Despite well-documented risks, injectable supplements containing high doses of vitamins are commonly used.
ObjectivesTo describe acute kidney injury (AKI) as a complication of vitamin intoxication.
MethodsOur series consisted of 16 patients with kidney complications resulting from the use of veterinary intramuscular injection supplements of vitamin A, D and E. The patients were admitted to two referral hospitals in Fortaleza (Brazil) between January 2010 and January 2015.
ResultsPatients’ mean age was 28.3±8.9 years (19–53 years), and 11 (68.7%) were male. Main signs and symptoms upon admission were nausea (68.7%), vomiting (62.5%), weight loss (43.7%), epigastric pain (31.2%) and headache (31.2%). At hospital admission the mean laboratory values were: hemoglobin 10±2.0g/dL (6.1–14.2), leukocytes 10,542±4871/mm3 (4100–15,100), creatinine 3.9±5.2mg/dL (0.7–22) and urea 91±88mg/dL (22–306), respectively. Serum calcium was 12±2.2mg/dL (8.8–15.5), 24-h urine calcium was 575±329mg (10.7–1058), serum PTH was 55±141pg/mL (2–406), and serum vitamin D concentration was 135±75ng/mL (22–265). Using KDIGO criteria, AKI was diagnosed in 13 patients (81.2%), classified as stage 1 (n=3), stage 2 (n=3) or stage 3 (n=7). No deaths occurred in the study period.
ConclusionsExcessive use of veterinary vitamin supplements containing high doses of vitamin A, D and E was associated with AKI. Hypercalcaemia, which was a common finding, appears to be a contributing factor to the development of this type of AKI.
Suplementos inyectables que contienen altas dosis de vitaminas son utilizados con frecuencia, a pesar de los riesgos bien documentados.
ObjetivoDescribir la ocurrencia de daño renal agudo (IRA) como complicación de intoxicación por suplementos vitamínicos.
MétodosEsta es una serie de 16 pacientes con complicaciones renales resultantes de la utilización de inyección intramuscular de suplementos veterinarios con vitaminas A, D y E. Los pacientes fueron ingresados en 2 hospitales de referencia en Fortaleza (Brasil), entre enero de 2010 y enero de 2015.
ResultadosLa edad media de los pacientes fue de 28,3±8,9 años (19–53 años) y 11 (68,7%) eran varones. Signos y síntomas principales al ingreso fueron náuseas (68,7%), vómitos (62,5%), pérdida de peso (43,7%), dolor epigástrico (31,2%) y cefalea (31,2%). Al ingreso en el hospital los valores medios de laboratorio fueron: hemoglobina 10±2,0g/dL (6,1–14,2), leucocitos 10.542±4.871/mm3 (4.100–15.100), creatinina 3,9±5,2mg/dL (0,7–22) y urea 91±88mg/dL (22–306), respectivamente. El nivel de calcio sérico fue de 12±2,2mg/dL (8,8–15,5), el de calcio en orina de 24h fue de 575±329mg (10,7–1.058), el de PTH sérico fue de 55±141pg/mL (2–406) y el nivel de vitamina D sérica fue de 135±75ng/mL (22–265). Utilizando criterios KDIGO, se diagnosticó IRA en 13 pacientes (81,2%); fueron clasificadas como clase 1 (n=3), clase 2 (n=3) y clase 3 (n=7). No hubo muertes en el período de estudio.
ConclusionesEl uso excesivo de suplementos vitamínicos veterinarios que contienen altas dosis de vitamina A, D y E se asoció con IRA. La hipercalcemia, un hallazgo común, parece ser un factor que contribuye al desarrollo de este tipo de IRA.
