Elderly patients and patients with co-morbidities such as hypertension, diabetes and heart disease are under risk of COVID-19.1 Anti-glomerular basement membrane (anti-GBM) disease is an autoimmune disease presenting with features of rapidly progressive glomerulonephritis and alveolar hemorrhage.2 It requires an aggressive immunosuppressive treatment.
We report the case of a 80-year-old female patient with anti-GBM disease who had a fatal course after acquiring a severe COVID-19 infection under immunosuppressive treatment. She had well-controlled hypertension and presented with fever, dyspnea, hemoptysis and hematuria for the last five days. Her laboratory values were as follows: Creatinine (Cre): 6.0mg/dL, blood urea nitrogen (BUN): 86.6mg/dL, sodium (Na): 139 mEq/L, potassium (K): 3.6mEq/L, uric acid (Ua): 12.6mg/dL, albumin (alb): 3.1g/dL, erythrocyte sedimentation rate (ESR): 64mm/h, CRP: 14.8mg/dL, BNP: 403pg/mL, procalcitonin: 1.14ng/mL. Urinalysis revealed 1226 erythrocytes/HPF and 86 leucocytes/HPF. 24-h proteinuria was 5.8g/day. Echocardiography revealed normal left ventricular functions with an ejection fraction of 66% and pulmonary hypertension (pulmonary artery pressure: 60mmHg).
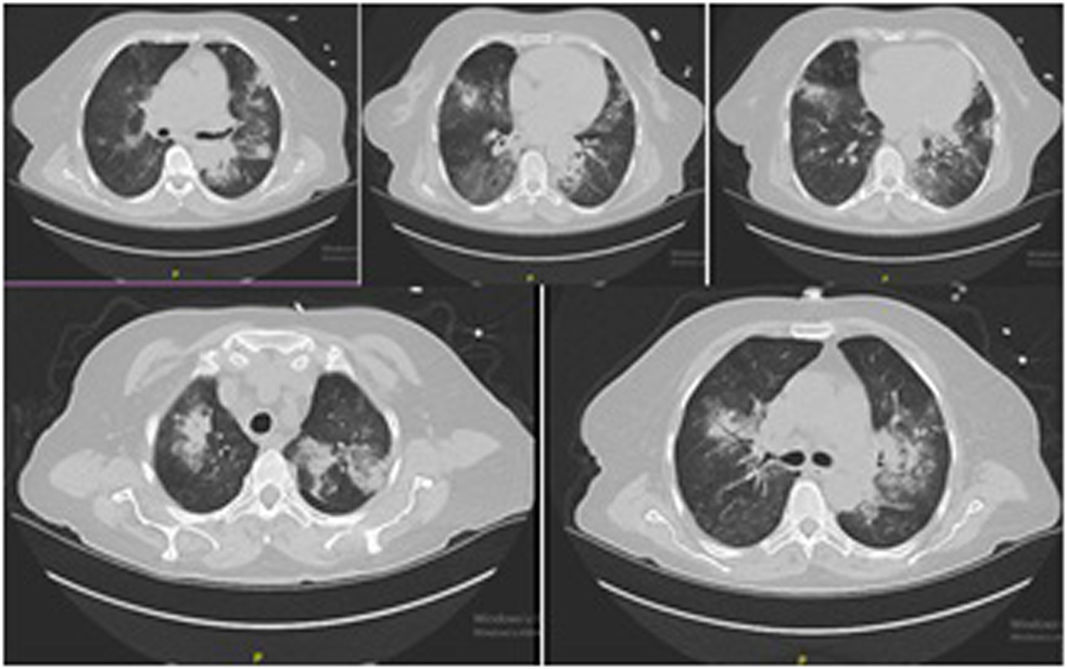
A non-contrast chest CT revealed alveolar hemorrhage and a suspicion of pneumonia. Nasopharyngeal swap for respiratory pathogens and PCR for COVID-19 was sent but there was no positivity. ANA, ANCA were negative, C3 and C4 levels were normal. However, testing for anti-GBM antibody was reported as positive.
