Renal fibrosis is a basic pathological change of almost all chronic kidney disorders. Epithelial–mesenchymal transition (EMT) and excessive extracellular matrix (ECM) accumulation play a crucial role in the process of fibrosis.
MethodsWestern blot and qRT-PCR were accomplished to analyze the expression levels of target proteins and genes, respectively. The fibrotic levels in the renal tissues of rats were confirmed utilizing Masson staining. Expression of ECM-related α-SMA in the renal tissues was determined by immunohistochemistry assay. The combination of GRB2 associated binding protein 1 (GAB1) and miR-200a was ensured by starBase database and luciferase reporter assay.
ResultsOur data uncovered that miR-200a was downregulated, but GAB1 was upregulated in the renal tissues of the rat experienced unilateral ureteral obstruction (UUO). Overexpression of miR-200a improved tissues fibrosis, suppressed GAB1 expression and ECM deposition, and inactivated Wnt/β-catenin in UUO rats. Moreover, miR-200a expression was inhibited, while GAB1 expression was facilitated in the TGF-β1-induced HK-2 cells. In TGF-β1-induced HK-2 cells, miR-200a overexpression inhibited GAB1 expression, also declined ECM-related proteins and mesenchymal markers expression. Oppositely, miR-200a overexpression facilitated epithelial marker expression in the TGF-β1-induced HK-2 cells. Next, the data revealed that miR-200a inhibited GAB1 expression through binding to the mRNA 3′-UTR of GAB1. Increasing of GAB1 reversed the regulation of miR-200a to GAB1 expression, Wnt/β-catenin signaling activation, EMT and ECM accumulation.
ConclusionOverall, miR-200a increasing improved renal fibrosis through attenuating EMT and ECM accumulation by limiting Wnt/β-catenin signaling via sponging GAB1, indicating miR-200a may be a promising objective for renal disease therapy.
La Fibrosis renal es un cambio patológico básico en casi todas las enfermedades renales crónicas. La transformación mesenquimal epitelial (EMT) y la acumulación excesiva de matriz extracelular (ECM) juegan un papel vital en el proceso de fibrosis.
MétodosWestern blot y qRT-PCR se utilizaron para analizar los niveles de expresión de proteínas y genes objetivo, respectivamente. La Tinción Masson se utilizó para confirmar los niveles de Fibrosis en el tejido renal de la rata. La inmunohistoquímica detecta la expresión de SMA relacionadas con ECM en el tejido renal. La Unión de la proteína de unión relacionada con GAB1 y miR-200a wa está garantizada a través de la base de datos starbase y el análisis del informe de luciferasa.
ResultadosNuestros datos muestran que en el tejido renal de las ratas con obstrucción ureteral unilateral (uo), miR-200a wa se redujo, pero gab1 se elevó. La sobreexpresión del miR-200a mejoró la Fibrosis tisular en ratas uuo, inhibió la expresión de GAB1 y la deposición de ECM y inactiva la wnt/β-catenina. Además, la expresión de miR-200a fue inhibida, mientras que la expresión de GAB1 fue promovida en células HK-2 inducidas por TGF-β1. En las células HK-2 inducidas por TGF-β1, la sobreexpresión de miR-200a inhibe la expresión de GAB1 y también reduce la expresión de proteínas relacionadas con ECM y marcadores mesenquimales. Por el contrario, la sobreexpresión de miR-200a promueve la expresión de marcadores epiteliales en células HK-2 inducidas por TGF-β1. A continuación, los datos muestran que miR-200a inhibe la expresión de GAB1 al unirse al arnm 3’-UTR de GAB1. El aumento de GAB1 revirtió la regulación del miR-200a sobre la expresión de GAB1, la activación de la señal de la proteína en cadena Wnt/β-catenin y la acumulación de EMT y ECM.
ConclusiónEn general, el miR-200a puede ser un objetivo prometedor en el tratamiento de la enfermedad renal al inhibir la transmisión de la señal Wnt/β-catenin, al inhibir la acumulación de EMT y ECM, y al aumentar la mejora de la Fibrosis renal a través de GAB1 esponjoso.
Chronic kidney disease (CKD) is defined as processive structural abnormalities, urine aberrations, or damaged excretory renal function. As reported, CKD has become one of the major public health problem, and the incidence of it is approximately 10% worldwide.1 Renal fibrosis is one of the common histopathologic manifestation of CKD and exacerbates the developing of CKD to end-stage renal disease. Extracellular matrix (ECM) accumulation, inflammation, epithelial–mesenchymal transition (EMT) and activation of myofibroblasts are the important characterizes for renal fibrosis.2,3 Multifarious pathological features decide the pathogenesis of renal fibrosis associating with complex signaling pathway, such as, mitogen-activated protein kinase signaling, transforming growth factor-β (TGF-β)/Smads signaling and Wnt/β-catenin signaling.4–6 In the renal tissues of the ischemia–reperfusion-induced acute kidney injury mice model, Wnt/β-catenin is activated and aggravates renal fibrosis.7 Similarly, Wnt/β-catenin signaling is animated in the renal tissues of the mice went through unilateral ureteral obstruction (UUO) and in the TGF-β1-induced rat renal tubular epithelial cells, and inhibiting the activation of Wnt/β-catenin signaling contributes to limit the EMT caused by TGF-β1 in vitro and the UUO-resulted renal fibrosis.8 Exploring the pathogenesis is the first and important thing for improving renal fibrosis, and even CKD.
