Kidney disease is frequent in HIV-patients. We present a case of a 44-year-old woman, with known uncontrolled HIV infection and chronic kidney disease due to HIV-associated nephropathy. After starting dolutegravir, the patient developed eosinophilia and worsening kidney function. A kidney biopsy confirmed the diagnosis of acute interstitial nephritis. Given the time relation with dolutegravir introduction, it was deemed the culprit medication. Dolutegravir was stopped, and corticosteroids were initiated, with moderate improvement in renal function. To our knowledge, this is the first reported case of acute interstitial nephritis to dolutegravir, which should raise awareness of previously undocumented renal effects of antiretroviral therapy.
La enfermedad renal es frecuente en pacientes con VIH. Presentamos el caso de una mujer de 44 años, con infección por VIH no controlada conocida y enfermedad renal crónica por nefropatía asociada al VIH. Después de comenzar con dolutegravir, el paciente desarrolló eosinofilia y empeoramiento de la función renal. Una biopsia de riñón confirmó el diagnóstico de nefritis intersticial aguda. Dada la relación temporal con la introducción de dolutegravir, se consideró al medicamento el culpable. Se interrumpió el tratamiento con dolutegravir y se iniciaron corticosteroides, con una mejoría moderada de la función renal. Hasta donde sabemos, este es el primer caso notificado de nefritis intersticial aguda por dolutegravir, lo que debería crear conciencia sobre los efectos renales previamente indocumentados de la terapia antirretroviral.
Kidney disease is increasingly frequent in HIV-patients, in an era where antiretroviral therapy (ART) complications are replacing opportunistic infections as the leading cause of morbidity and mortality in those patients. HIV-patients are at increased risk for kidney disease not only for ART nephrotoxicity, but also HIV-associated nephropathy (HIVAN), immune complex diseases and other comorbidities, such as diabetes or hypertension.1 Clinicians must be aware of the newly widely available antiretroviral agents’ renal complications. The authors present a case of worsening kidney function related to ART, which is, to the best of our knowledge, the first described case of acute interstitial nephritis (AIN) to dolutegravir (DTG).
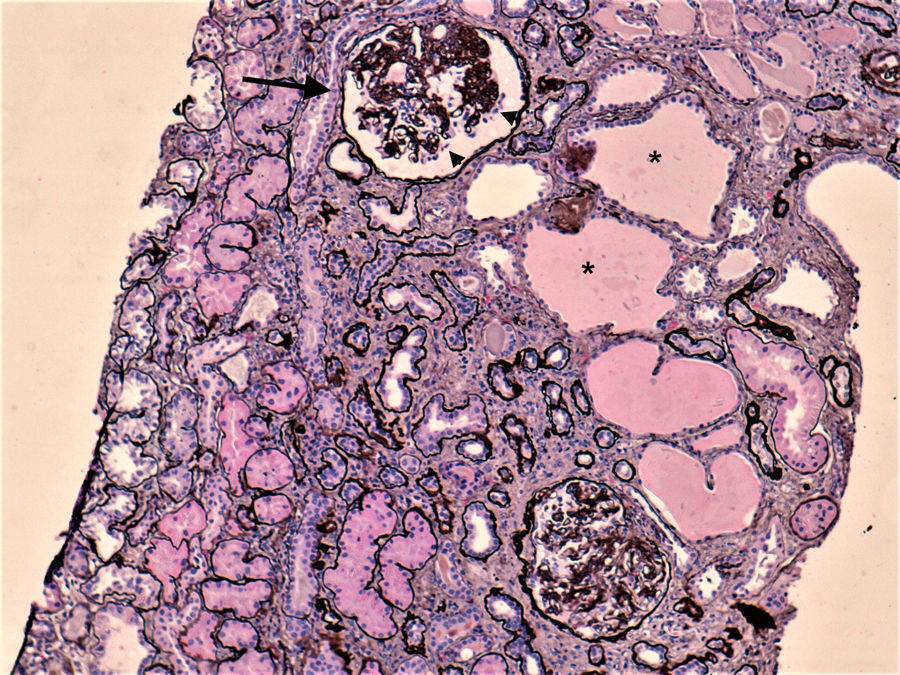
Case reportWe present a case of a 44-year-old woman with past medical history of HIV-1 infection for 14 years, with suboptimal adherence and persistent uncontrolled viremia. She had multiple antiviral resistances, with several therapy adjustments throughout the years. She also had a stage III chronic kidney disease (CKD) due to biopsy-proven HIVAN (Fig. 1), with baseline serum creatinine (sCr) of 1.88mg/dL (estimated glomerular filtration rate [eGFR)] of 32mL/min./1.73m2); hypertension, at the time controlled without medication; dyslipidemia, treated with pitavastatin (1mg/day); and depression disorder, treated with sertraline (100mg/day).
