Focal segmental glomerulosclerosis (FSGS) is a histopathological lesion characterized by scarring in specific sections of some glomeruli, accompanied by podocyte injury. Worldwide, the prevalence of FSGS and its temporal trends have not been sufficiently studied. However, some reports suggest an increase in the frequency of FSGS in recent decades. Understanding the epidemiology of FSGS is crucial for clinicians to improve diagnosis and treatment.
ObjectiveThis study critically evaluates global prevalence trends of FSGS over the past 32 years (1992–2024), highlighting variations between countries through a systematic review.
MethodsA systematic search of Medline, Embase and ScienceDirect was conducted to identify relevant studies. The reliability of prevalence data was assessed by critical appraisal of selected publications.
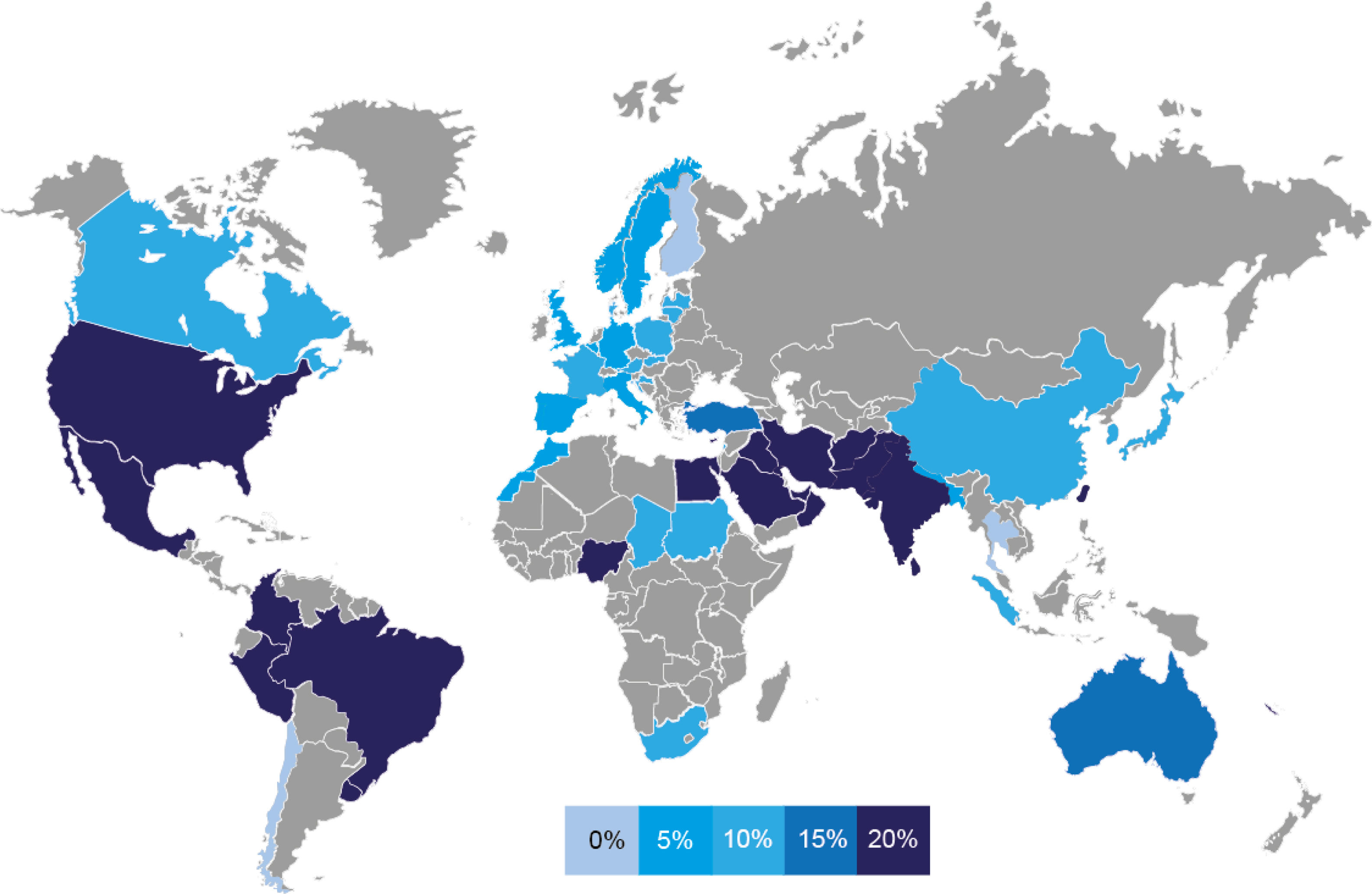
ResultsThe prevalence of FSGS varies significantly between regions. East Asian countries have a relatively low prevalence, with a mean around 7%. In contrast, countries in South Asia, the Middle East and the Americas have a higher prevalence of around 18%. European countries show an intermediate prevalence of about 11%. African countries do not show a clear pattern, with high and low prevalence rates in different countries.
ConclusionsThe prevalence of FSGS differs by geographic region and ethnicity. While South Asian countries have maintained a consistently low prevalence, other regions have experienced an increase in FSGS cases over time. This study improves the understanding of global patterns of FSGS, providing valuable epidemiological insights for clinicians and researchers.
La glomeruloesclerosis focal y segmentaria (GEFS) es una lesión histopatológica caracterizada por la cicatrización en secciones específicas de algunos glomérulos, acompañada de lesión de podocitos. A nivel mundial, la prevalencia de la GEFS y sus tendencias temporales no han sido suficientemente estudiadas. Sin embargo, algunos informes sugieren un aumento en la frecuencia de la GEFS en las últimas décadas. Comprender la epidemiología de la GEFS es crucial para que los médicos mejoren el diagnóstico y el tratamiento.
ObjetivoEste estudio evalúa críticamente las tendencias de prevalencia mundial de FSGS en los últimos 32 años (1992-2024), destacando las variaciones entre países a través de una revisión sistemática.
MétodosSe realizó una búsqueda sistemática en Medline, Embase y ScienceDirect para identificar estudios relevantes. La fiabilidad de los datos de prevalencia se evaluó mediante una evaluación crítica de publicaciones seleccionadas.
ResultadosLa prevalencia de la GEFS varía significativamente de una región a otra. Los países de Asia Oriental tienen una prevalencia relativamente baja, con una media de alrededor del 7%. En contraste, los países del sur de Asia, Medio Oriente y las Américas tienen una mayor prevalencia, con un 18%. Los países europeos muestran una prevalencia intermedia de alrededor del 11%. Los países africanos no muestran un patrón claro, con tasas de prevalencia alta y bajas en diferentes países.
ConclusionesLa prevalencia de la GEFS difiere según la región geográfica y la etnia. Mientras que los países del sur de Asia han mantenido una prevalencia sistemáticamente baja, otras regiones han experimentado un aumento de los casos de GEFS a lo largo del tiempo. Este estudio mejora la comprensión de los patrones globales de FSGS, proporcionando información epidemiológica valiosa para médicos e investigadores.