MicroRNAs (miRNAs) are a class of short non-coding RNA, without the potential that translation into proteins. Nevertheless, miRNAs play an important role in the regulation of gene expression in post-translational level.9 In recent years, numerous studies indicated that miRNAs involve in regulating renal fibrosis development. Zhang et al. discovered miR-181 expression that is downregulated in UUO mice and in Ang-II-induced rat renal tubular epithelial cells, while the renal fibrosis in UUO mice and the expression of ECM accumulation marker α-smooth muscle actin (α-SMA) in Ang-II-induced cells are significantly suppressed by miR-181 overexpression.10 Another miRNA, miR-21, was proved to be increased in the exosome secreted by the TGF-β1-induced rat renal tubular epithelial cells, and the miRNA contributes to renal fibrosis in the mice went through UUO.11 In addition, a previous study reported that two members of miR-200 family, miR-200a and miR-141, are decreased in the renal tissues of UUO mice at the early phase, and which may be regulate renal fibrosis development through affecting the expression of EMT-related proteins.12 The study accomplished by Wu et al. illustrated that miR-200a might be developed positive effects in attenuating the renal fibrosis in diabetic nephropathy mice model.13 Anyway, the mechanism of action of miR-200a in regulating renal fibrosis remains unclear.
Through three databases (TargetScan, starBase and miRDB), we predicted GRB2 associated binding protein 1 (GAB1) is a downstream target of miR-200a. At present, there is no relative studies focus on the role and the regulatory mechanism of GAB1 in renal fibrosis. However, some studies indicated that high expression of GAB1 promotes tissue fibrosis formation. For instance, in bleomycin-induced Systemic sclerosis mice model, silencing of GAB1 contributes to attenuate the inflammation and pulmonary fibrosis.14 Another study indicated that GAB1 facilitates pulmonary fibrosis in mice through regulating the polarization of macrophages.15 Feng et al. discovered that the expression of GAB1 is upregulated in the keloid tissues, and inhibiting GAB1 expression could effectively suppress keloid fibroblasts proliferation and migration.16 Thus, we hypothesized that GAB1 as a downstream target of miR-200a, and it mediated the regulating of miR-200a in renal fibrosis.
Briefly speaking, we established renal fibrosis rat model through UUO surgery, and TGF-β1-induced HK-2 served as cell model to explore the role and regulatory mechanism of miR-200a in the EMT, ECM accumulation and finally renal fibrosis. Our data may provide a novel idea for the treatment of renal tissues.
Materials and methodsReagentsThe lentivirus encoding miR-200a mimics or its negative control was purchased from the GenePharma (Shanghai, China). Recombinant human TGF-β1 was provided by the MedChemExpress (China). miR-200a mimics and the negative control (mimics NC), and miR-200a inhibitor and the negative control (inhibitor NC) were synthetized by the RiBoBio Co. (Guangzhou, China). Masson trichrome staining kit, DAB reagent and hematoxylin were obtained from the Solarbio (Beijing, China). Primary antibodies and secondary antibodies were purchased from Abcam (USA). Lipofectamine 3000, TRIzol reagent, SYBR Premix Ex Taq™ II assay and RIPA lysis buffer were obtained from the Invitrogen (USA). RevertAid™ First Strand cDNA Synthesis kit and the kit for miRNA-specific TaqMan MicroRNA assay were obtained from the Thermo Fisher Scientific (Waltham, MA, USA).
Rats and experimental groups in vivoFor carrying out animal experiment, healthy Wistar rats (male, weighting at about 180g, and aging at 6–8 weeks) were obtained from the Shanghai Experimental Animal Center (Shanghai, China). All rats were raised in accordance with standard conditions, including but not limit to free food and water, constant temperature at 22°C and 12h cycle of light–dark. At one week after acclimatization, rats were randomly divided into 7 groups (n=6): Sham, UUO-7d, UUO-14d, Sham+mimics NC, Sham+miR-200a mimics, UUO+mimics NC and UUO+miR-200a mimics. UUO surgery was carried out according to previous study.17 In brief, after anaesthetization with pentobarbital sodium (1%), the left ureter was uncovered and subsequent ligated at two points. The rats in Sham group were anaesthetized and exposed the left ureter, but without ligation. The rats in Sham+mimics NC, Sham+miR-200a mimics, UUO+mimics NC, and UUO+miR-200a mimics groups were injected with the lentivirus (1.0×1012vg) encoding miR-200a mimics or mimics negative control through tail vein at one week after UUO surgery. At the 14 days after operation, rats were euthanatized, and then renal tissues were collected for next study. All animal procedures were approved by the Committee of Animal Use and Care of Zhuzhou Central Hospital. All animal experiments were carried out strictly in accordance with the Guideline for Animal Research.
Masson trichrome stainingIn order to analyze the fibrotic levels in renal tissues, the tissues were embedded with paraffin, and then were cut into 4μm-thick slices. After that, slices were soaked successively with dimethylbenzene I for 10min, dimethylbenzene II for 10min, ethanol absolute I for 1min, ethanol absolute II for 1min, 95% ethanol for 1min, 90% ethanol for 1min, 90% ethanol for 1min, and 85% ethanol for 1min, and were washed with double distilled water. Subsequently, slices were maintained with Masson trichrome staining according to the manufacture's protocol.
