quality appraisal checklist. The level of bias risk was considered: low<50% (+); moderate 50–70% (++); high>70% (+++); Certainty of evidence was assessed according to the Oxford CEBM level.
In two separate studies,36,37 researchers explored the potential significance of poor nutrient intake, particularly zinc deficiency, as a critical parameter for assessing malnutrition in dialysis patients.
In two studies, a double-blind randomized study36 aimed to determine the impact of zinc supplementation on taste perception in 15 haemodialysis patients. Seven patients received zinc sulphate (220mg) over six days, while the other eight received a placebo pill. The Visual Analog Scale was utilized to measure taste scores along with blood chemistry tests. The study revealed a disturbance in taste perception for sour taste in haemodialysis patients, and interestingly, this disturbance was not corrected by zinc supplementation. Despite previous evidence highlighting the importance of zinc in taste perception, none of the existing studies have proposed a persuasive mechanism to explain this phenomenon.
In a preceding study,37 designed as a cross-over study with 20 haemodialysis patients using zinc supplements (440mg) and placebos, researchers assessed taste acuity, serum zinc, zinc concentration in hair samples, and daily caloric intake. In contrast to the findings of Matson et al.,36 this study demonstrated that after zinc supplementation, taste acuity improved in 95% of patients, and hair zinc concentration increased in 85% of patients. Additionally, patients reported a significant improvement in appetite, and the average caloric intake increased in nineteen out of 20 patients. This seminal yet enduring study suggests that zinc supplementation proves highly beneficial in improving malnutrition among these patients.
In contrast to previous studies,36,37 a singular cross-sectional study38 aimed to investigate the potential association between taste alterations and the presence of malnutrition. The study encompassed 298 patients divided into two groups: the first group (n=145) underwent conservative non-dialysis management, while the second group (n=153) underwent renal replacement therapy. The researchers employed a 5-point Likert scale, termed iPOS-renal, ranging from none to overwhelming, to assess taste changes. Additionally, they evaluated various symptoms, including gastrointestinal symptoms, nausea, vomiting, diarrhoea, anorexia, constipation, and sore/dry mouth. Furthermore, the nutritional status was determined using the 7-point Subjective Global Assessment Tool, and biochemical parameters, such as serum urea, estimated glomerular filtration rate, and albumin, were also collected. Taste changes were reported by 38% of the 298 patients, and these changes were found to be associated with upper gastrointestinal symptoms and malnutrition in CKD patients. In a separate investigation,8 taste perception was assessed using a sensory questionnaire, which rated flavour intensity on a scale from 0 to 100. Unadjusted flavour intensity showed no discernible differences between dialysis patients and the control group. However, upon adjustment for deionized water taste, significant differences emerged between the groups (p=0.04), particularly for monosodium glutamate, sodium chloride, and sodium phosphate solutions. The study also noted significant differences in serum parameters, especially potassium, and sensory ratings. In the most recent study,39 researchers delved into whether gustatory function was correlated with frailty, utilizing both objective measures (taste strip kit) and subjective assessments (visual analogue scale). Among the 78 CKD patients involved, 14 patients were identified as frail (17.9%). The researchers employed three validated instruments: Edmond Frail Scale (EFS), FRAIL scale, and Study of Osteoporotic Fracture scale (SOF) and to assess frailty in patients. Their findings suggested that gustatory dysfunction might be a significant risk factor in CKD patients.
DiscussionMalnutrition is prevalent among CKD patients, influenced by various comorbidities such as anorexia, deficient caloric intake, and gastrointestinal symptoms, representing one of the major determinants of morbidity and mortality in these population.40
This systematic review aimed to comprehensively explore the interconnections between olfactory and gustatory dysfunctions and malnutrition in CKD patients. The study uncovers significant insights, providing a comprehensive understanding of the relationship and clinical implications of these sensory alterations in the context of malnutrition.
Various studies were analyzed to investigate gustatory and olfactory dysfunction associated with malnutrition in CKD patients. Concerning anosmia, a cross-sectional study35 demonstrated that impaired smell identification was not found in ESRD patients with a normal nutritional status but was impaired in patients with worse nutritional status and higher C-reactive protein (CRP) levels. More recently study33 observed that a reduction in odour identification was linked to higher SGA scores and lower levels of biochemical measures of nutritional status, indicating olfactory deficit as a potential mechanism underlying malnutrition. They also introduced a novel intervention involving intranasal theophylline to enhance olfaction in ESRD patients, suggesting a new avenue for potential therapy to alleviate olfactory deficits and malnutrition in kidney disease patients.