Body sculpting is an increasingly common practice in modern society.1 Some enthusiasts resort to substance abuse to boost results, despite the serious health risks involved. A number of commercially available substances, from anabolic steroids (hormones promoting muscle anabolism) to oily compounds (increasing muscle volume by way of retention, without contributing to anabolism), are known to cause severe damage to the organism.2
One such substance is the compound “ADE”, a veterinary product containing liposoluble vitamin A, D and E, indicated for the treatment of vitamin deficiency and infection in cattle and horses. According to the manufacturers, animals should not be injected with more than 5mL per 120-day period of fattening. However, much higher doses are used by human body sculptors.2 The injection of ADE produces a local granulomatous reaction and encapsulation. If encapsulation fails, or if the substance enters the blood stream, embolism may occur, in some cases followed by death.2 In Brazil, human ADE use was first described in the late 1980s, but may have started earlier.3
The purpose of the present study was to evaluate the occurrence of acute kidney injury (AKI) or chronic kidney disease (CKD) in a series of patients admitted to two Brazilian referral hospitals due to complications from excessive and prolonged intramuscular injection of ADE vitamins.
Patients and methodsStudy designThis is a descriptive study based on a case series. We have evaluated a series of 16 patients with kidney complications resulting from the use of veterinary intramuscular vitamin supplements containing high doses of vitamin A, D and E. The patients were admitted to two referral hospitals in Fortaleza (Northeastern Brazil) between January 2010 and January 2015. Patients with a history of renal failure, hypertension, diabetes mellitus, nephrolithiasis, nephrotoxic drug use, or any comorbidity potentially detrimental to renal function, were excluded from the analysis.
Vitamin supplements usedAll studied patients have used vitamin supplements containing vitamins A, D and E. In each 100mL the following composition was observed: vitamin A 20,000,000IU, vitamin D 35,000,000IU and vitamin E 6000IU, which represents a very high dose, higher than the recommended daily intake for humans.
Study parametersInformation was collected on demographics, length of hospital stay, clinical manifestations, laboratory findings, need for dialysis, treatment and mortality. Clinical information included all clinical signs and symptoms registered upon admission and during hospitalization. The laboratory tests included complete blood count, serum urea, creatinine, sodium, potassium, calcium, aspartate aminotransferase, alanine aminotransferase, parathyroid hormone (PTH), vitamin D and urinalysis. Twenty-four-hour urinary calcium levels were also registered.
DefinitionsAKI and CKD were diagnosed and classified according to KDIGO criteria.4 Due to lack of information on serum creatinine levels prior to admission, all patients were assumed to have a baseline glomerular filtration rate of 100mL/min/m2. The patients were classified as the highest KDIGO stage observed during hospitalization. Oliguria was defined as a urine output <0.5mL/kg/h. Dialysis was indicated for patients who remained oliguric after effective hydration for at least 24h, for refractory hypervolemia, severe metabolic acidosis or uremia. High blood pressure was defined as systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mmHg.
Ethical aspectsThe study protocol was reviewed and approved by the Research Ethics Committees of the General Hospital of Fortaleza and the Walter Cantídio University Hospital.
ResultsPatients’ mean age was 28.3±8.9 years (19–53 years), and 11 (68.7%) were male. Mean length of hospital stay was 15±13 days (1–52 days). Upon admission, all patients reported a history of intramuscular injection of vitamin supplements containing vitamins A, D and E for esthetic purposes. Six patients reported concurrent use of anabolic steroids. All were body building practitioners and attended gyms regularly. The median time between the last injection and hospitalization was 10 months (range: 1–48 months). The median time between the first use and hospitalization was 28 months (range: 4–60 months). Table 1 summarizes patients’ clinical and laboratory characteristics.
Clinical and laboratory characteristics of 16 patients with acute kidney injury due to vitamin supplements abuse.