Immunohistochemistry (IHC) detectionThe expression of α-SMA in the renal tissues of the rats experienced UUO or without was measured by immunohistochemistry assay. The 4μm-thick slices also were soaked successively with dimethylbenzene and ethanol. After that, the slices were maintained successively with 3% H2O2 for 20min at room temperature, 0.01M trisodium citrate dihydrate for 10min at 95°C, 10% normal goat serum for 20min at room temperature, and anti-α-SMA primary antibody (1:200 dilution). Subsequently, the slices were soaked with the goat anti-rabbit antibody for 30min at 37°C. All slices were maintained with HRP-labeled streptavidin for 20min at 37°C. Finally, the slices were maintained with DAB reagent and hematoxylin. Five fields were randomly selected from each slices at ×200 magnification, and the integrated optical density in positive area was analyzed utilizing the Image J software (NIH, Bethesda, MD).
Cell culture, induction, and transfectionFor carrying out cell experiments, human renal proximal tubular epithelial cells HK-2 were obtained from the China Center for Type Culture Collection. Cell culture was carried out in accordance with the previous study.3 Recombinant human TGF-β1 at a dosage of 5ng/ml was utilized to induce HK-2 cells for 12, 24, or 48h. In addition, to overexpress miR-200a, miR-200a mimics and the negative control (mimics NC) was synthesized. To downregulate miR-200a, a specific inhibitor of miR-200a and the negative control (inhibitor NC) was synthesized. To overexpress GAB1, the recombined pcDNA 3.1 vector expressing GAB1 was established, and the empty vector served as control. According to the different experiment aims, above mimics, inhibitor, GAB1 overexpression vector or the corresponding control were transfected into TGF-β1-induced HK-2 cells utilizing Lipofectamine 3000. 48h after TGF-β1 induction or transfection, cells were collected for next step.
Gene expression detectionqRT-PCR was carried out to indicate the relative levels of miR-200a, Collagen I, α-SMA, N-cadherin, E-cadherin and GAB1 genes. Total RNA was isolated from renal tissues or HK-2 cells in accordance with the protocol of TRIzol reagent. Then, cDNA was synthesized using the RevertAid™ First Strand cDNA Synthesis kit. After that, real-time PCR reactions for mRNA or miR-200a were accomplished utilizing the SYBR Premix Ex Taq™ II assay or a miRNA-specific TaqMan MicroRNA assay, respectively. The relative expression of mRNAs was normalized to GAPDH, and the relative expression of miR-200a was normalized to U6. Relative expression of mRNA and miR-200a was analyzed utilizing the 2−ΔΔCt method. The primers sequences as follows: α-SMA: 5′-AAAAGACAGCTACGTGGGTGA-3′ (F) and 5′-GCCATGTTC TATCGGGTACTTC-3′ (R); E-cadherin: 5′-CGAGAGCTACACGTTCACGG-3′ (F) and 5′-GGGTGTCGAGGGAAAAATAGG-3′ (R); U6: 5′-CTCGCTTCGGCAGCAC A-3′ (F) and 5′-AACGCTTCACGAATTTGCGT-3′ (R); GAPDH: 5′-CACCCACTCC TCCACCTTTG-3′ (F) and 5′-CCACCACCCTGTTGCTGTAG-3′ (R); Collagen I: 5′-G AGGGCCAAGACGAAGACATC-3′ (F) and 5′-CAGATCACGTCATCGCACAAC-3′ (R); N-cadherin: 5′-GCTTATCCTTGTGCTGATGTTT-3′ (F) and 5′-GTCTTCTTCTC CTCCACCTTCT-3′ (R); GAB1: 5′-GAAGTTGAAGCGTTATGCGTG-3′ (F) and 5′-T CCAGGACATCCGGGTCTC-3′ (R).
Protein expression detectionWestern blot was carried out to determine the relative expression of Collagen I, α-SMA, N-cadherin, E-cadherin, GAB1, p-Ser9GSK3β, GSK3β, p-Ser675β-catenin and β-catenin. Total protein was extracted from renal tissues and HK-2 cells utilizing RIPA lysis buffer. An equal quality (25μg) proteins from each group were separated by 12% SDS-PAGE. Following transferring all proteins into PVDF membranes, the membranes were blocked with 5% non-fat milk, and subsequent were maintained with the primary antibodies (1:2000 dilution) for overnight at 4°C. Then, membranes were maintained the horseradish peroxidase-labeled secondary antibodies, and were then exposed to the enhanced chemiluminescence reagent for protein bands visualization. The relative levels of proteins were analyzed utilizing the Image J software.
Target genes prediction and verificationThe target genes of miR-200a were predicted through three databases, including TargetScan Human, starBase and miRDB. Three binding site sequence between miR-200a and GAB1 3′-UTR were predicted by starBase and verified utilizing luciferase reporter assay. Three wide type (WT-1, WT-2 and WT3) and three mutant (Mut-1, Mut-2 and Mut-3) human GAB1 3′-UTR sequences which containing the predicted binding sites for miR-200a were sub-cloned into pMIR vector. The GAB1 3′-UTR-WT-1, 3′-UTR-WT-2, or 3′-UTR-WT-1 and GAB1 3′-UTR-Mut-1, 3′-UTR-Mut-2 or 3′-UTR-Mut-3 were co-transfected with miR-200a mimics or mimics NC into 293T cells for 48h. Then, the luciferase activity of each group cells was examined utilizing a luciferase reporter system (Promega, WI, USA)
Statistical analysisAll data were showed as mean±standard deviation, and were analyzed using the GraphPad Prism software. Student's t-test or one-way ANOVA followed by the Bonferroni's multiple comparison test were utilized to the comparison between two groups or among multiple groups, respectively. P<0.05 was recognized as statistically significant.