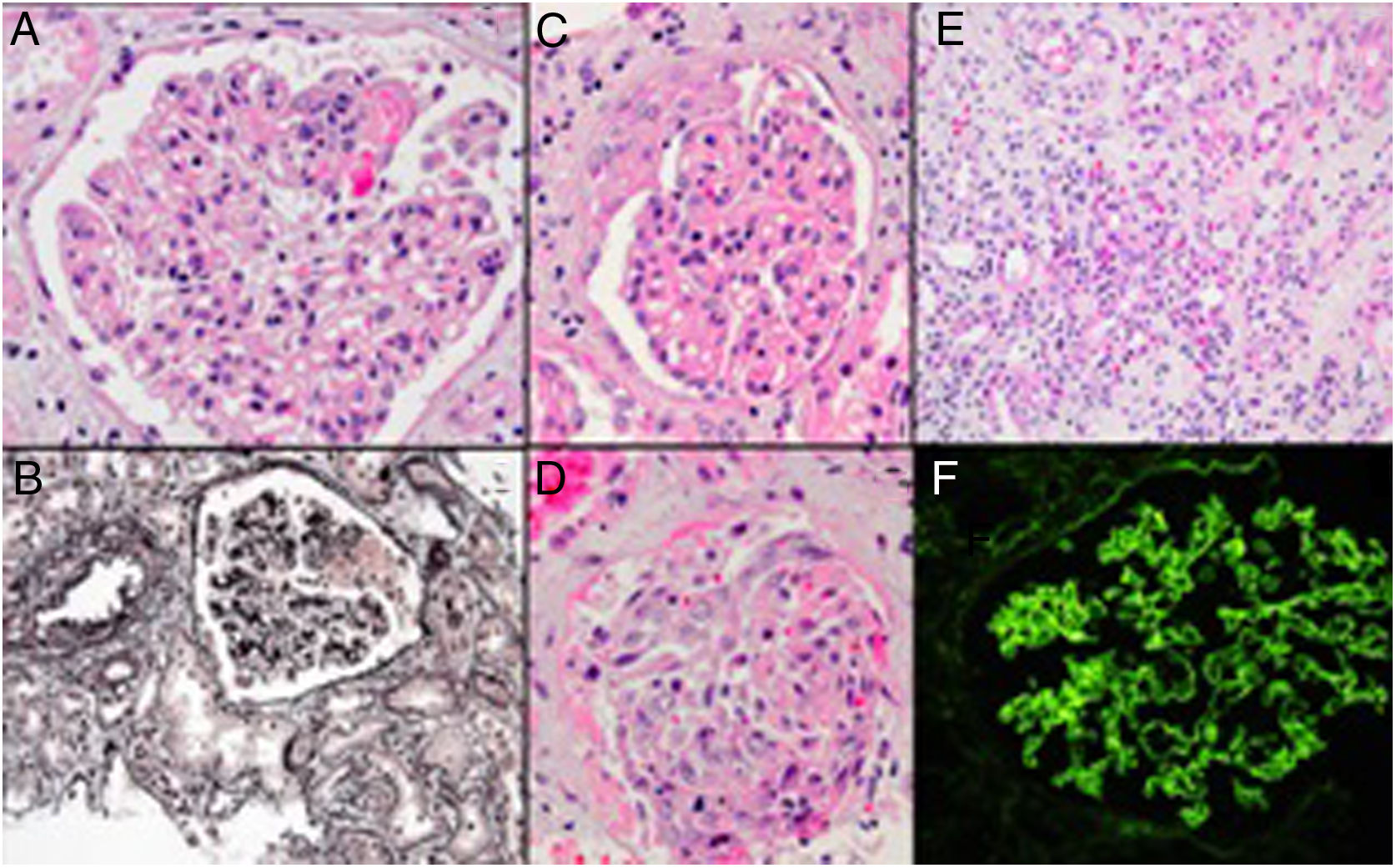
Renal biopsy revealed a focal necrotizing extra-capillary proliferative glomerulonephritis and acute tubulointerstitial nephritis. Immunofluorescence microscopy showed presence of linear IgG along the glomerular basement membrane. A diagnosis of anti-GBM disease with a comment about supervening possible drug reaction was made (Fig. 1).