Focal segmental glomerulosclerosis (FSGS) is a histopathological lesion characterized by scarring (sclerosis) affecting specific sections (segmental) of some glomeruli (focal), accompanied by podocyte injury.1 Although previously considered a single disease, FSGS is now recognized as a heterogeneous condition with diverse etiologies, clinical presentations and responses to treatment. The defining characteristic of FSGS is podocyte injury and loss, which can occur as a primary disorder or as a secondary response to various glomerular stressors. As the disease progresses, localized segmental sclerosis can spread to involve entire glomeruli, eventually culminating in complete glomerular dysfunction.1
Since the 1970s, FSGS has attracted increasing attention due to its role as a leading cause of end-stage renal disease (ESRD) worldwide.2
The morphological classification of FSGS, based on the Columbia system,3 classified the disease into five variants: collapsing, tip, cellular, perihilar, and not otherwise specified. However, relying solely on this framework to make therapeutic decisions proved to be insufficient, as it did not take into account key genetic factors or syndromic presentations, often leading to ineffective treatment. In response, a more comprehensive approach has emerged that considers both clinical and pathological features, redefining FSGS into primary, secondary, genetic and indeterminate forms.4 Primary FSGS is thought to be caused by an unknown circulating factor, in the absence of an identifiable underlying cause. In recent years, several studies have identified anti-nephrin antibodies in a substantial proportion of patients with primary FSGS, suggesting their potential role in the disease.5 These antibodies have been proposed as candidate permeability factors; however, their direct involvement in the pathophysiology of FSGS has not yet been conclusively demonstrated. Other proposed candidates include soluble urokinase plasminogen activator surface receptor (suPAR), apolipoprotein A1b (APOA1b), cardiotrophin-like cytokine factor 1 (CLCF1), and anti-CD40 antibodies.6 In contrast, secondary FSGS develops as a consequence of systemic conditions or external influences, such as viral infections, exposure to toxins or drugs, and glomerular hyperfiltration due to factors such as obesity, congenital renal anomalies, solitary kidney or reflux nephropathy.4 Genetic FSGS, on the other hand, is the result of mutations in genes essential for podocyte function and structural integrity, leading to progressive renal damage.7
Although it is recognized that the overall incidence of FSGS has increased,8 its prevalence varies significantly depending on factors such as geographic region, study period, race, age, and sex distribution. The objective of this review is to critically evaluate prevalence studies published in the last 32 years, providing valuable information on trends, regional differences, and changes in disease rates. By conducting a systematic analysis of the global prevalence and incidence of FSGS, we aim to improve understanding of its epidemiological patterns and suggest future research and health care strategies.
MethodsThe search strategy followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines. A comprehensive search of different information sources was carried out in Medline, Embase, and ScienceDirect databases (1992–2024) was performed using the terms “glomerulonephritis”, “nephropathy”, “kidney disease”, “prevalence”, “incidence”, “epidemiology”, “focal segmental glomerulosclerosis”, and/or “focal segmental glomerulosclerosis epidemiology”, The eligibility criteria for the included studies required them to present original research, were published in English or Spanish, and contained relevant FSGS epidemiological information about trends, epidemiology or incidence of FSGS. Each record was independently reviewed by two researchers based on its title and abstract. In cases of disagreement regarding a study's inclusion, a third researcher evaluated the record and made the final decision. All studies were considered and reviewed from 10 January 2023 to 30 December 2024, regardless of their focus or age group, as long as they contained information according to the inclusion criteria. Exclusion criteria included duplicate publications, insufficient information on incidence, unavailability of full text or incomplete data, and reporting bias. Review articles, transplant registries, conference abstracts, unpublished manuscripts, small sample size (less than 100 patients) in countries with multiple reports and studies on recurrent FSGS were also excluded. Only descriptive statistics were used from the original article. Alternative meta-analyses or advanced statistical models were not performed due to several limitations inherent to the study. These include the use of registry data that may not accurately reflect the general population in some countries, discrepancies in the time periods covered by the various registries, and significant heterogeneity in renal biopsy practices across countries. This variability includes differences in clinical indications for biopsy, procedural strategies, and diagnostic criteria, all of which limit the comparability of the data. We evaluated the language of publication biases, but only 8 articles were discarded as being written in Hebrew, Italian, French, Croatian, German, Chinese, Hungarian and Romanian. Titles and abstracts were reviewed by the authors, and selected studies were evaluated in more detail. We considered frequency and prevalence data reported by the authors, incorporating information from national biopsy registries, case reports, and case series. This work was not registered in PROSPERO because the project had already commenced before registration was considered, and it was later decided to proceed without registration given the stage of progress at that time.
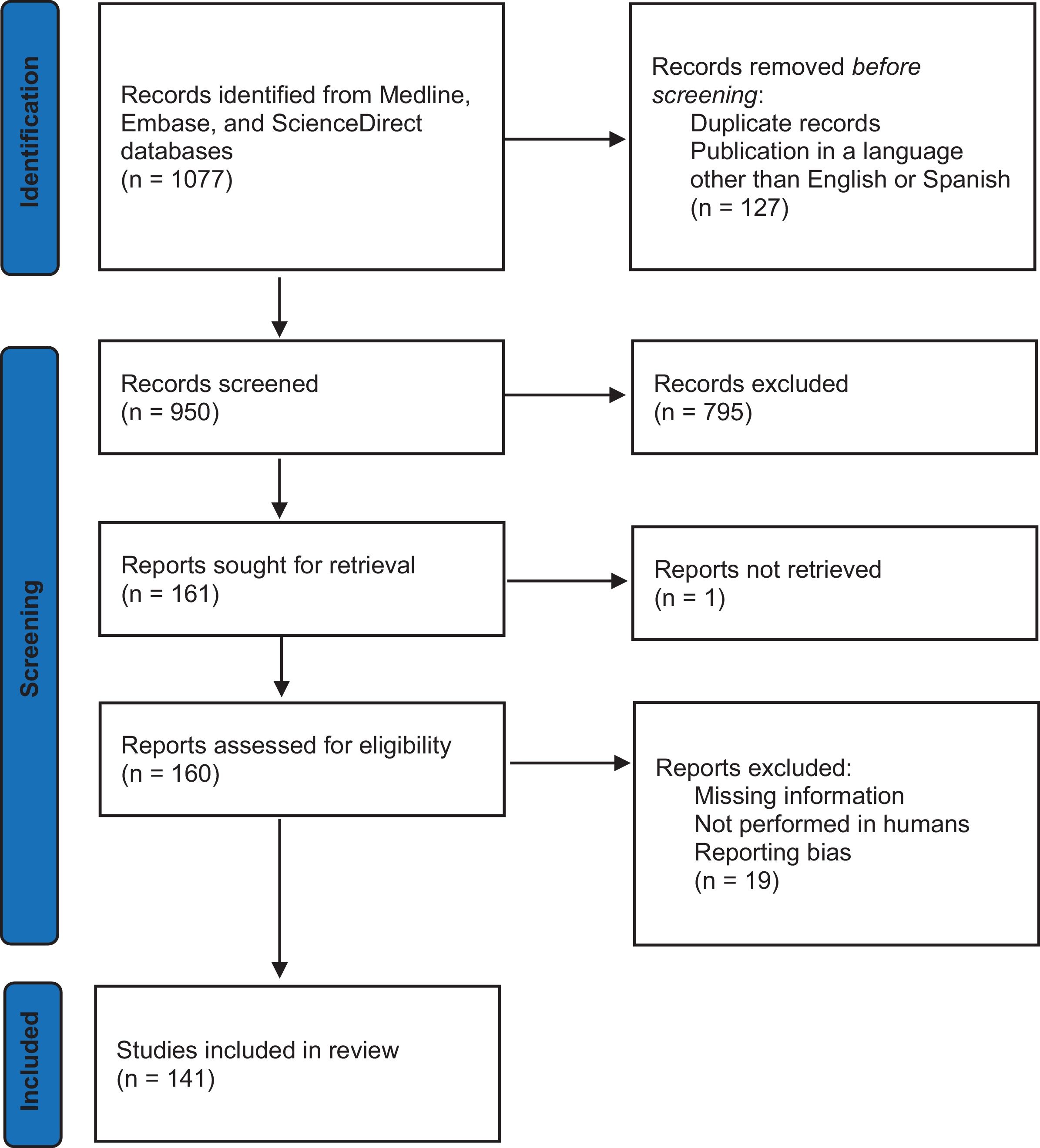
ResultsThe initial literature search identified 1047 articles, which were systematically selected according to predefined inclusion and exclusion criteria. After this screening process, only 130 original studies met the analysis criteria. A detailed breakdown of the screening process, including the number of articles included and excluded at each stage, is presented in Fig. 1. Across all continents, Asia was the continent with the highest number of reports, 67 in total. Among them, China contributed the largest number of studies with 16, followed by India with 10, Japan with 7 and Pakistan with 4. South Korea and Iran provided 4 studies, while Saudi Arabia, Turkey and Nepal contributed 3 each. Lebanon and Jordan submitted 2 reports, while the United Arab Emirates, Taiwan, Thailand, Oman, Iraq, Bangladesh and Sri Lanka, Kuwait and Singapore contributed 1 study each. Europe provided 33 reports, with Italy leading with 5, followed by Romania with 3, Poland, Spain, the Czech Republic, Denmark, Serbia, Belgium, Germany and Croatia with 2 each. Sweden, France, Cyprus, Lithuania, Estonia, Portugal, Finland, Norway and United Kingdom received 1 report each. The Americas contributed 22 reports, with Brazil leading the way with six studies, followed by the United States and Colombia with five each, Mexico with 2 and Chile, Uruguay, Peru and Canada with 1 each. Africa presented 9 reports, 4 from Nigeria and 1 each from Egypt, South Africa, Morocco, Chad and Sudan. Oceania was the continent with the fewest reports, with only 3, 2 from Australia and 1 from New Caledonia. Table 1 provides an overview of the main characteristics of the studies included in this analysis, and Supplementary Table 1 contains the complete list of studies.