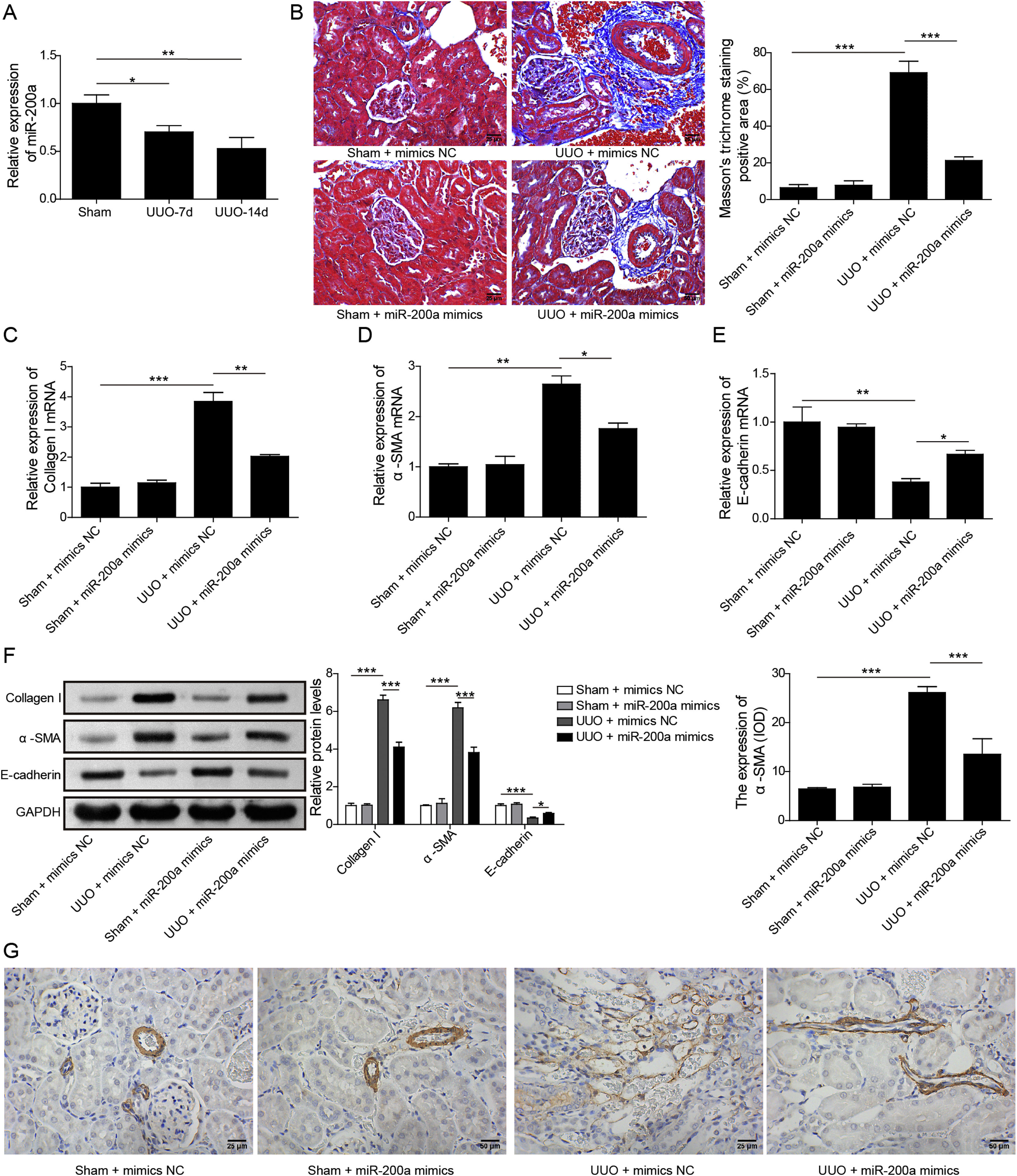
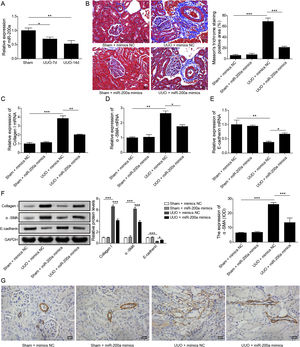
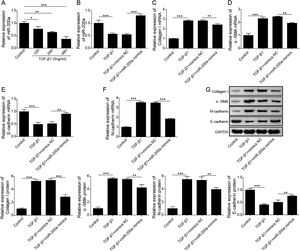
ResultsUpregulation of miR-200a ameliorates ureteral obstruction-induced renal fibrosisIt was uncovered that miR-200a is downregulated in the renal tissues of UUO rats, and may be participate in ureteral obstruction-induced renal fibrosis.12 Consistently, we also found decreased miR-200a in the renal tissues of UUO rats after 7 days or 14 days of surgery, and the decreasing following with the longer time after operation (Fig. 1A). Then, to dig the functions of miR-200a in the ureteral obstruction-induced renal fibrosis, we overexpressed it in normal and UUO rats utilizing the lentivirus which expressing miR-200a mimics. Masson staining results exhibited fibrotic renal tissues in UUO rats. Overexpression of miR-200a had no influence on the renal tissues in normal rats, but obviously diminished renal fibrosis in the rats experienced UUO (Fig. 1B). The importantly component of extracellular matrix, Collagen I and α-SMA, and E-cadherin are three major indicators for tissues fibrosis.18 Here, the data revealed that Collagen I and α-SMA mRNA levels were enlarged in UUO rats when compared to normal rats. Overexpression of miR-200a declined Collagen I and α-SMA mRNA levels in UUO rats, but had no effect on these gene expression in normal rats (Fig. 1C and D). Oppositely, E-cadherin mRNA level was decreased in the rats went through UUO. Overexpression of miR-200a obviously facilitated E-cadherin mRNA level in UUO rats, but not normal rats (Fig. 1E). Western blot data showed the consistent results with qRT-PCR assay. Collagen I and α-SMA were increased, but E-cadherin was decreased in the renal tissues of UUO rats. miR-200a overexpression dropped the expression of Collagen I and α-SMA, while facilitated