| n | % | |
|---|---|---|
| Age (years) | 28.3±8.9 | – |
| Gender | ||
| Male | 11 (68.7%) | 68.7 |
| Female | 5 (31.3%) | 31.3 |
| Length of hospital stay (days) | 15±13 | – |
| Median time between first use and admission (months) | 28 | – |
| Signs and symptoms at admission | ||
| Nausea | 11 | 68.7 |
| Vomiting | 10 | 62.5 |
| Weight loss | 7 | 43.7 |
| Epigastric pain | 5 | 31.2 |
| Headache | 5 | 31.2 |
| Fever | 4 | 25 |
| Paresthesia (limbs) | 4 | 25 |
| Polyuria | 3 | 18.7 |
| Syncope | 3 | 18.7 |
| Anorexia | 3 | 18.7 |
| Low back pain | 2 | 12.5 |
| Edema | 2 | 12.5 |
| Nocturia | 2 | 12.5 |
| Pallor | 2 | 12.5 |
| Gynecomastia | 1 | 6.2 |
| Lymphadenopathy | 1 | 6.2 |
| AKI | 13 | 81.2 |
| Laboratory tests | ||
| Hbadm (g/dL) | 10±2.0 | – |
| Leukocytesadm (1×/mm3) | 10,542±4871 | – |
| Cradm (mg/dL) | 3.9–5.2mg | – |
| Crmax (mg/dL) | 4.3±5.2 | – |
| Crdis (mg/dL) | 1.6±1.8 | – |
| Uradm (mg/dL) | 91±88 | – |
| Urmax (mg/dL) | 102±86 | – |
| Urdis (mg/dL) | 45±28 | – |
| Caadm (mg/dL) | 12–2.2 | – |
| Camax (mg/dL) | 13±2.2 | – |
| 24h-Ca (mg/dL) | 575±329 | – |
| PTH (pg/mL) | 55±141 | – |
| Vitamin D (ng/mL) | 135±75 | – |
Mean±SD and %.
Hb, hemoglobina (g/dL); Ur, urea (mg/dL); Cr, creatinine (mg/dL); Ca, calcium (mg/dL); 24h-Ca, 24h calciuria (mg/dL); adm, at admission; max, maximum at hospital stay; dis, at hospital discharge.
Main signs and symptoms upon admission were nausea (68.7%), vomiting (62.5%), weight loss (43.7%), epigastric pain (31.2%) and headache (31.2%), fever (25%), paresthesia in the upper and lower limbs (25%), polyuria (18.7%), syncope (18.7%) and anorexia (18.7%). Patients also reported low back pain, edema, nocturia and pallor (12.5% each), and gynecomastia and lymphadenopathy (6.2% each).
At hospital admission the mean laboratory values were: hemoglobin 10±2.0g/dL (range 6.1–14.2), leukocytes 10,542±4871/mm3 (4100–15100), creatinine 3.9±5.2mg/dL (0.7–22) and urea 91±88mg/dL (22–306), respectively. Serum calcium level was 12±2.2mg/dL (8.8–15.5), 24-h urine calcium level was 575±329mg (10.7–1058), median PTH level was 55±141pg/mL (2–406), and serum vitamin D concentration was 135±75ng/mL (22–265). During hospital stay maximum creatinine ranged from 0.8 to 8.6mg/dL (mean 4.3±5.2mg/dL) and serum calcium from 10.2 to 16.8mg/dL (mean 13±2.2mg/dL). Laboratory findings for each patient are summarized in Table 2.
Laboratory tests during hospital stay of 16 patients with kidney injury due to vitamin supplements abuse.