Summary of the studies analyzed in this paper to estimate the worldwide prevalence of focal segmental glomerulosclerosis (FSGS).
| Continent | Country | Period and reference | No. biopsies | Prevalence (%) | Population | Description |
|---|---|---|---|---|---|---|
| Asia | China | 1979–200229 | 13,519 | 6 | Adults. A single unit in China and 57.3% were male. | Average age was 32.7±12.2 (9–83) years. Biopsies with PGD 9278, FSGS was present in 557 cases. |
| 1987–201230 | 11,618 | 5.82 | General; All patients were Asian, 6646 male cases and 4972 female cases (1.33:1). | PGD 8209 cases, median age of 35 years (range 3–85 years old). Among the PGD FSGS was present in 458 patients of 8209. | ||
| 1993–201731 | 5398 | Prevalence of FSGS was 2.7 in 1st period (1993–1997), 3.3 in 2nd (1998–2002) and 3.8 in 3rd period (2003–2007). | Adults | One center, patients (age ≥14 years) were included, 3331 PGD, 110 FSGS (71 male/39 female). | ||
| 1994–201432 | 4931 | The proportion of FSGS increased from 1.74% in period 1(1994–1999) and 1.55% in period 2 (2000–2004) to 5.57% in period 3 (2005–2009), but then it decreased to 3.09% in period 4 (201–2014). | Adults | Ten hospitals in China. Average age was 35.2 years, 2254 patients (45.7%) were men. PGD were 81.55%, FSGS was 146 patients (3.63%), 85 male and 61 female. | ||
| 2001–201533 | 10,779 | 2.5 | General | A single center, patients aged (≥15 years old. Average age was 40±14.8 years old | ||
| 2001–201934 | 9448 | FSGS was in 477 patients (5.05%), group 1 (01/01/2001 to 12/21/2004) was 51 patients (5.06%), group 2 (01/01/2005 to 12/31/2009) was 109 patients (6.04%), group 3 (01/01/2010 to 12/31/2014) was 205 patients (6.26%), and group 4 (01/01/2015 to 12/31/2019) was 112 patients (3.33%). | Adults | Average age was 41.2 years for males and 41.60 years or females. 60.39% male. | ||
| 2003–201435 | 40,759 | FSGS was 6% in period 1979–2002 and changes frequencies at 7.34% to period of 2003–2014. | Adults | Patients > 14 years. Renal Biopsy Registry of the National Clinical Research Center of Kidney Diseases. Average age was 36.59±14.12 years. 52% male. PGD was 67.1% of cases. | ||
| 2004–201436 | 7962 | FSGS was 6% in male and 4% in female patients. 5% in patients to 0–12 year and 6% in 13–18 year. FSGS showed a significant decreasing trend, starting at 14% in the first period (2004–2007) and decreasing to 6% and 4% in the second (2008–2011) and third periods (2012–2014). | Children | Average age was 13.5±4.1 years, 64% boys. Included 115 hospitals. | ||
| 2007–201637 | 2725 | 2.68 | Adults | One hospital in northeast China. Only >14 years. Average age was 41.2±15.1 years. FSGS was 73 patients. | ||
| 2008–201838 | 10,996 | 8 | General | A single center, retrospective study of native kidney biopsies, PGD was 69.42%, with 8% for GSFS. | ||
| 2008–201739 | 4910 | 3.1 | General | 3593 cases of PGD (73.2%). Average age was 42.6±15.7 years (range: 7 to 84 years), 2629 males and 2281 females (ratio: 1.15:1). | ||
| 2008–201340 | 3722 | 8.78 | Adults | Shandong Province of China. FSGS patients average was 37.9±12.5 years FSGS frequency was 327 patients. | ||
| 2009–201841 | 35,783 | FSGS frequency was in 850 patients (2.45%). FSGS had distribution peaks in the 20–39-year age group. In elderly patients FSGS was 2.92% and in children (-14 years) FSGS was 3.32%. | General | Adults 54.27% male patients, ratio male. Female 1.19:1, 31,256 were adults, with age of adults 40.8±15.2 years. | ||
| 2011–202042 | 9310 | 14.97 | Adults | One center, two periods 2011–2015 and 2016–2020. PGD were in 66.93% cases (6166 cases), among these FSGS was 14.97% patients. In patients with age 14–24 years FSGS was 14.04%, in 25–44 years 13.39% and 45–59 years 18.33%. | ||
| 2014–201643 | 1445 | 21.6 | Adults | Included 17 centers, of PGD FSGS was 21.6% cases (133). Average age was 50.4±17.7 years, 55.6% male, 14.3% had diabetes and 50.4% had hypertension. | ||
| 2014–201844 | 7917 | 4.3 | Adults | Nationwide cross-sectional survey of kidney biopsies in China. 59.6% male. FSGS was in 331 patients. | ||
| 2015–201745 | 62,569 | FSGS was in 2526 (4.4%) of patients, 199 (4.7%) pediatrics and 2327 (4.4%) in adult patients | General | Retrospective study, including 56,880 native biopsies at 1211 hospitals across China. Children were 4274 patients y adults 52,606 patients. | ||
| India | 1990–200846 | 1849 | 15.3 | General | Average age was 32.2±18.3 years. 1091 males and 758 females. FSFGS was 195 of 1278 patients, median age 25 years. Male 2.25: 1 Female | |
| 1997–201347 | 423 | 25.8 | Children | A single center. Average age was 10.4±4.5 years, 57.9% male. PGD was 360 patients, and of these FSGS was 109 patients. | ||
| 2006–201648 | 3257 | FSGS was the most common PGD accounting for 18.2%. | Adults | A single center study in northern India. Average age was 33.2±14.2 years, 61.9% male. | ||
| 2007–201849 | 254 | 12.2 | Children | Median age was 15 years, 57% female. | ||
| 2008–201350 | 335 | 8.02 | Children | Average age was 7.91±3 years, 68.1% males. PGD was the most common in 81.8%, and FSGS was 8.02%. | ||
| 2009–201651 | 270 | 13.7 | Adults | Retrospective study. Average age was 36.92 years. FSGS was 37 out of 270) most common PGD. | ||
| 2010–201252 | 666 | 22.58 | Adults | Average age was 28±14.6 years. Of 527 cases, primary FSGS was 119 patients. | ||
| 2016–202153 | 409 | 17.4 | Children | 353 PGD, FSGS was present in 71 patients. | ||
| 2017–202054 | 347 | 17.02 | Adults | Mean age was 41.4±15.7 years, 58.5% were males. NS 36.3% of cases; 69% was PGD, FSGS was 32 patients. | ||
| Japan | 2007–200855 | 2126 | 3.62 | Adults | Japan Renal Biopsy Registry. Prospective registry system, J-RBR, 23 renal centers. Average age was 44.4±21 years, 1281 male. FSGS was present in 77 patients. | |
| 2007–201056 | 438 | 10.3 | Adults | J-RBR. Mean age 73 years, 226 males. All with elderly (aged ≥65 years) Japanese primary nephrotic syndrome (NS), reported FSGS in 45 patients. | ||
| 2007–201157 | 2802 | FSGS present in 99 patients from 1259 patients in elderly (≥65 years old) and 83 patients (1.7%) in control group (20–64 years) of 5021 cases. | Adults | J-RBR of the Japanese Society of Nephrology included 1596 males. Renal disease in the elderly (age ≥65 years old) and very elderly (age ≥80 years old) Japanese. | ||
| 2007–201658 | 1409 | 3 | Adults | JRBR. Average age was 47 years, 59.4% were male. The annual incidence of FSGS accounted for 3.5–4.5% Percentage of primary FSGS were constant at approximately 3% of all during 2007 to 2016. | ||