E-cadherin expression in only UUO rats (Fig. 1F). The inhibition of miR-200a to upregulated α-SMA protein level in UUO rats also was proved by immunohistochemistry assay (Fig. 1G). Overall, miR-200a overexpression significantly alleviated the renal tissues in rats after UUO surgery.
The influence of miR-200a overexpression on the renal fibrosis of the rats experienced UUO. (A) The expression of miR-200a in renal tissues was analyzed utilizing qRT-PCR after the 7 days and 14 days of UUO surgery. The normal and UUO rats were injected with the lentivirus expressing miR-200a mimics or mimics negative control at the 7 days post-UUO. Next, (B) the fibrotic level of renal tissues was analyzed through Masson staining after the 14 days of UUO rats. (C–E) The relative expression levels of Collagen I, α-SMA, and E-cadherin mRNA in the renal tissues of rats were analyzed using qRT-PCR assay. (F) The relative expression levels of Collagen I, α-SMA, and E-cadherin proteins were determined through Western blot. (G) The expression level of α-SMA in renal tissues of rats was also evaluated through immunohistochemistry assay.
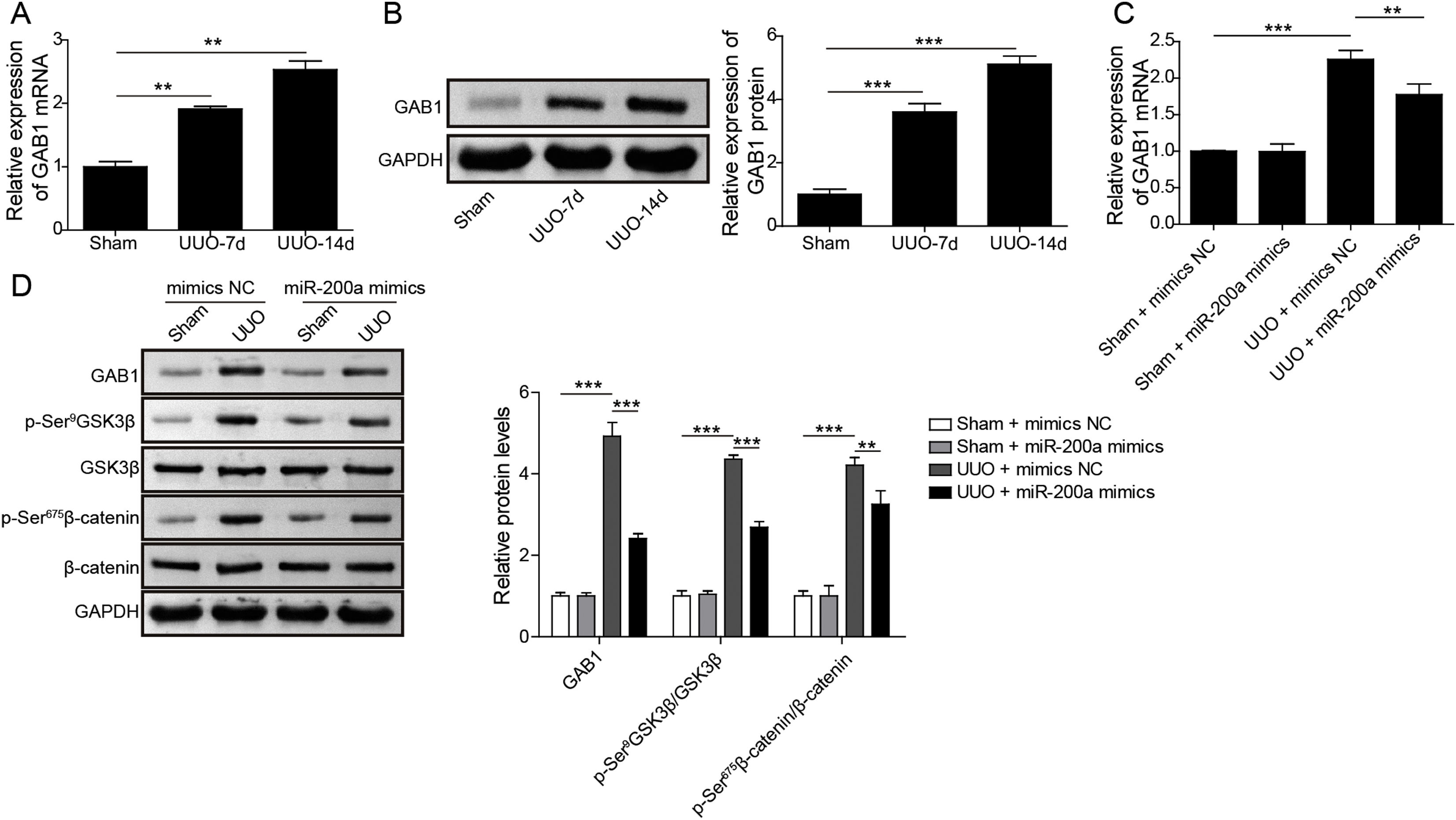
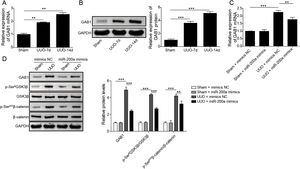
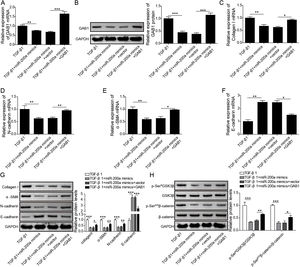
Through the miRDB, TargetScan Human, and starBase databases, we predicted that GAB1 was a downstream target of miR-200a (Fig. 4E). We then inspected the mRNA and protein levels of GAB1 in the renal tissues of the rats experienced UUO. GAB1 gene and protein expression was enhanced in the renal tissues of the rats experienced UUO, and the expression of them positively associated with the time after UUO surgery (Fig. 2A and B). Crucially, miR-200a overexpression had no influence on the expression of GAB1 gene and protein in normal rats, but noticeably suppressed GAB1 gene and protein expression in UUO