Glomerulonephritis associated with celiac disease (CD) is rarely reported. Here we report a rare case of nephrotic syndrome associated with C3 glomerulopathy (C3G) in a patient with CD. A 19-year-old female with bilateral leg swelling and hypoalbuminemia was referred with an initial diagnosis of nephrotic syndrome. She had also abdominal pain and diarrhea. First a duodenal biopsy confirmed the diagnosis as CD. Nephrotic range proteinuria was detected so then a percutaneous renal biopsy was performed. Renal biopsy revealed membranoproliferative glomerulonephritis (MPGN) with C3 staining. Introduction of a gluten-free diet, ramipril, steroid and mycophenolic acid ameliorated clinical and laboratory parameters at three years follow up. Edema and hypoalbuminemia are common sharing clinical and laboratory parameters presented in not only patients with CD but also with glomerulonephritis. Urine analysis is very important in patients with CD to detect proteinuria. It is important to keep the possible link between CD and glomerulonephritis in mind for clinicians when working up a patient with diarrhea and nephrotic syndrome.
La glomerulonefritis asociada con la enfermedad celíaca (EC) se reporta raramente. Aquí informamos un caso raro de síndrome nefrótico asociado con glomerulopatía C3 (C3G) en un paciente con EC. Una mujer de 19 años con edema bilateral de piernas e hipoalbuminemia fue referida con un diagnóstico inicial de síndrome nefrótico. También presentó dolor abdominal y diarrea. Primero, una biopsia duodenal confirm el diagnóstico como EC. Se detectó proteinuria en rango nefrótico, por lo que se realizó una biopsia renal percutánea. La biopsia renal reveló glomerulonephritis membranoproliferativa (GNMP) con tinción C3. La introducción de una dieta libre de gluten, ramipril, esteroides y ácido micofenólico mejoró los parámetros clínicos y de laboratorio a los tres años de seguimiento. El edema y la hipoalbuminemia son parámetros clínicos y de laboratorio comunes que se presentan no solo en pacientes con EC sino también con glomerulonefritis. El análisis de orina es muy importante en pacientes con EC para detectar proteinuria. Es importante que los médicos Tengan presente el posible vínculo entre la EC y la glomerulonefritis cuando evalúan a un paciente con diarrea y síndrome nefrótico.
Celiac disease (CD) is an autoimmune disorder characterised by malabsorption and chronic inflammation of the small intestinal mucosa, triggered by oral intake of gluten. CD is one of the most common autoimmune disorders, with a reported prevalence of 1–0.5% of the general population.1 Glomerulonephritis associated with celiac disease is rarely reported. IgA nephropathy (IgAN) is the most common glomerular abnormality in CD patients.2
C3 glomerulopathy (C3G) is defined based on a common pathogenesis of excessive activation of the complement alternative pathway, manifesting as C3-dominant staining on renal biopsy after the exclusion of other diseases. On the basis of electron microscopy (EM), C3G is subdivided into dense deposit disease (DDD) and C3 glomerulonephritis(C3GN).3
We report a rare case of nephrotic syndrome associated with C3G in a patient with CD.
Case historyA 19-year-old female patient with past medical history of hypothyroidism admitted to our hospital with bilateral leg swelling on April 2019. She had also abdominal pain and diarrhea for last three years. She was referred to the Nephrology Department upon detection of proteinuria. On physical examination, her body mass index (BMI) was 20.8kg/m2 and her blood pressure was 120/70mmHg. She had bilateral ++ edema of the lower limbs.
Her medication: Levothyroxine, 25μg once daily, in the morning.