| # | Age | Gender | Ht adm | Hb adm | WBC adm | Platelets adm | Ur adm | Ur max | Ur dis | Cr adm | Cr max | Cr dis | AST/ALT adm | AST/ALT max | AST/ALT dis | Na adm | K adm | Ca adm | Ca max | Ca dis | Vit D | 24h calciuria | PTH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | F | 27.5 | 9.2 | 7584 | 397,709 | 30 | 45 | 33 | 1.6 | 1.6 | 1.1 | 21/14 | – | – | 140 | 3.5 | 11.9 | 12.4 | 11.1 | >150 | – | – |
| 2 | 29 | M | 30 | 10.1 | 8214 | 463,100 | 62 | 73 | 66 | 1.6 | 2.4 | 1.7 | 19/35 | 25/52 | 20/52 | 139 | 4.4 | 11.7 | 14.6 | 10 | >150 | 499.8 | 3.4 |
| 3 | 34 | F | 26.5 | 8.9 | 12,530 | 288,800 | 46 | 80 | 78 | 2.5 | 2.5 | 1.4 | 24/14 | 47/26 | – | 146 | 3.5 | 15.5 | 16.8 | 10.1 | – | 422.8 | 7 |
| 4 | 23 | M | 34.7 | 11.6 | 4100 | 200,000 | 61 | 61 | 48 | 2.5 | 2.5 | 1.4 | 117/73 | 117/73 | – | 139 | 4 | – | 12.8 | 9.1 | – | – | – |
| 5 | 29 | F | 33.3 | 11.5 | 10,800 | 313,000 | 32 | 32 | 30 | 1.4 | 1.5 | 1.2 | 58/75 | 58/75 | – | 136 | 2.9 | 11.8 | 12.8 | 11.7 | 182.4 | 484.16 | 8.41 |
| 6 | 28 | M | 29.6 | 9.16 | 6060 | 286,000 | 32 | – | 29 | 1.1 | – | 0.9 | – | – | – | 139 | 3.8 | 9.5 | – | 8.8 | 154.4 | – | – |
| 7 | 19 | M | 34.8 | 11.8 | 23,900 | 594,000 | 54 | 35 | 30 | 3.5 | 3.5 | 0.8 | – | – | – | 137 | 4.2 | 13.3 | 13.3 | 7.9 | – | 1058 | – |
| 8 | 45 | F | 34.4 | 11.5 | 10,540 | 250,000 | 32 | 57 | 15 | 1.5 | 1.5 | 0.8 | – | 15/22 | 15/22 | 139 | 3.8 | 11 | 11.3 | 9.8 | >160 | 428.4 | – |
| 9 | 26 | M | – | – | 5500 | – | 55 | 77 | 15 | 2.7 | 3.7 | 0.6 | – | – | – | 135 | 3 | 10.6 | 16.8 | 8 | 116.7 | 1039.5 | – |
| 10 | 53 | M | 27.7 | 9.44 | 15,100 | 320,000 | 68 | 68 | 42 | 3.2 | 3.2 | 1.3 | 40/32 | 50/25 | 35/22 | 139 | 4.8 | 14 | 14 | 9.6 | 160 | 536.3 | 5.15 |
| 11 | 24 | M | 30.6 | 10.9 | 8350 | 60,000 | 200 | 200 | 56 | 8.6 | 8.6 | 1.8 | 30/22 | – | – | 141 | 3.4 | 13.8 | 14.2 | – | 53.9 | 330.2 | 6.32 |
| 12 | 21 | M | 43.9 | 14.2 | 13,000 | 247,000 | 22 | 28 | 16 | 0.7 | 1 | 0.7 | 33/44 | 33/44 | 22/30 | 140 | 4.6 | 9.2 | 10.2 | 10 | – | – | – |
| 13 | 25 | F | 25.3 | 8.02 | 7480 | 457,000 | – | – | – | 0.79 | 0.8 | 0.8 | – | – | – | – | – | 10.01 | 10.73 | 10.73 | 129.1 | – | 2 |
| 14 | 25 | M | 19.8 | 6.1 | 12,540 | 31,200 | 247 | 247 | 56 | 5.8 | 5.8 | 1.3 | 31/29 | 39/48 | – | 148 | 4.9 | 15 | 15 | 12 | – | – | – |
| 15 | 24 | M | 20.4 | 6.8 | 12,440 | 114,600 | 306 | 306 | 124 | 22 | 22 | 8.5 | – | – | – | 120 | 4.6 | 8.8 | 9 | 8.6 | 22.5 | 10.73 | 406 |
| 16 | 27 | M | 33 | 10.8 | – | – | 122 | 122 | 37 | 4.1 | 4.1 | 1.3 | 43/45 | 43/111 | 36/111 | 136 | 5.3 | 14.9 | 14.9 | 9.3 | 265.25 | 1012 | 4.1 |
Ht, hematocrit (%); Hb, hemoglobina (g/dL); WBC, white blood count (1×/mm3); platelets, 1×/mm3; Ur, urea (mg/dL); Cr, creatinine (mg/dL); AST, aspartate aminotransferase (IU/L); ALT, alanine aminotransferase (IU/L); Na, sodium (mEq/L); K, potassium (mEq/L); Ca, calcium (mg/dL); Vit D, vitamin D (ng/mL); PTH, parathyroid hormone (pg/dL); adm, at admission; max, maximum at hospital stay; dis, at hospital discharge.