rats (Fig. 2C and D). Meantime, we determined the activation of Wnt/β-catenin signaling that has been proved to be activated in the renal tissues of rats following UUO surgery.19 Our results revealed that the phosphorylation level of GSK3β and β-catenin was increased in UUO rats. Overexpression of miR-200a does not change the level of p-GSK3β and p-β-catenin in normal rats, but markedly declined GSK3β and β-catenin phosphorylation in UUO rats (Fig. 2D). In summary, miR-200a overexpression limited GAB1 expression and Wnt/β-catenin signaling activation.
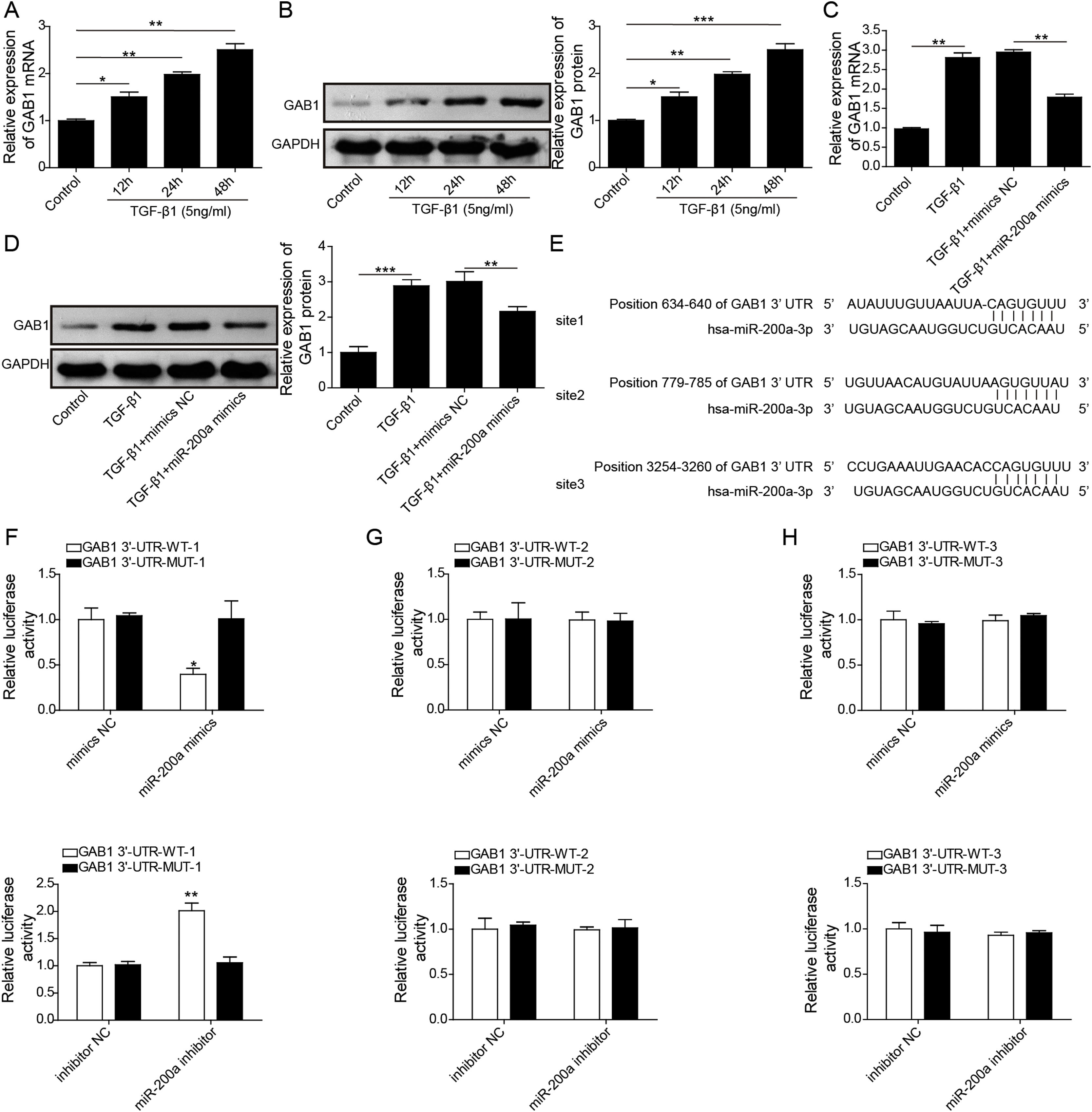
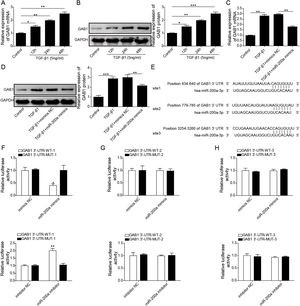
The regulation of miR-200a to GAB1 expression. (A, B) TGF-β1 (5ng/ml) was used to induce HK-2 cells for 0, 12, 24, and 48h, and then qRT-PCR was accomplished to analyze GAB1 gene expression and Western blot was accomplished to measure GAB1 protein expression. (C, D) The TGF-β1 (5ng/ml)-induced HK-2 cells were transfected with miR-200a mimics and mimics negative control for 48h. Next, qRT-PCR and Western blot were carried out to analyze the relative expression levels of GAB1 mRNA and proteins in the cells. (E) Three different binding sites sequences of miR-200a and GAB1 mRNA 3′-UTR was predicted utilizing starBase database. (F–H) Luciferase activity reporter assay was carried out to verify which binding sites truly effect the regulation of miR-200a to GAB1 expression.
The regulation of miR-200a to GAB1 expression and activation of Wnt/β-catenin signaling. (A, B) The relative levels of GAB1 mRNA and protein in renal tissues was analyzed utilizing qRT-PCR and Western blot after the 7 days and 14 days of UUO surgery, respectively. After that, (C) the relative expression level of GAB1 mRNA in renal tissues of the rats, which injected with miR-200a mimics- or mimics negative control-expressed lentivirus, was determined at the 14 days post-UUO. (D) After the 14 days of UUO surgery, the relative expression levels of GAB1, GSK3β, and β-catenin proteins, and the phosphorylation of GSK3β and β-catenin in renal tissues were analyzed through Western blot.