| 2007–201759 | 32,254 | 3.4 in adults and 3.7 in pediatrics | General | Japan Renal Biopsy Registry (J-RBR) 2007–2017. 973 patient adults with FSGS and 129 pediatrics patients with FSGS. | ||
| 2009–201060 | 8697 | 5.3 | Adults | JRBR. Predominantly males 53.6% in 2009 and 54.9% in 2010, included 4016 biopsies in 2009 and 4681 cases in 2010, average age (years) were 46.7±19.9 and 46.7±20.6 respectively. From 7034, FSGS was present in 378 patients. | ||
| 2015–201822 | 6036 | 10.85 | Adults | JNSCS patients with primary NS; FSGS was 655 patients, age 39 years, 57.5% males. | ||
| Pakistan | 1997–201361 | 423 | 25.8 | Children | Average age was 10.48±4.58 years, 57.9% were males (245). FSGS in 109 patients. | |
| 1998–200525 | 415 | Primary FSGS in 18.3% of patients. | Children | Pediatric kidney biopsies between 3–15 years, male: female ratio was 1.6:1. | ||
| 2010–201526 | 108 | 25.9 | Children | A tertiary care hospital. Average age in FSGS patients was 7.0±4.2 years, 56.5% were male, FSGS was present in 28 patients. | ||
| 2011–202062 | 307 | Primary FSGS in 40.4% (124 patients) | Children | . FSGS cases, mean age was 8.8±3.0 years while most of the children, 70 (56.5%) were above 10 years of age; 64 cases were male. | ||
| South Korea | 1973–199563 | 2097 | FSGS was present in 154, Adults 97 (4.6%) and 57 Children (4%). | General | 82.5% PGD. Male: female ratio was 1.5:1. | |
| 1987–200664 | 1818 | 5.6 | Adults | Average age was 36 years, male: female ratio was 1.02:1. | ||
| 1992–201165 | 818 patients, adult group (18–59 years) included 758 cases, and the older group (≥60 years) included 60 cases. | FSGS was 3.5% in 18–59 years (N=621) and 12.8% in ≥60 years (N=43 patients) | Adults | Average age was 37.2 years, male-to-female ratio was 1.2:1. | ||
| 2001–201366 | 1924 | FSGS was in 130 patients (6.8%). People over 65 years of age, FSGS was in 18 patients out of 155 (11.6%). | Adults | A single center. Average age was 37.7±16.5 years, 56% were male. | ||
| Iran | 2006–201867 | 2975 | 15.9 | Adults | The mean age of the patients was 27.4 years old; 51.6% were male. | |
| 2009–201468 | 1054 | 24.8 | Adults | Average age was 33.1 (±18.5) years, 43.3% were female. | ||
| 2011–201769 | 774 | 188 patients were FSGS of 774 biopsies of primary glomerulonephritis (24.2%). | Adults | A Single Center. Average age was 33.9±17.5 years, 58% men. | ||
| 2007–201870 | 2975 | 15.9% | Adults | A Single Center. 51.6% was male. The mean age was 27.4 years. | ||
| Saudi Arabia | 1989–202071 | 350 | 60 patients of 350 had FSGS (17.1%). | Children | A Retrospective Study. Included 39 pediatric patients and 21 adults. Male were 55% in both groups. The mean age of the pediatric patients was 7.13±5.18 years, while that of the adult patients was 35.8±14.3 years. | |
| 1995–200872 | 242 | FSGS was 32 cases (13.2%) | Children | Only 183 patients had glomerular disease. Patients were 2 days to 17 years old, 122 were males. | ||
| 2005–200973 | 348 | 27.6 | General | Retrospective study, a single center, 176 were on adult males, 127 were on adult females and 45 were on children. | ||
| Turkey | 1990–200674 | 614 | 7.3 | Children | 376 with PGD (61.2%). Mean age was 10.4 years, 604 boys. | |
| 1991–201075 | 3892 | 16.46 | Children | Turkish Society of Nephrology Registry System (TSNRS), 2438 were glomerular diseases The age range was 0 to 15 years. Male/female ratio was 1:1, FSGS was 641 patients. | ||
| 2009–201976 | 3875 | 21.9 | Adults | A multicenter study by the Turkish Society of Nephrology Glomerular Diseases Working Group, 47 centers of PGD of Turkish Society of Nephrology Glomerular Diseases (TSN-GOLD) Working Group. Average age was 41.5±14.9 years, 56.3% were male. | ||
| Jordan | 2010–201677 | 99 | 23 | Children | Retrospective study of FSGS, a tertiary care hospital. Average age 3.71±2.59 years, 66% were male. | |
| 2013–202078 | 106 | 11.32 | Adults | A Single-Center. Average age was 34±12.7 years, females were 53.7% | ||
| Nepal | 2014–201679 | 175 | 18.28% (18 patients) had FSGS. | Adults | A single center. Tertiary care center. Average age was 35.3±13.5 years. 59.4% female, ratio female: male ratio of 1.5. The mean age of patients with FSGS was 36.89 years. | |
| 2001–200780 | 137 | 8 | General | Average age was 30.6 years for males and 32.9 years for females. The male to female ratio was 1.6: 1 | ||
| 2022–202281 | 213 | Primary FSGS in 10.33 | Adults | One Center | ||
| Lebanon | 2003–200782 | 1048 | In Age≤15 FSGS was 6.6%, in 15<Age≤60 FSGA was 13.9% and age>60 FSGS was 12.1%. | General | Average age was 36.76±20 years (range 1–84), 54.4% were male, 20% pediatric population, 67.3% young adult group and 12.7% elderly group. | |
| 2014–201583 | 144 | Children were FSGS (5.7%) 31 adult patients were FSGS (28.4%) | General | 35 children (mean age 11±5.6 years) and 109 adults (mean age 41.6±16.5 years). 74.2% were male in children group and 45.8% were male in adult patients, | ||
| United Arab Emirates | 1978–199684 | 490 | 18.3 | Adults | Data from the United Arab Emirates Renal Diseases Registry. 378 PGD. Adults aged 14–66 years. FSGS were 69 patients. | |
| Taiwan | 2014–201685 | 1445 | FSGS were in 133 of 599 PGD patients (25%), | Adults | National Renal Biopsy Registry with 17 medical centers. Average age was 48.4±16.6 years, 53.8% cases male samples. Average age 50.4±17.1 years, 55.6% male in FSGS group. | |
| Thailand | 1983–200586 | 3355 | 2.8 | Adults | A single center, FSGS was 61 patients. | |
| Oman | 2011–201587 | 596 | Primary FSGS in 18.3% patients. | Children | Pediatric kidney biopsies between (3–15 years), male: female ratio was 1.6:1. | |
| Iraq | 2012–201388 | 662 | 22 | Adults | Average age was 27.3±17.6, 53% were male. FSGS was 135 patients. | |
| Bangladesh | 2008–200989 | 95 | 11.58 | Adults | Average age was 30.29 years, 60% female; male to female ratio of 1:1.5. | |
| Sri Lanka | 2018–201990 | 140 | 22.1 | Adults | One center, 55.7% females. Mean age was 46±15.3 years. FSGS in 31 patients. | |
| Kuwait | 2013–201891 | 356 | 21.9 | General | Report of four public hospitals, 356 adult native kidney biopsies, average age was 39.8 (12 to 90 years) years, 61.2% were male. FSGS came third place with78 (21.9%) of the cases, 31 patients were less than 18 years. 37 with sub-nephrotic proteinuria plus AKI, 15 with nephrotic syndrome, 11 with nephrotic syndrome plus AKI, 11 with sub-nephrotic proteinuria and 4 with unexplained renal impairment. | |