Three patients had imaging findings compatible with nephrocalcinosis and seven had nephrolithiasis. Using KDIGO criteria, AKI was diagnosed in 13 cases (81.2%) and classified as stage 1 (23%), stage 2 (23%) or stage 3 (54%). No deaths occurred in the study period.
Treatment consisted primarily of controlling calcium levels and complications such as acute pancreatitis due to hypercalcemia (18.7%) and local infections (18.7%) resulting from intramuscular injections of vitamin supplements without sterilization. Hypercalcemia was treated with vigorous hydration, steroids and loop diuretics. Due to severe AKI, two patients required dialysis. One (#11) received 5 hemodialysis sessions during hospitalization. The other (#15) developed chronic kidney disease and was started on regular hemodialysis three times a week. Six previously non-hypertensive patients presented hypertensive peaks upon admission. Hypertension persisted in four cases who were discharged with prescriptions for anti-hypertensive drugs.
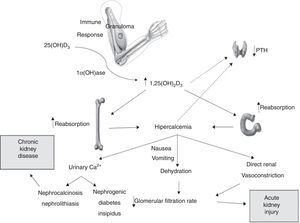
DiscussionA considerable number of patients with severe kidney complications (including chronic kidney failure) from excessive use of vitamin supplements are described in this study. Kidney injury due to vitamin abuse is seldom reported in literature and it seems to be a frequent complication, at least among patients taking very high doses of vitamins (veterinary doses).5 The possible mechanisms involved in the pathophysiology of kidney injury associated with hypercalcemia and injectable vitamins A, D and E supplements is schematized in Fig. 1.
As in other granulomatous conditions, such as tuberculosis, sarcoidosis and fungal infections, vitamin D is converted extra-renal into its active form by granuloma cells (especially macrophages). This increases the intestinal absorption of vitamin D-dependent calcium and raises serum calcium levels.6 Hypercalcemia and high level of 1.25(OH)2D3 concentrations have a negative impact on the parathyroid glands, reducing PTH production and serum levels.7 This pattern was observed in most of our patients and in most patients reported in the literature.5,8–10
On the other hand, some believe hypervitaminosis is the result of the absorption of injected vitamin D. Further studies are necessary to clarify the mechanisms involved, but the fact that a similar reaction occurs when other substances (such as paraffin) are injecting into the muscle, along with the observed late onset of hypercalcemia (months later), point to granuloma formation and extra-renal conversion as the most likely explanation.3 If the absorption of injected vitamin D were the culprit, the onset of hypercalcemia would be acute.11–13
The effects of hypervitaminosis A and E in humans are less studied than hipervitaminosis D. It is known that after consumption of high doses of vitamin A the concentrations of retinoids and metabolites are elevated in plasma. Toxicity following high consumption of vitamin A can be acute (intake >660,000IU) or chronic, when intake lasts for months or years (a dose >100,000IU for more than 6 months is considered toxic),14,15 and our patients consumed a dose very higher than the levels considered toxic. The main effects of hypervitaminosis A are related to bone metabolism. There is association between high vitamin A intake and osteoporosis and pathological fractures.14 Other signs and symptoms of vitamin A toxicity includes include dry skin, nausea, headache, fatigue, irritability, anorexia, liver disease, hair loss and alopecia, hyperostosis, high cholesterol and increased cerebrospinal fluid pressure.15 Hypervitaminosis E is rare, but it is possible to be more frequent once consumption of this vitamin is increasing due to its potentially antioxidant and antiatherogenic effects. Toxic effects of hypervitaminosis E includes prolongation of prothrombin time and hemorrhage (due to antagonist effects of vitamin E against vitamin K), hypertension, angina and stroke.16 Our patients had nausea, vomiting and headache, which could be attributable to hypervitaminosis A and E, but these symptoms could also be consequence of AKI-associated uremia.