Four months before the current episode, ART was adjusted due to a raise in viremia and new drug resistance profile, adding DTG (50mg/day) to abacavir (ABC) [600mg/day], lamivudine (3TC) [300mg/day] and darunavir/cobicistat (DRV/c) [800/150mg/day]. After initiation, the patient complained of generalized pruritus, and blood analysis revealed worsened kidney function (sCr 2.5mg/dL) and de novo eosinophilia (1.42×109/L). After temporary suspension of DTG, with slight improvement of renal function (sCr 2.15mg/dL), DTG was restarted under close clinical surveillance.
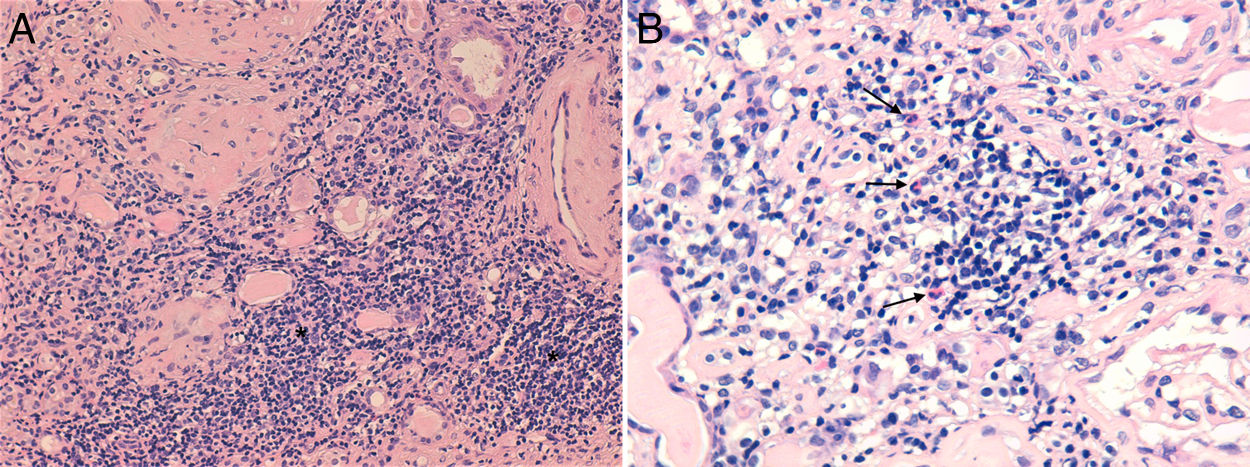
Two months after the reintroduction of DTG, the patient sought medical attention for itching, hyperpigmented macules on both feet and left groin, and vomits. She denied other complaints. At the time, her renal function had deteriorated further (sCr 6.87mg/dL). Suspecting of superimposed bacterial infection of the groin lesions, the patient was treated with oral flucloxacillin, with no improvement. She was admitted to the hospital for further treatment. The urinalysis showed proteinuria of 600mg/dL and discrete erytrocituria and leucocyturia. A renal ultrasound excluded obstruction. The serologic study of infectious and autoimmune causes of AKI was unremarkable, apart from slightly elevated anti-streptolysin O antibody titer (ultimately considered a false positive). After adequate hydration, there was no recovery of renal function. Kidney biopsy (Fig. 2) revealed intense mononuclear interstitial infiltrate, along with eosinophils, findings consistent with AIN. There also moderate interstitial fibrosis ant tubular atrophy (about 40%), apart from the already diagnosed HIVAN. Skin lesion biopsy showed hyperkeratosis, acanthosis, and an unspecific mono and polynuclear infiltrate, consistent dyshidrotic eczema was made.
Since the beginning of symptoms were coincident with DTG introduction, along with eosinophilia and renal function worsening, it was deemed the culprit drug. DTG was stopped, and corticosteroids were started 3 weeks after the begging of symptoms (intravenous methylprednisolone, 250mg for 3 days, followed by oral prednisolone, 30mg, tapered over one month). After a slight improvement in renal function (sCr 4.13mg/dL), the patient was discharged, and raltegravir (RAL) was added to her previous ART, replacing DTG.
Unfortunately, the patient's renal function did not return to its previous baseline level, with a nadir sCr of 3.61mg/dL. It further deteriorated after an episode of urinary infection, and renal replacement therapy was started almost 1 year after the initial admission due to AIN.