Laboratory findings revealed the following: hemoglobin (Hb), 12.9g/dL; blood urea nitrogen (BUN), 10mg/dL; serum creatinine (SCr), 0.38mg/dL; serum albumin, 2.9g/L and total cholesterol, 324mg/dL. Thyrotropin, 4.07 (normal range: 0.43–4.00) μIU/mL; thyroperoxidase antibody, 201.8 (normal range: 0–33.9) IU/mL and thyroglobulin antibody, 14 (normal range: <4) IU/mL. Erythrocyte sedimentation rate was 12mm/h. Additional analysis showed as folic acid, 2.1 (normal range: 3.1–19.9) μg/L; vitamin B12, 108 (normal range: 126.5–505) ng/L; iron, 50 (60–180) μg/dL, total iron binding capacity, 236 (225–450) μg/L and ferritin, 182.8 (5.8–274) μg/L. Urinalysis showed proteinuria and sediment with 32 RBC/hpf. 24-h urine protein excretion was 6482mg/day with a microalbuminuria of 1899mg/day. Hepatitis B and C serologies were negative. No monoclonal protein was detected in serum electrophoresis examination. Immunological assays including antinuclear antibodies (ANA), anti-double stranded DNA (Anti-dsDNA), anti-neutrophilic cytoplasmic antibodies (ANCAs), rheumatoid factors and cryoglobulins were negative. Decreased C3 level was detected (0.53g/L, normal range: 0.9–1.8g/L) and C4 level was normal. The renal echography was normal.
The patient also admitted Gastroenterology Department because of gastrointestinal symptoms and laboratory findings including folic acid and vitamin B12 deficiencies. Antiendomysial antibody was found positive. Elevated tissue transglutaminase (TTG) IgA antibody (>200, normal range: 0–20RU/mL), elevated anti-gliadin IgA antibody (44.82, normal range: 0–24RU/mL) and elevated anti-gliadin IgG antibody (38.96, normal range: 0–24RU/mL) were detected. Upper gastrointestinal endoscopy with duedonum biopsy revealed villus atrophy with intra-epithelial lymphocytic infiltrates consistent with CD.
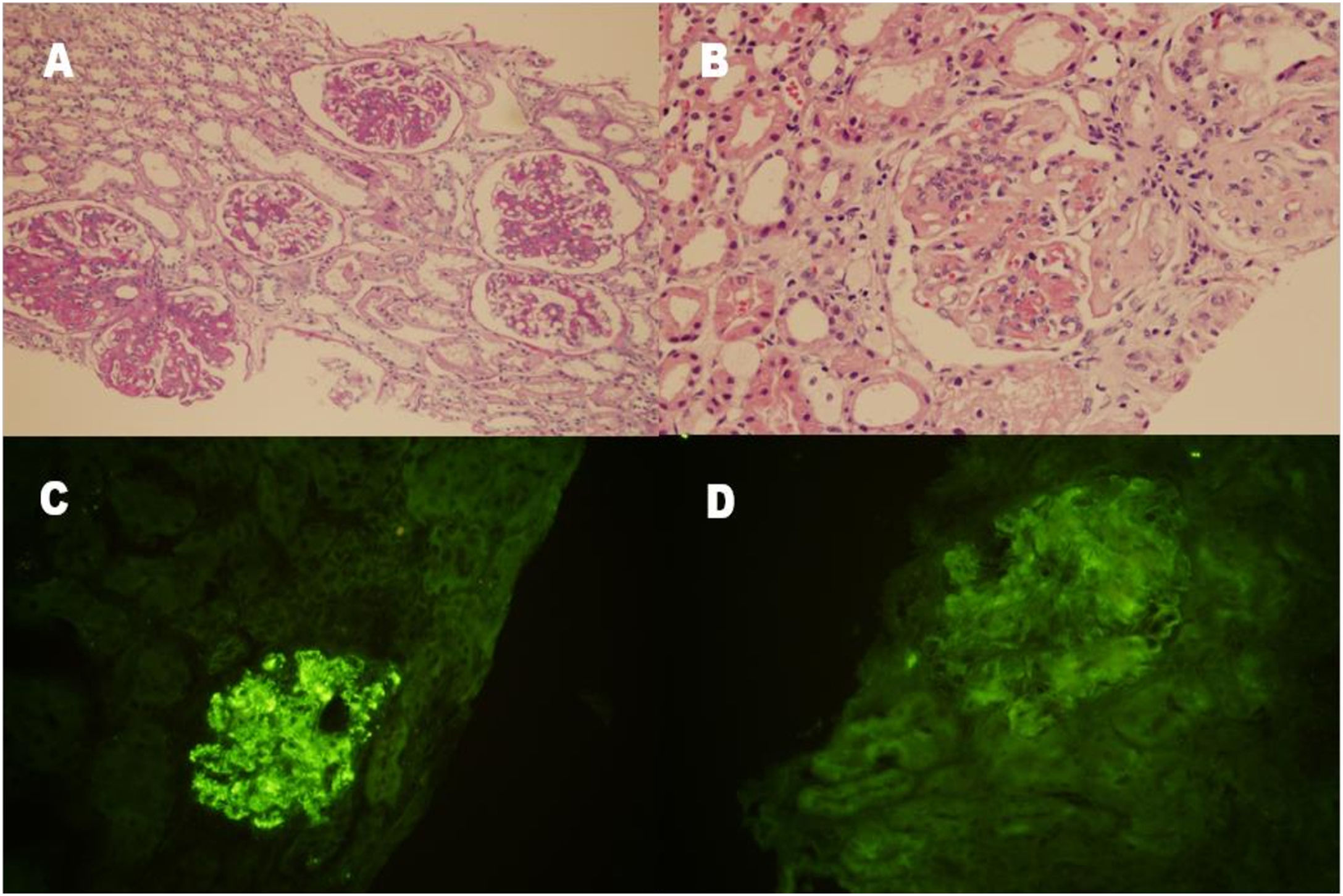