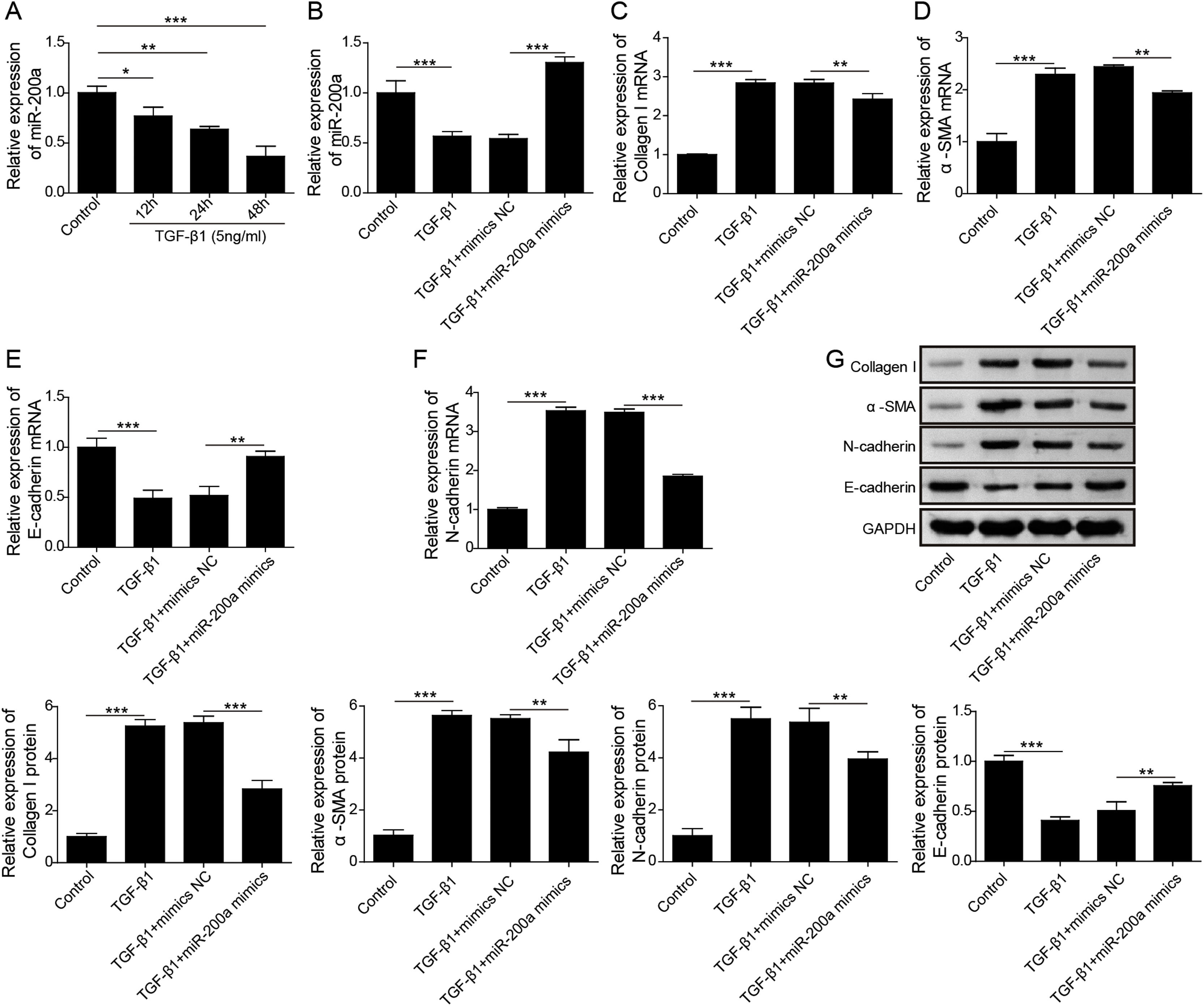
According to above data, we guessed that overexpression of miR-200a contributes to the improvement of ureteral obstruction-induced renal fibrosis. We further examined the influence of miR-200a overexpression on the TGF-β1-indued human renal proximal tubular epithelial (HK-2) cells EMT considered as a pivotal change in the development of fibrosis. Our results showed that the level of miR-200a was declined in the TGF-β1-induced HK-2 cells in a time-dependent manner (Fig. 3A). However, TGF-β1-induced downregulating in miR-200a expression was markedly recused by miR-200a mimics transfection (Fig. 3B). In addition, our results corroborated that the gene expression of ECM-related proteins, Collagen I and α-SMA, was facilitated in TGF-β1-indued HK-2 cells, but it was reversed by miR-200a overexpression (Fig. 3C and D). The gene expression of epithelial marker E-cadherin was suppressed, but the gene expression of mesenchymal marker N-cadherin was facilitated in the HK-2 cells treated with TGF-β1, however, there were partly recused by miR-200a expression (Fig. 3E and F). The western blotting assay also confirmed that TGF-β1 treatment resulted in an increasing in Collagen I, α-SMA, and N-cadherin protein expression, and decreasing in E-cadherin protein expression. Whereas, overexpression of miR-200a notably suppressed Collagen I, α-SMA, and N-cadherin protein expression, and enhanced the protein expression of E-cadherin in the HK-2 cells treated with TGF-β1 (Fig. 3G). All in all, miR-200a overexpression effectively alleviated TGF-β1-induced EMT and ECM deposition in HK-2 cells.