| Singapore | 1978–200892 | 3282 biopsies with PGD | 11.88 | General | Average age was 47.9±13.5 years with a range from 15 to 85 years, predominantly in males in the first 3 decades. Retrospective study over 40 years. 390 cases of GSFS (11.88%). Frequency of FSGS was 5% in the 1st decade (1978–1988), 6% in 2nd decade (1988–1998), 15% in 3rd decade (1998–2008) and 25% in 4th decade (2008–2018). | |
| Europe | Italy | 1970–199493 | 1926 | FSGS present in 150 patients (7.8%), changes over time: 5.2% during 1907–1974, 6.3% 1975–1979, 6.7% 1980–1984, 9% in period 1985–1989 and 8.8% in period 1990–1994. | Adults | Mean age of patients undergoing biopsy (from 29.3±12.2 years to 47.0±17.8 years). In PGD predominance of males (>2:1). |
| 1977–2005 94 | 3269 | 19.8 | Adults | 66% PGD. Mean age 42 year, 59% males | ||
| 1979–201424 | 213 | 11.6 | General | A single tertiary pediatric hospital. Median 10.4 years (range 0.6–24 years), 43.2% female. | ||
| 1987–199320 | 13,835 | FSGS was 11.8% of glomerulonephritis. The annual frequency of PGD to FSGS was 10.4% in 1993, 14.4% in 1990. | Adults | The Italian Group of Renal Immunopathology. Male sex was predominant in PGD (65%). | ||
| 1996–200095 | 14,607 | FSGS was 16.9% of PGD with NS | Adults | Italian Immunopathology Group, Date from 128 rental units in Italy were reported. PGD were in 6990 patients and were more frequent in males (64%), | ||
| 1998–201096 | 4378 | 13.5 | Adults | |||
| Romania | 1995–200497 | 635 | 11.5 | General | Two regional renal biopsy databases. PGD was 401 cases. FSGS incidence was 10 p.m.p/year | |
| 2005–201098 | 514 | Overall, 288 patients, incidence of FSGS was 13.5%, (0.51) p.m.p/year | Adults | Average age was 41.9±2.8 years, 58.5% were male. FSGS had an incidence of 0.70p.m.p./year. | ||
| 2011–201999 | 1101 | 4.8% of PGD (2.9% of 442 cases) | Adults | Biopsy reports were divided into 3 periods, 320 from 1994 to 2004 (period 1), 239 from 2005 to 2010 (period 2) and 442 biopsies between 2011 and 2019 (period 3). Mean age of the renal biopsy population during period 3 was 39.2±13.8 years, 65.2% were male. PGD was 59.5% of the cases. | ||
| Poland | 1990–2010100 | 746 | FSGS was 58 of 607 PGD patients (9.5%). | Adults | Adults (>18 years), in a single tertiary nephrology center serving an area of Central Poland. 607 PGD. Average age was 40.5±8 20.8 years, 411 male patients. | |
| 2009–2014101 | 8843 | 15 | General | Polish Society of Nephrology. A total of 1939 (21%) biopsies were performed in patients <18 years of age, 6394 (68.7%) in those 18–64 and 955 (10.3%) in elderly individuals (defined as ≥65 years of age). FSGS was present en 997 patients. Average age was 47 years (19–87 years) in FSGS patients and 55.1% were males. | ||
| Spain | 1994–1999102 | 7016 | In children FSGS was 15.2% and 10.8% in adult patients; in the elderly FSGS was 6%. | General | 93 medical renal units. Median (range) <15 years (487), 15–65 years (4827) and >65 years (1510). Male/female ratio in children of 1.2, in adults of 1.5 and in the elderly of 1.4. | |
| 1994–2019103 | 18,852 | 9 | Adults | The age range was 15 to 65 years. Male/female ratio was 1.5. | ||
| Sweden | 2014–2029104 | 913 | 8.4 | Adults | FSGS was present in 77 patients. | |
| Czech Republic | 1994–2000105 | 3294 biopsies in adults and 710 biopsies in <15 years | 10.8 | General | Czech Registry of Renal Biopsies included 28 centers. Mean age 10 years for children and 42 years for adults, 57.9% males. | |
| 1994–2011106 | 10,472 | 12.6 | General | 31 centers, 57.8% male. Mean age for children was 10 years and for adults 44.5 years FSGS incidence was 3.9 p.m.p/year, mean age for FSGS group was 40 years, 56.8% males. | ||
| Denmark | 1985–1997107 | 2380 | 13.66 | Adults | Danish Renal Biopsy Register (DANYBIR). Average age was 42.6±20.2 years. FSGS was 325 patients. Incidence 5.7 pmp/year, age FSGS group was 43.4±19 and 34% were female. | |
| 1985–2014108 | 5043 | 7.93 | Adults | Danish Renal Biopsy Registry and Patobank registries. FSGS was 400 patients of 5043 biopsies. | ||
| Serbia | 1987–2006109 | 1626 | FSGS was 15.1% causes of nephrotic syndrome among 872 native kidney diseases. | Adults | Average age was 39.1±13.8 years, 51.2% were male. | |
| 2001–2010110 | 150 | 20.9 | General | A single center. Mean age was 11.5 years, 56% were female patients. PGD was 57.4%. | ||
| Croatia | 1996–2012111 | 922 | 15.8 | Adults | Average age was 48 years (range 16–84 years), patients were ≥16 years old. | |
| Belgium | 2017–2019112 | 2054 | 9.3 | Adults | Median age 61.10 years, 62.1% males. 26 nephrology centers in Flanders (Belgium) 1152 glomerular final clinical diagnoses, FSGS incidence rate 12.1p.m.p/year | |
| 2017–2020113 | 148 | 11.1% | Children | Diagnosis was often determined by results of genetic analysis | ||
| France | 1976–1990114 | 480 | 10.6 | General | Western France in the north of Brittany (Cotes d’Armor Department) | |
| Germany | 1990–201318 | 1208 | FSGS accounted 6.1% of all diagnoses (N=1208). Over time FSGS was 4% in first period (1990–1997), 10% in second period (1998–2005) and 10% en 3rd period (2006–2013), being significant the trend in the incidence of GSFS in the study period | General | A single center in Central Europe over a period of 24years. 23 children (≤15 years) and 1185 adults. 706 (58.4%) was PGD. Age averaged 50±17.5 years, 63% male. | |
| 2002–2008115 | 222 | Primary FSGS was 12%, secondary FSGS was 9%. FSGS had an incidence of 11.2 pmp, with 43% linked to an underlying etiology. Secondary glomerulonephritis had an incidence of 17.5 pmp. | Adults | A single center study. The male-to-female ratio was 0.9, and the rate of elderly persons aged 60 years and more was 26.2% | ||
| Cyprus | 2006–2015116 | 153 | FSGS was 41% of PGD, 12% primary and 29% secondary form. | Adults | A tertiary referral hospital. Average age was 45.7 years, 51% male; 56% was PGD. | |
| Lithuania | 1994–2012117 | 2165 | FSGS was 285 cases (13.2%) in all three-time intervals. In 404 child patients FSGS was 56 cases (13.9%). | General | Nationwide renal biopsy data in Lithuania. Average age was 43.2±20 years. Male: female ratio was 1.4:1. | |
| Estonia | 2000–2010118 | 578 | 16.1 | General | Average age was 38.7±17.7 years, predominantly male; 45.4% PGD | |
| Portugal | 1998–2021119 | 228 | 7.9 | Children | One center. The most common indication for kidney biopsy was nephrotic syndrome (42.9%). Primary glomerular diseases were found in 153 cases (67.1%) and FSGS corresponded to 7.9% (18 cases) | |
| Finland | 1976–2000120 | 2057 | 3.9 | Children | Six hospitals, predominantly male. FSGS was 81 cases (3.9%) and, in patients <15 years was 3%. | |