A percutaneous renal biopsy that was performed in July 2019 revealed membranoproliferative glomerulonephritis (MPGN). Twenty glomeruli were observed, with global sclerosis in one glomerulus and segmental sclerosis in two glomeruli. Specifically, segmental mesangial matrix expansion, mesangial hypercellularity and thickening of the glomerular capillary loops with double contours were noted under light microscope. Lobular appearance was seen in some glomeruli. There were segmental endocapillary proliferations in five glomeruli and adhesions to bowman capsule in two glomeruli. Interstitial fibrosis was mild with mononuclear inflammatory cell infiltrations (Fig. 1A and B). Kongo staining was negative. On immunofluorescence examination, C3 was present along the capillary loops and in the mesengium (+++) (Fig. 1C). IgG detected (+) along the capillary loops in a granular pattern (Fig. 1D). IgM, IgA, C1q, fibrinogen, kappa and lambda were not detected. There was no C4d deposition. EM could not be examined.
Renal histopathological findings. (A and B) Mesangial hypercellularity and thickening of the glomerular capillary wall with double contour formation, giving the glomeruli a lobular appearance (periodic acid staining, 100× and hematoxylin–eosin staining, 200×). (C) Granular C3 deposition (+++) along the capillary wall and in the mesangium (indirect immunofluorescence staining on frozen tissue, 200×). (D) Segmental IgG deposition (+) along the capillary wall and in the mesangium (indirect immunofluorescence staining on frozen tissue, 200×).
Genomic DNA extracted from blood sample of patient was used to amplify and bidirectionally sequence the coding regions and intron–exon boundary junctions of C3 (NM_000064), CFH (NM_000186), CFHR5 (NM_030787), CFB (NM_001710) and CFI (NM_0001331035). Four single nucleotide polymorphisms in CFH and C3-namely CFH c.1204C>T;p.His402Tyr (rs1061170), CFH c.184 G>A;p.Val62IIe (rs800292), C3 c.2863+7C>T (rs2287845) and C3 c.2246-8C>T (rs406514) were found.
An antibody test against C3 convertase (C3NeF) was not performed.