DiscussionHIV-patients are at increased risk of either acute and chronic kidney injury. Even though HIVAN is the most classical kidney manifestation in these patients, it has become less common with the advent of ART, with increased prevalence of drug related nephrotoxicity.1
Some ART drugs have well documented renal complications, like tenofovir disoproxil fumarate (TDF), which is associated with acute tubular injury, tubule-interstitial nephritis, and proximal tubulopathy; or protease inhibitors lopinavir, atazanavir and indinavir, which are associated with renal stones formation. Moreover, some ARV drugs, like cobicistat, are known to reversibly inhibit tubular creatinine secretion, increasing its serum levels, and prompting a misdiagnose of renal disfunction.2
DTG is a second generation protease inhibitor, given in a dosage of 50mg/day (or twice daily, if there are known mutations in integrase).3 The renal effects of DTG have been widely studied.4 A study on the effect of DTG in two different dosages (50mg daily and 50mg twice daily) on glomerular filtration and renal blood flow concluded that DTG did not have an impact on those parameters, although it may increase sCr by 10–14%.5 That effect is a direct consequence of DTG inhibition of OCT2 (a renal transporter).3,6 Additional studies of renal effects of DTG, as SPRING-1 trial and SPRING-2 trial, also reported a mild, nonprogressive increase in Scr, without the need to stop DTG.7,8
Drug-induced AIN represents about 20% of causes of unexplained acute kidney injury (AKI), and can also lead to CKD. Though almost every drug can cause AIN, the most common culprits are antibiotics, non-steroidal anti-inflammatory drugs, and proton pump inhibitors. While some patients present with AKI, others show a slower but progressive deterioration of renal function. Systemic manifestations of AIN are often discreet and unspecific. Urinary findings are also unreliable, ranging from normal to the classical findings of eosinophiluria, pyuria and leucocytes.9,10 However, even though studies have shown a correlation between urine eosinophils and AIN, it is a poor diagnostic tool to distinguish AIN from other causes of AKI.11 The definite diagnosis relies on kidney biopsy.
The treatment for AIN consists in early detection and discontinuation of the causative drug, as earlier suspension is associated with better renal outcomes. The use of corticosteroids is not consensual, although several studies show beneficial results in patients treated with corticosteroids.10 An observational study detected no difference in median sCr in patients who received steroids therapy versus patients on conservative therapy.12 Another observational retrospective study with one of the largest series of biopsy-proven AIN (61 patients) found a significant difference in complete renal recovery in patients treated with steroids (54%) compared to patients who did not receive it (33%).13 Furthermore, better results seem to be related to early initiation of steroids, preferably in 1 or 2 weeks after the diagnosis.9
Despite the conflicting evidence, many centers would treat patients with corticosteroids when kidney function does not improve after withdrawing the drug, and in patients with less than 75% interstitial fibrosis/tubular atrophy on kidney biopsy. When used, steroids dose is not consensual, with many studies using 250–500mg intravenous methylprednisolone followed by 1mg/kg/day of oral prednisone, or only oral prednisone alone. The duration of treatment is also controversial, but it should not be continued beyond 1–1.5 months.9
As far as we are aware, no other renal manifestation associated with DTG have been reported. However, we highlight a postmarketing pharmacovigilance report from Food and Drug Administration about a case of a 12-year-old boy with AIN after introduction of ABC/DTG/3TC. However, more information is lacking, including concomitant medication and follow-up. So, although it could represent a case of AIN to DTG, more information is needed to make that conclusion.14
Our case represents the first report of AIN to DTG. Although it is difficult to be ascertain of the culprit drug in those cases, many features suggest that DTG was the causative agent. First, pruritus and eosinophilia started immediately after DTG introduction, suggesting an ongoing allergic reaction. There was also a slight increase in sCr, which could also be the effect of DTG on tubular creatinine secretion. It should be noted that no other drug had been started at that time. Furthermore, the complaints improved with first suspension, and came back after its reintroduction, this time along with severe AKI, supporting our diagnostic hypothesis. It is worth noting that flucloxacillin was introduced after the patient had already systemic allergic manifestations and severe AKI, making this antibiotic a poor candidate as culprit drug.
The serologic study was inconclusive, and the urinary abnormalities unspecific. Kidney biopsy confirmed the diagnosis. The advanced interstitial fibrosis and tubular atrophy found may reflect the progression of her previous diagnosed kidney disease, in a non-adherent patient. However, it may also be a consequence of an AIN that was developing since its introduction, with initial acute inflammation giving way to fibrosis.
The decision to treat this patient with corticosteroids was not straightforward, as there was significant fibrosis on kidney biopsy, and the risks associated with this medication (namely poor glycemic control and further immunosuppression). We decided to treat with methylprednisolone pulses, and give an inferior dose of prednisone (0.5mg/kg/day). The sCr improvement may reflect either the discontinuation of DTG, or the effects of corticosteroids. Given the amount of fibrosis, and the time elapsed since the begging of symptoms, prolongation of the corticosteroid treatment probably wouldn’t have achieved a greater recovery of the renal function. Despite its initial partial recovery, suggesting some response to our therapeutic approach, the patient renal function kept deteriorating during the following months. It should be noted that the patient was taking cobicistat, known to increase sCr, as explained. However, the had started it long before the present clinical condition.
In conclusion, our case should raise awareness to this previously undocumented renal effect of ART, and a need for monitoring of renal function. We believe that a serial measurement of sCr, serum ionogram and urinalysis may help early detection of renal disfunction, especially after recent introduction of new drugs.
Informed consentInformed consent was obtained from the individual participant whose case was reported.
All authors have seen this version, discussed the results and contributed to the final manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declared no potential conflicts of interest with respect to the research, authorship or publication of this article.