One notable aspect explored across several dysgeusia studies was the potential role of poor nutrient intake, particularly zinc deficiency, as a critical determinant of malnutrition in individuals undergoing dialysis.36,37 Some studies investigated the efficacy of zinc supplementation in ameliorating taste perception and nutritional outcomes among haemodialysis patients. While the double-blind randomized study36 failed to demonstrate significant improvements in taste perception following zinc supplementation, a cross-over study37 revealed promising outcomes, including enhanced taste acuity, increased appetite, and improved caloric intake post-supplementation. These contrasting findings underscore the complexity of taste perception mechanisms and highlight the need for further research to elucidate the underlying mechanisms driving these responses.
Moreover, a cross-sectional study38 explored the association between taste alterations and malnutrition in CKD patients. Taste changes were found to correlate with upper gastrointestinal symptoms and malnutrition, emphasizing the multifactorial nature of dysgeusia in this population.
Additionally, investigations into taste perception differences between dialysis patients and controls revealed nuanced findings. While unadjusted flavour intensity did not differ significantly between groups, adjustments for taste perception of deionized water unveiled notable distinctions, particularly for specific taste solutions. These findings underscore the importance of considering contextual factors and control conditions when assessing taste perception in CKD patients. Lastly, a recent study39 delved into the correlation between gustatory function and frailty among CKD patients. The identification of gustatory dysfunction as a potential risk factor for frailty highlights the broader implications of taste perception impairments on overall health outcomes in this population.
Overall, the findings from these studies underscore the multifaceted nature of anosmia, dysgeusia and malnutrition in CKD patients. These sensory dysfunctions serve as important indicators for assessing nutritional status, especially in CKD patients susceptible to protein-energy wasting.41 Further research is warranted to elucidate the underlying mechanisms driving olfactory and taste perception alterations and to explore targeted interventions aimed at improving nutritional outcomes and overall quality of life in this vulnerable patient population.
The result of this systematic review demonstrates as anosmia and dysgeusia have a relatively high impact on malnutrition just as other chronic pathologies, like oncological disease or cancer where patients undergoing oncological therapies develop these complications.42 Taste and smell disorder are often underestimated in these patients.43 Furthermore, taste disorders condition quality of life in these patients as demonstrated in a study, which highlights how it's important evaluate taste and smell disorders during and after treatment with oncological therapy.44
Strength and limitationsWhile the present review offers a comprehensive analysis of the available literature on anosmia in CKD patients, it is important to acknowledge certain limitations. Our study is inherently constrained by the limited number of studies available, particularly concerning anosmia. Additionally, many of the included studies had small sample sizes and lacked relevance to malnutrition. Despite these limitations, some studies underscore the importance of not underestimating symptoms such as anosmia and dysgeusia in haemodialysis patients. Furthermore, these symptoms may serve as indicators of nutritional status in both CKD and ESRD patients. Thus, while our study has its constraints, it emphasizes the significance of recognizing these symptoms and their potential implications for nutritional management in CKD and ESRD patients.
ConclusionIn summary, our systematic review provides valuable insights into the relationship between olfactory and gustatory dysfunctions and malnutrition in CKD patients. This emphasizes the need for increased clinical attention to these sensory alterations and emphasizes the importance of ongoing research to optimize the management of malnutrition in this patient population.
Funding statementThe authors received no funding for this research, neither from internal nor from external bodies.
Authors’ contributionsGF; SMP, MS, SM: conceptualization, methodology, writing original draft, review & editing, interpretation, visualization, coordinator; GA, MP, LG, DG: review & editing, interpretation, visualization; LG: review, data analysis. All authors read and approved the final manuscript. GF & SMP provided an equal contribution as first author in drafting the manuscript; MS & SM provided an equal contribution as first author in drafting the manuscript.
Systematic review protocol registrationThe protocol of this SR was registered in the International prospective register of systematic reviews (PROSPERO) of the National Institute of Health Research available at https://www.crd.york.ac.uk/prospero/ with protocol registration number: CRD42023469530.
Conflict of interestNone.