| Norway | 1988–2021121 | 575 | 8.17 | Children | Norwegian Kidney Biopsy Registry (NKBR) and in the Norwegian Renal Registry (NRR). Average age was 10.7 (6.1 to 14.1) years, 313 (54.4%) were boys, FSGS was 47 patients. | |
| United Kingdom | 1976–2005122 | 1844 | 5.7 | Adults | Mean age was 49±17.8 years, 61% were male. PGD was 907 patients and 52 was FSGS. Incidence de FSGS of 0.15 php/year, per hundred thousand adult population per year. | |
| The Americas | United States | 1974–2003123 | 195 | 16.9 | Adults | Average age was 44±20 year, 111 were male, 33 of 195 cases were FSGS. Annual incidence for FSGS was 1.8 per 100,000. |
| 1994–2013124 | 370 | 16 | Adults | 281 PGD. FSGS was 46 patients with PGD. | ||
| 2000–2011125 | 2501 | 38.9 | Adults | Average age was 50.6±16.7 years, 45.7% were women. FSGS was in 973 patients, mean age in FSGS group was 51.1±16.2 years and 56.9% were male. | ||
| 2001–2005126 | 1228 | 9.6 | Adults | FSGS were in 435 of 1228 adult patients. | ||
| 2004–2014127 | 710 | 22.25 | Adults | A single center, patients >18 years. FSGS were 158 patients. Mean age of the group with FSGS was 54±19.09 years, the ratio male: female was 1.72:1 in FSGS group. | ||
| Brazil | 1992–2016128 | 582 | 28.8 | General | 9 AMICEN (Minas Gerais Association of Nephrology Centers). Age means 35, 50.9% male sex. PGD was 75.3% of cases, FSGS was present in 126 cases. | |
| 1993–2007129 | 9617 | 24.6 in adults and 23.5% in Children | General | Retrospective study, mean age of the general population was 35.07±18.65 years, 51% female. (51.0% were PGD and FSGS was present in 1135 cases. | ||
| 1998–2016130 | 1151 | 43 | Adults | Average age was 35.0±15.3, 41% male, 670 biopsies of native kidneys on patients. | ||
| 1999–2005131 | 2086 | PGD in 29.7 | Adults | Paulista Registry of Glomerulopathies. 1131 were PGD. Average age was 34.5±14.6. PGD were more frequent in males (55.1%). | ||
| 2000–2018132 | 1051 | PGD in 37.3% and 10% secondary FSGS. | Adults | Average age was 44.9±16.1 years. Female 52.9%, FSGS in 60.3%. | ||
| 2000–2018133 | 1057 | 37.3% | Adults | Temporal variation across the three time periods showed a statistically significant reduction in FSGS over time | ||
| Colombia | 1998–200723 | 1040 | 34.8 in adults and 28.7% in children. | Adults | FSGS was present in 288 biopsies (109 were male); in children were 76 patients (male were 48 patients). | |
| 1998–2009134 | 1412 | Primary FSGS in 20.6 | General | Median age of patients was 26 years; 56.7% were males, 291 had the confirmed diagnosis of primary FSGS, 74 patients (25.4%) were <15 years of age. | ||
| 2003–2015135 | 9911 | 20.1 | Adults | Over 18 years old. Male: female ratio was 42.6%: 57.4%. 1992 patients had FSGS. | ||
| 2007–2017136 | 241 | 11.6 | Children | Average age was 11±4.3 years, 58% female. FSGS was present in 28 patients. | ||
| 2008–2018137 | 871 | 19 | Adults | The mean age was 39±14 years, 67% female. | ||
| Mexico | 2003–2011138 | 163 | 47 | Adults | Single second level hospital center. Retrospective analysis, Average age was 32.6±13.3, 55% were female. In FSGS group male: female ratio was 1.4:1 and average age was 25.9±10.4 years. | |
| Canada | 1985–2014139 | 6434 | The relative frequencies of FSGS was 13.73% (1985–1989), 16.13% (1990–1994), 17.93% (1995–1999), 15.99% (2000–2004) and 17.9% (210–2014). | Adults | Mean age 47.9±19.8 years, 58% were male. | |
| Chile | 1999–2020140 | 550 | 14.1 | Adults | 63.5% females, Mean age at diagnosis was 47.8±18.2 years. | |
| Uruguay | 1980–2003141 | 2058 | FSGS was the most frequent PG in 29.3% and decreased from 36.3% in 1995–1999 period to 19.1% in 2000–2003 period. | Adults | Incidence FSGS was 6.4 per million population (pmp). Average age was 39.1±19.6 years, males was >50% on all periods. | |
| Peru | 1985–1995142 | 1263 | Global FSGS was 13.9 and 15.8 in prospective patients. | Adults | FSGS was present in 144 patients 171 were idiopathic. In addition, 101 cases were analyzed in prospective and FSGS was present in 16 patients with mean age 31 years, sex male/female 5/11. | |
| Africa | Nigeria | 1997–2013143 | 162 | 31.8 between 1997–2001 and 43 between 2006–2013 | Children | Between 1997 and 2001 FSGS predominated in 56 patients, and between 2006 and 2013, native kidney biopsy of 106 children, FSGS was in 46 patients. |
| 2002–2011144 | 165 | 27.8 | General | Single center. Average age was 15.4±12 years, 64.8% male. | ||
| Egypt | 2003–2008145 | 924 | 21.21 of PGD | General | Mean age was 26.5±14.6 years. | |
| South Africa | 2000–2009146 | 1284 | 10.5 | Adults | A single-center renal biopsy database. Average age was 36.8±14 years, 54% were female. | |
| Morocco | 2000–2007147 | 161 | 5.9 | Adults | A single center renal biopsy database. PGD were in 84 patients. Average age was 40.4±15 years, 101 males, FSGS was in 8 patients. | |
| Sudan | 2002–2007148 | 321 | 13.7 | Children | Mean age was 8.71, 60.2% were male. FSGS was in 44 patients. | |
| Oceania | Australia | 1982–2005149 | 653 renal biopsies on indigenous people and 249 biopsies non-indigenous patients. | In 249 non-indigenous patients FSGS was 16.1%, in aboriginal non-remote 12%, in Torres Strait Islander was 9.5%, in aboriginal remote/very remote FSGS was 19.6%. | Adults | Age range non-indigenous 42.5±16.6 years, aboriginal remote/very remote (n=455) age was 39.2±13.9. Non-indigenous 40.2% female, and aboriginal remote/very remote 55.4% female. |
| 2002–201117 | 3697 | FSGS was relative frequency of 20.9% in patients with nephrotic syndrome and 6.4% in nephritic syndrome, undefined renal dysfunction 15.9% and nephrotic proteinuria was 35.3% of cases. | Adults | Average age was 48±17 years. Male was ∼60%, FSGS was in 1.02php/yr. | ||
| New Caledonia | 1986–1993150 | 181 | 20.4 | Adults | FSGS was present in 37 cases. | |
FSGS: Focal Segmental Glomerulosclerosis; JNSCS Japan Nephrotic Syndrome Cohort Study; J-RBR: Japan Renal Biopsy Registry; PGD: Primary glomerular diseases; NS: Nephrotic Syndrome SRNS: Steroid-resistant nephrotic syndrome.
In Asia, the prevalence of FSGS varies significantly from region to region. In general, South Asian countries, such as India, Pakistan and Sri Lanka, together with Middle Eastern countries, such as Iran, Jordan, Saudi Arabia, Iraq, Lebanon, United Arab Emirates and Oman, have a high prevalence of FSGS, with a mean of about 19%, although some countries, such as Bangladesh and Nepal, show more moderate levels. In contrast, East Asian countries, including China, Korea, Japan and Thailand, tend to have a lower prevalence, with an average of approximately 6.5%, with some exceptions, such as Taiwan, where the prevalence is slightly higher.