Glomerulus with focal fibrinoid necrosis (A, hematoxylin-eosin), another glomerulus with fibrinoid necrosis, which can be seen as the pink argyrophobic area in the silver stain (B, Jones methenamine silver). Glomerulus with partial cellular crescent formation (C, hematoxylin–eosin stain), another glomerulus with extensive extracapillary proliferation filling the Bowman space and part of a tubule filled with a red blood cell cast (D, hematoxylin–eosin stain). Accompanying interstitial inflammation with abundance of eosinophils (E, hematoxylin–eosin stain). Immunofluorescence microscopy showing lineer IgG along the glomerular basement membrane (F).
500mg intravenous metylprednisolone (mps) for three days and 500mg once a week cyclophosphamide treatment was started. She was discharged on oral mps treatment since there was no fever and progression in the infiltrations in the control CT imaging.
Three days after discharge, patient presented to the emergency service with tachypnea and hemoptysis. She was admitted to intensive care unit. A control chest CT showed similar findings with the previous ones (Fig. 2).
Considering her hospitalization history during COVID-19 outbreak and CT findings, a control PCR for COVID-19 was sent which was reported as positives. She had leukocytosis with lymphopenia and high level of acute phase reactants.
Combination treatment of hydroxychloroquine and azithromycin was started. In the fifth day of treatment she had to be intubated and vasopressor treatment was started. Favipravir and intravenous immunoglobulin were added because of resistant COVID-19 infection. Patient underwent continuous renal replacement therapy due to severe oliguric acute kidney injury and died on the 14th day of hospitalization.
This case report demonstrates a patient with anti-GBM disease who died in a short period of time after acquiring COVID-19 infection under immunosuppressive treatment. Although immunosuppressive treatment may theoretically complicate the course of an infectious disease,3 there is no firm evidence of increased complication for COVID-19 infections in patients under immunosuppressive treatment.4–6 However, it is impossible to completely exclude any detrimental effect of immunosuppression for specific disorders such as anti-GBM disease on COVID-19 infection. The unfavorable clinical course of this patient may be related either to the immunosuppression or the primary disease affecting the lungs, as it is well known that patients with lung diseases are at increased risk.
The PCR test for COVID-19 is far from perfect as approximately 30% of the patients have an initial false-negative result.7 This creates a dilemma for the clinicians when encountering a patient who has or may have an alternative diagnosis requiring immunosuppression but also has some features of COVID-19 infection. In this particular patient, since the initial PCR for COVID-19 was negative, a prompt response to antibacterial treatment was observed and there was no progression in the control CT imaging we excluded COVID-19 during the initial hospitalization. However, when the patient presented with a more severe condition in the second admission, considering her previous hospitalization history in the same ward with COVID-19 positive patients and her severe lymphopenia a repeat PCR test for COVID-19 was ordered which was found positive. The patient was therefore considered as new onset COVID-19 infection under immunosuppressive treatment.
Another point that should be considered however is the potential direct pathogenetic effect of the COVID-19 infection on the kidneys. Anti-GBM disease is known to be associated with infectious triggers and to present as mini-epidemics concurrent with influenza outbreaks.8–9 Considering reports of false negativity of patients at initial PCR testing there is thus the possibility that COVID-19 can be the causative factor of the anti-GBM disease. A Chinese study that examined post mortem renal biopsy findings in 26 COVID-19 infection cases however did not mention any cases with necrotizing extracapillary proliferative glomerulonephritis that could suggest possibility of anti-GBM renal disease.10 Thus to date there is no known case of anti-GBM glomerulonephritis in a covid19 patient.
Ethical approvalThe article does not contain any studies with human participants or animals performed by any of the authors.
Informed consentSince the patient that is described in the case report was died, her daughter has given consent for publication
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no conflict of interest.