A gluten-free diet and ramipril 2.5mg/day was started. After 3 months, patient was reevaluated. At this time, she had proteinuria of 5969mg/day with SCr, 0.36mg/dL and serum albumin, 3.1g/L. We decided to start immunosuppressive treatment. The patient was initiated on steroids (methylprednisolone 48mg/day) as well as an additional immunosuppressive agent (mycophenolic acid [MMF] 1500mg/day). Over the next 38 months, the patient gained 9kg and the oedema resolved; the proteinuria decreased to 237mg/day, serum albumin and C3 rose to 4.4g/L and 1.17g/L, respectively with a normal renal function (SCr: 0.48mg/dL). Also TTG IgA antibody decreased to <2RU/mL (normal range: 0–20RU/mL).
DiscussionBiopsy findings of our case presented with nephrotic syndrome and CD showed mesangial cell and matrix proliferation with C3 deposition consisted with C3G. However, we could not further classify based upon ultrastructural features observed on EM as DDD or C3 glomerulonephritis.
CD is associated with a variety of autoimmune diseases such as type 1 diabetes mellitus, autoimmune thyroid disorders, Sjogren's syndrome and IgA nephropathy.4 The prevalence of CD in patients with autoimmune thyroid disease (Graves disease and Hashimoto disease) is around 2–7% and the risk of autoimmune thyroid disease in CD is 3 times higher than in the healthy population.5 The co-existence of CD and autoimmune disease is thought to be partly due to a common genetic predisposition. CD and autoimmune thyroid disease are reported to be associated with the gene encoding cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), a candidate gene for conferring susceptibility to thyroid autoimmunity.6,7
Patients with celiac disease suffer from diarrhea, malabsorption, weight loss, iron deficiency, and anemia. It has been hypothesised that circulating immune complexes originating in the small intestine could be responsible for other diseases associated with CD.8,9 In terms of renal involvement it has been reported that glomerular diseases may ensue during the course of CD.10 Glomerulonephritis in association with enteropathy is a recognised, though rare, entity. IgA nephropathy is the most common glomerular abnormality reported in patients with CD.9 By far the main association between CD and glomerular disease is IgA nephropathy (IgAN) with 22–77% of patients with IgAN having IgA anti-gliadin antibodies and 3–4% of these having CD.11,12 A few case series described other glomerulopathies in the context of CD, such as membranous nephropathy and membranoproliferative nephropathy.13–18
The relation of autoimmune enteropathy and glomerulonephritis is related to the elevation of gut permeability and the presence of increased intra-epithelial T-lymphocytes in the duodenal mucosa of patients with primary glomerulonephritis.19,20 It was assumed that the antigen responsible might result from increased mucosal permeability to antigens, might be a product of gluten or could be derived from damaged intestinal mucosa. Increased intestinal permeability, typically seen in inflammatory bowel disease and celiac disease, has also been reported in patients with IgA nephropathy.21–23
Few studies have examined the possibility of complement involvement in CD pathogenesis. Untreated CD patients typically have high levels of IgG1 and IgG3 anti-gliadin antibodies in their serum both of which are capable of activating complement.24 Sub-epithelial IgA-TG2 deposits, found in the early stages of CD might also play a role, and polymeric IgA has been shown to activate complement via the MBL pathway.25,26 This could explain that patients with untreated celiac disease show C3 hypocomplementemia and have circulating immune complexes that disappear after gluten-free diet but reappear along with C3 split products shortly after gluten challenge along with C3 split products shortly after gluten challenge.27–30
The alternative pathway activation in C3GN was driven by inherited or acquired defects. Complement C3 and CFH are key inhibitors of the alternative pathway. C3 c.2863+7C>T variant was associated with severe preeklampsia,31 overall survival and prognosis in patients with early stage non-small cell lung cancer after surgical resection.32 The c.1204c>T; p.His402Tyr andCFH c.184 G>A;p.Val62IIe variants in the CFH gene had significant associations with an increased risk of developing age-related macular degeneration (AMD).33,34
ConclusionWe are able to report a good renal course in the context of remission of enteropathy by the maintenance of a gluten-free diet lasting and MMF plus steroid treatment to a three-years follow up. Thus, it is important to keep this association in mind for primary care physicians, internists, gastroenterologists and nephrologists when working up a patient with diarrhea and nephrotic syndrome.
FundingThe author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestsThe author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.