ChinaThe 20 reports from China span a study period from 1979 to 2020, encompassing data from several medical institutions, with some temporal overlap between studies. Together, these studies analyzed a total of 181,384 samples, with individual sample sizes ranging from 162 to 62,569. The reported prevalence of FSGS remained relatively low, with a mean of 7.01%, and values ranging from 2.45% to 21.6%. Despite this variability, prevalence rates were generally consistent across studies. Based on five studies conducted in the adult population, the prevalence of FSGS has shown slight variations over time. From 1993 to 1997, it was estimated at approximately 3.48%, increasing to 5.47% from 1998 to 2002. From 2003 to 2019, the prevalence remained relatively stable at around 4.53%. Distinguishing differences in prevalence between children and adults is challenging, as many studies analyze both groups together. However, based on data from three studies, the estimated prevalence of FSGS in children (≤14 years old) is approximately 4.67%. In contrast, adults (>14 years old), for whom more reports are available, show a slightly higher mean prevalence of approximately 7.06%.
IndiaStudies conducted in India covered the period from 1990 to 2020, with some temporal overlap between studies, and reported a higher prevalence of FSGS compared to those in China. Across all studies, the overall mean prevalence was 18.26% among the 8325 biopsies analyzed, with values ranging from 8.02% to 22.58%. The number of samples analyzed per study ranged from 65 to 3257. When looking at specific age groups, six studies focusing on the pediatric population (≤14 years old) estimated a prevalence of approximately 14.53%, while eight studies in adults reported a slightly higher prevalence of around 17.05%.
JapanThe studies conducted in Japan covered the period from 2007 to 2021, with some temporal overlap and the inclusion of data from multiple medical institutions, including some large-scale studies. According to these studies, the mean incidence of FSGS was 6.36%, based on an analysis of 49,171 biopsies. The number of samples analyzed per study ranged from 438 to 32,254, with a median of 2802. The overall variation in prevalence was small, ranging from 3.5% to 10.85%. Only one study focused on the pediatric population, reporting a prevalence of 3.7% during the period 2007 to 2017. In contrast, the adult population was analyzed in seven studies, with a mean prevalence of 6.24%.
PakistanThe six studies conducted in Pakistan analyzed a total of 1375 kidney biopsies over a period from 1997 to 2021. Most reports focus on the pediatric population (≤14 years of age) and reported a high prevalence of FSGS, with a mean of 25.54% and a range between 12% and 40.4%. Only one study examined the adult population and reported an even higher prevalence of 30.86%.
South KoreaThe South Korean population studies spanned from 1973 to 2013 and analyzed a total of 6657 biopsy samples. Like China and Japan, the prevalence of FSGS among the Korean population is generally low, at 6.21%. However, this rate varies by age group. In pediatric patients (≤14 years of age), one study reports a prevalence of 4%. Among adults under 65 years of age, two studies estimate it at 5.15%, while among those over 65, the prevalence increases to 12.2%.
IranSimilar to India and Pakistan, the prevalence of FSGS in adult Iranian population is relatively high, averaging 21.63% based on three studies. These studies, conducted between 2006 and 2018, analyzed a total of 4803 biopsy samples.
Saudi ArabiaIn Saudi Arabia, three studies conducted between 1989 and 2020 reported an average FSGS prevalence of 19.3% based on an analysis of 940 biopsy samples.
TurkeyIn Turkey, the mean prevalence of FSGS was approximately 15.22%, based on an analysis of 8381 biopsies in three studies. Two of these studies focused on the pediatric population and reported a mean incidence of 11.88%, while the third study, conducted in adults, found a prevalence of approximately 21.9%.
JordanIn Jordan, the mean prevalence of FSGS is estimated at 18.44% according to three studies. However, the total sample size was relatively small, with only 260 biopsies analyzed between 2006 and 2020. Two studies reported a prevalence of 22% in the pediatric population, while one study in adults found a mean prevalence of 11.32%.
NepalIn Nepal, the prevalence was moderate, estimated at approximately 14.85% based on three studies conducted between 2001 and 2023. However, this finding is based on a limited sample size, with only 549 biopsies analyzed.
EuropeOverall, the prevalence of FSGS in Europe appears to be relatively constant in all countries for which data were available (Italy, Romania, Poland, Spain, Sweden, Czech Republic, Denmark, Serbia, Belgium, France, Germany, Cyprus, Lithuania, Estonia, Croatia and the United Kingdom). In all these regions, prevalence remains moderate, with an average of around 10%.
ItalyThe mean prevalence of FSGS in Italy was about 13.57%, based on 38,228 biopsies analyzed in seven studies conducted between 1970 and 2010, involving multiple medical institutions with some overlap between studies. Based on two studies in pediatric patients, the prevalence in this population was about 10.05%, while based on five studies in adult patients, it was slightly higher at 14.08%. Over time, the prevalence has gradually increased, as indicated by two studies: from 5.2% between 1907 and 1974, to 6.3% between 1975 and 1979, to 6.7% between 1980 and 1984, to 9% between 1985 and 1989, and to 11.2% between 1990 and 1994.
RomaniaIn Romania, the mean prevalence of FSGS was approximately 9.3%, according to four reports covering the period from 1995 to 2023, in which a total of 2127 biopsies were analyzed. Incidence rates varied among the studies, with one reporting an incidence of 0.7 cases per million people per year and another reporting 10 cases per million people per year.
The AmericasAlmost all countries studied in the Americas, including the United States, Brazil, Colombia, Mexico, Uruguay, Peru and Canada, report a high prevalence of FSGS, with some exceptions, such as Chile. Notably, Brazil is the country with the highest prevalence in the region.
BrazilIn Brazil, the prevalence of FSGS was remarkably high, ranking as the second highest among all the countries studied. However, Brazil had a much larger sample size than Nigeria, which reported the highest prevalence. A prevalence of approximately 33.13% was found in 15,666 biopsies analyzed in six studies conducted between 1992 and 2018.
United StatesIn the United States, the prevalence of FSGS was relatively high, with a mean of 20.13% according to six studies analyzing 5173 biopsies collected between 1974 and 2020, with some overlap between studies. The incidence was recorded at approximately 1.6 cases per 100,000 people per year according to two studies, with an increase observed when comparing the periods 1994–2003 and 2004–2013.
ColombiaIn Colombia, the estimated prevalence of FSGS was high, around 20.39%, based on 12,354 biopsies analyzed in four studies conducted between 1998 and 2017. Among the pediatric population (≤15 years old), the prevalence was estimated at 21.9%, while in the adult population it was higher, at 25.17%, based on three studies each.
AfricaIn African countries, prevalence varies from moderate, around 11% in South Africa, Morocco, Chad and Sudan, to high, above 20% in Nigeria and Egypt. However, these estimates are based on a limited number of samples tested.
NigeriaNigeria had the highest prevalence of all the countries studied, with a mean of 33.84%. However, this finding is based on a relatively small sample, with only 360 biopsies analyzed in five studies conducted between 1997 and 2022. Notably, most of these studies focused on children, and only one included both children and adults.
OceaniaIn Oceania, the prevalence of FSGS is relatively high, averaging approximately 18.53%. This estimate is based on an analysis of 4531 samples collected in Australia and New Caledonia between 1982 and 2011.
DiscussionThe prevalence of FSGS varies significantly from region to region (Fig. 2). In general, in Asia, South Asian and Middle Eastern countries have a high prevalence, with an average of about 19%, while East Asian countries have lower rates, with an average of 6.5%. In Europe, the prevalence of FSGS remains relatively uniform across countries, with an average of 10%. In the Americas, most countries have a high prevalence. Africa shows a spectrum of prevalence, ranging from moderate to high rates exceeding 20%, but data are limited. A systematic review and meta-analysis in African populations reported a pooled FSGS prevalence of 26.1%. Prevalence rates were similarly high across sub-Saharan regions [West (34.9%), East (33.5%), and South (34.8%)], while notably lower in North Africa (19.8%).9 Oceania has a relatively high average prevalence of 18.5%.