In the present case series, kidney injury ranged from relatively mild and reversible acute injury to severe disease progressing to end-stage chronic kidney failure (as observed in patient #15). Persistent hypercalcemia with serum calcium levels >11mg/dL may in up to 20% of cases develop into nephrogenic diabetes insipidus, which usually reverts with the resolution of the hypercalcemic condition. Though not yet fully understood, the mechanism of nephrogenic diabetes insipidus possibly involves the inhibition of reabsorption of sodium chloride in the thick ascending limb of the loop of Henle (in detriment to countercurrent exchange) and of vasopressin-mediated water permeability in the terminal collecting duct.17
Hypercalcemia has been shown to downregulate aquaporine 2 protein expression in rat kidney medulla, interfering with the ability to concentrate urine.18 Calcium deposition in the medulla (a cause of tubulointerstitial injury) appears to play an important role in this process.19 The activation of calcium-sensing receptor can affect the urinary-concentrating mechanism by acting in both the loop of Henle and the collecting tubules.20
Hypercalciuria secondary to hypercalcemia also leads to the deposition of calcium in the renal parenchyma, eventually causing nephrocalcinosis and irreversible damage and predisposing toward the development of chronic nephropathy. When the concentration of calcium phosphate exceeds solubility, Randall's plaques are formed in the basement membranes of the thin limbs of the loop of Henle in the inner medullary interstitium.21–23 These calcium phosphate plaques can extend into the surrounding interstitial tissue, producing medullar nephrocalcinosis, or penetrate the urothelium where they are believed to provide a nidus for intratubular stone formation, leading to nephrolithiasis, obstructive uropathy and kidney injury.24
Thus, nephropathy induced by vitamin supplement abuse can indeed lead to chronic kidney disease. This sheds light on the findings and outcome of patient #15 in our series. Unlike the other patients, #15 had low calcium and vitamin D levels and high PTH levels. Combined with anemia and the need for persistent dialysis, these findings are an indication of terminal chronic kidney injury. In the absence of other offending factors, it may be inferred that the condition of this 25-year old patient was specifically the result of vitamin supplement abuse.
Most of our patients experienced acute kidney injury, but did not develop chronic kidney disease (nephrocalcinosis or hydronephrosis). The observed kidney injury may have involved other mechanisms. Thus, serum calcium levels in excess of 12–15mg/dL can acutely induce a reversible decrease in glomerular filtration by direct renal vasoconstriction of the arterioles and decreased extracellular fluid volume due to anorexia, vomiting and inability to concentrate urine (nephrogenic diabetes insipidus).25 Vomiting was a common symptom in our patients, most likely due to hypercalcemia. In such cases, kidney function is often fully restored by early treatment. On the other hand, non-renal function recovery or partial recovery in patients with chronic hypercalcemia with tubular degeneration, fibrosis and nephrocalcinosis are observed.26
High blood pressure observed in these patients is due mainly to hypercalcemia or kidney injury. Hypercalcemia affects the vascular smooth muscle directly increasing vascular resistance, and indirectly increasing catecholamines levels. However, the impact is much stronger on renal vascular resistance than on peripheral vascular resistance.27 There are also reports associating vitamin A intoxication with hypercalcemia.28,29 Its mechanism is still poor understood and may include up-regulation of osteoclasts by retinol metabolites.29
In conclusion, excessive use of veterinary vitamin supplements that containing high doses of vitamin A, D and E is associated with AKI and prolonged use with CKD. Hypercalcemia, a common finding, appears to be a contributing factor for this outcome. The importance of this report is to alert the medical community to the ongoing and dangerous practice of vitamin and supplement abuse by young individuals.
Study limitationsAs this is a descriptive study, there are some limitations. We have described a small number of patients, but this is representative of a rare complication (AKI-associated to vitamin supplements abuse), which is still not well described in literature. Some laboratory tests, such as creatine kinase (CK), to rule out rhabdomyolysis, and serum vitamin D were not available for all patients due to technical and economic problems. It was not possible to perform a powerful statistical analysis, so it was not possible to draw statistically significant associations between possible causal factors for AKI.
Conflict of interestThe authors have no conflicts of interest to disclose.