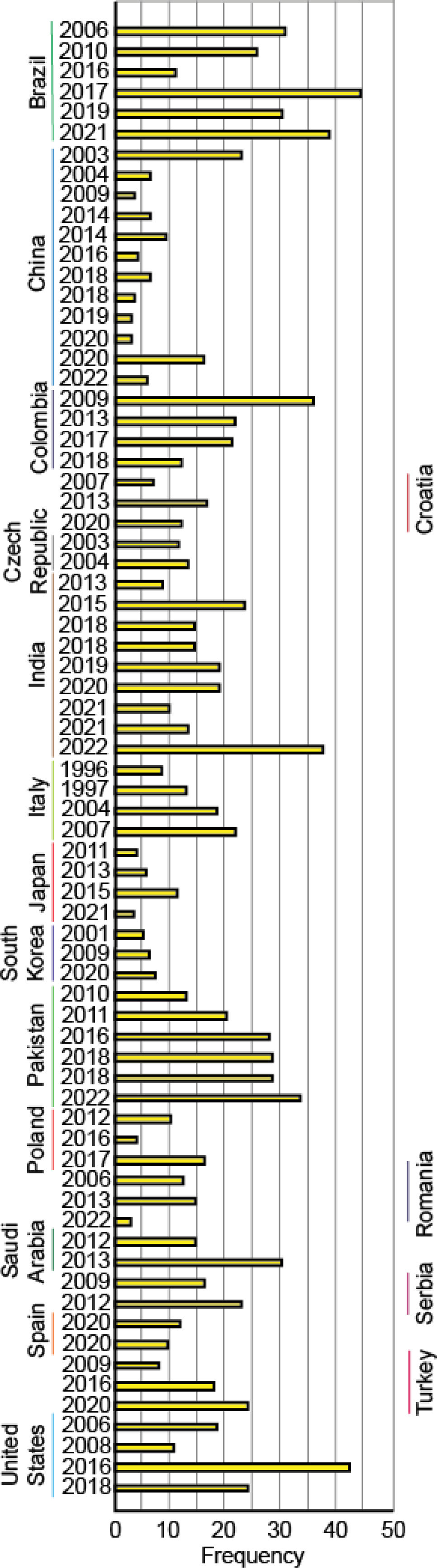
It is important to note that data from many countries are based on limited samples, not numerically representative of the population, which means that actual prevalence rates of FSGS may differ from those reported. In addition, the reported incidence may vary according to the type of healthcare facility, such as primary, secondary or tertiary level hospitals, which may directly influence the observed prevalence of primary and secondary glomerulopathies. Despite these limitations, this study provides valuable information on an estimate of the overall prevalence of FSGS in different countries and, in some cases, highlights trends and changes over time (Fig. 3).
The prevalence of FSGS is influenced by a complex interaction of genetic, dietary, environmental and health factors. An example of this is that in African countries with a high prevalence of FSGS, such as Nigeria and Egypt, a high presence of high-risk variants of the APOL1 gene has been identified.10 In contrast, populations in East Asian countries such as China, Japan and Korea have a lower prevalence of these high-risk APOL1 variants, which may partly explain the lower incidence of FSGS in these regions.11 Dietary patterns also play a critical role. In Western countries, such as the United States and Brazil, diets high in sodium and protein are common. These dietary factors can exacerbate hypertension and hyperfiltration injury, contributing to the higher rates of FSGS observed in these populations.12 In contrast, the diets of East Asian countries, rich in omega-3 fatty acids due to high fish consumption, have anti-inflammatory properties that may provide protective effects against glomerular damage.13 Similarly, the Mediterranean diet, predominant in Southern Europe, is associated with lower rates of cardiovascular and metabolic diseases, key risk factors for kidney disease.14 Additionally, rapid urbanization in countries like India and Brazil has led to lifestyle changes, including increased sedentary behaviors and dietary shifts, which can amplify metabolic risk factors for kidney disease. Notably, countries like Japan and Korea have maintained relatively low obesity rates, which could also contribute to their reduced prevalence of FSGS and other kidney-related conditions.15 It is important to note that disparities in access to genetic testing across countries may also contribute to the higher prevalence of FSGS observed in biopsy samples from lower-resource settings. In wealthier countries, genetic forms of glomerular disease (e.g., APOL1-associated nephropathy) are more often diagnosed through genetic testing, potentially reducing the need for renal biopsy in these cases. As a result, such cases may be underrepresented in biopsy registries from high-resource countries and overrepresented in those from regions with limited access to genetic diagnostics.
Historical reports highlight the global variation in FSGS incidence over time. In 2011, McGrogan et al. documented an incidence of 0.2–1.1 cases per 100,000 person-years,16 while Jegatheesan et al. in 2016 reported an incidence of 1.02 cases per 100,000 person-years.17 In Germany, the incidence increased from 0.1 cases per 100,000 person-years (1990–1997) to 0.6 (2006–2013).18 Similarly, Uruguay reported stable incidence rates across five time periods (1990–2014), ranging between 6.93 and 12.01 per million person-years.19 Italy also observed an increasing trend, with Schena et al. reporting an incidence of 2.3 per million population (pmp) in 1993 and FSGS accounting for 16.6% of nephrotic syndrome cases by 2004.20 Meanwhile, in India, prevalence has varied widely, ranging from 13.1% to 30.6% across different cohorts, reflecting diverse genetic and environmental influences.
Recent trends in FSGS prevalence and incidence reveal changing patterns over time. In North America, Molnár et al. (2023) observed a prevalence of 17.7%, noting a downward trend in the past three years.21 Conversely, Nakagawa et al. (2023) documented stabilization of FSGS incidence in Europe after a decline from 18.6 pmp in 2000 to 14.5pmp in 2013.22 In Latin America, countries like Colombia reported an increase in prevalence from 20.6% in 2013, while in Brazil, prevalence peaked at 55.4% between 2000 and 2005 before declining to 25.8% by 2018.
Age and gender significantly influence FSGS prevalence. Males are predominantly affected, with higher frequencies observed in pediatric populations, particularly among those with corticosteroid-resistant nephrotic syndrome. In contrast, geriatric populations exhibit much lower rates of this podocytopathy.6 A study in Uruguay spanning 25 years (1990–2014) showed FSGS was most prevalent among individuals aged 15–50 years, with declining prevalence in those over 65 years of age.19 Pediatric populations are especially affected, with prevalence rates ranging from 8.5 to 11.6% in Italy, 18.3 to 25.9% in Pakistan, and 28.7% in Colombia, while India reported 9.23%, with 36.2% of cases being steroid-resistant.23–27 A systematic review on the epidemiology of childhood nephrotic syndrome in Africa reported a rise in the prevalence of FSGS from 14% in studies conducted before 1990 to 40% in those published after 1990. Regional differences were also observed, with the highest proportion of FSGS found in Central Africa (33%), followed by Southern (22.8%) and Northern Africa (21.7%). The lowest proportions were reported in Eastern (19.4%) and Western (19.1%) Africa.28
Despite the inherent limitations of data collection and representation, this analysis significantly improves our understanding of global patterns of FSGS. By offering detailed epidemiological insight, it provides clinicians and researchers with a valuable basis for identifying regional trends, underlying risk factors, and variations in prevalence. This information is crucial for tailoring prevention strategies, refining diagnostic approaches, and guiding future research efforts aimed at addressing the global burden of FSGS.
Author contributionsConceptualization, COC and VCV; Methodology, VCV; Formal analysis, COC, VCV and FALR. Investigation, COC and VCV; Data curation, VCV and FALR; Writing-original draft preparation, VCV, AGG and FALR; Writing-review and editing, COC, VCV, AGG and FALR; Project administration, VCV. All authors have read and agreed to the published version of the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflict of interest.
Data availability statementData will be made available on request.