The regulation of miR-200a to EMT and ECM deposition in HK-2 cells caused by TGF-β1. (A) 5ng/ml of TGF-β1 was utilized to induce HK-2 cells for 0, 12, 24, and 48h. Then, the relative level of miR-200a in the cells was analyzed by qRT-PCR. After that, TGF-β1-induced HK-2 cells were transfected with miR-200a mimics or mimics negative control for 48h. (B) The relative level of miR-200a in the cells was examined by qRT-PCR. (C, D) The relative mRNA levels of ECM-related proteins, Collagen I and α-SMA, in the cells were analyzed by qRT-PCR. (E, F) The relative gene expression levels of EMT-related markers, N-cadherin and E-cadherin, in the cells were examined by qRT-PCR. (G) Collagen I, α-SMA, N-cadherin, and E-cadherin proteins expression in the cells was measured by Western blot.
To probe whether miR-200a regulates TGF-β1-induced EMT and ECM deposition via targeting GAB1, we analyzed the relationship between miR-200a and GAB1. Our data proved that GAB1 mRNA and protein levels was upregulated in the TGF-β1-induced HK-2 cells in a time-dependent manner (Fig. 4A and B). Nevertheless, the promotion of TGF-β1 to GAB1 mRNA and protein levels in HK-2 cells was limited by miR-200a overexpression through transfection of miR-200a mimics (Fig. 4C and D). Through the miRDB, TargetScan Human, and starBase databases, we conjectured GAB1 maybe a downstream objective of miR-200a. Based on the prediction, the three different binding sites between miR-200a and GAB1 mRNA 3′-UTR was shown in Fig. 4E. Subsequently, we verified the combination of miR-200a and GAB1 mRNA 3′-UTR through luciferase reporter assay. The results confirmed that miR-200a bound with GAB1 mRNA 3′-UTR through the first binding site (Fig. 4F–H). In conclusion, miR-200a inhibited GAB1 expression via binding GAB1 mRNA 3′-UTR region.
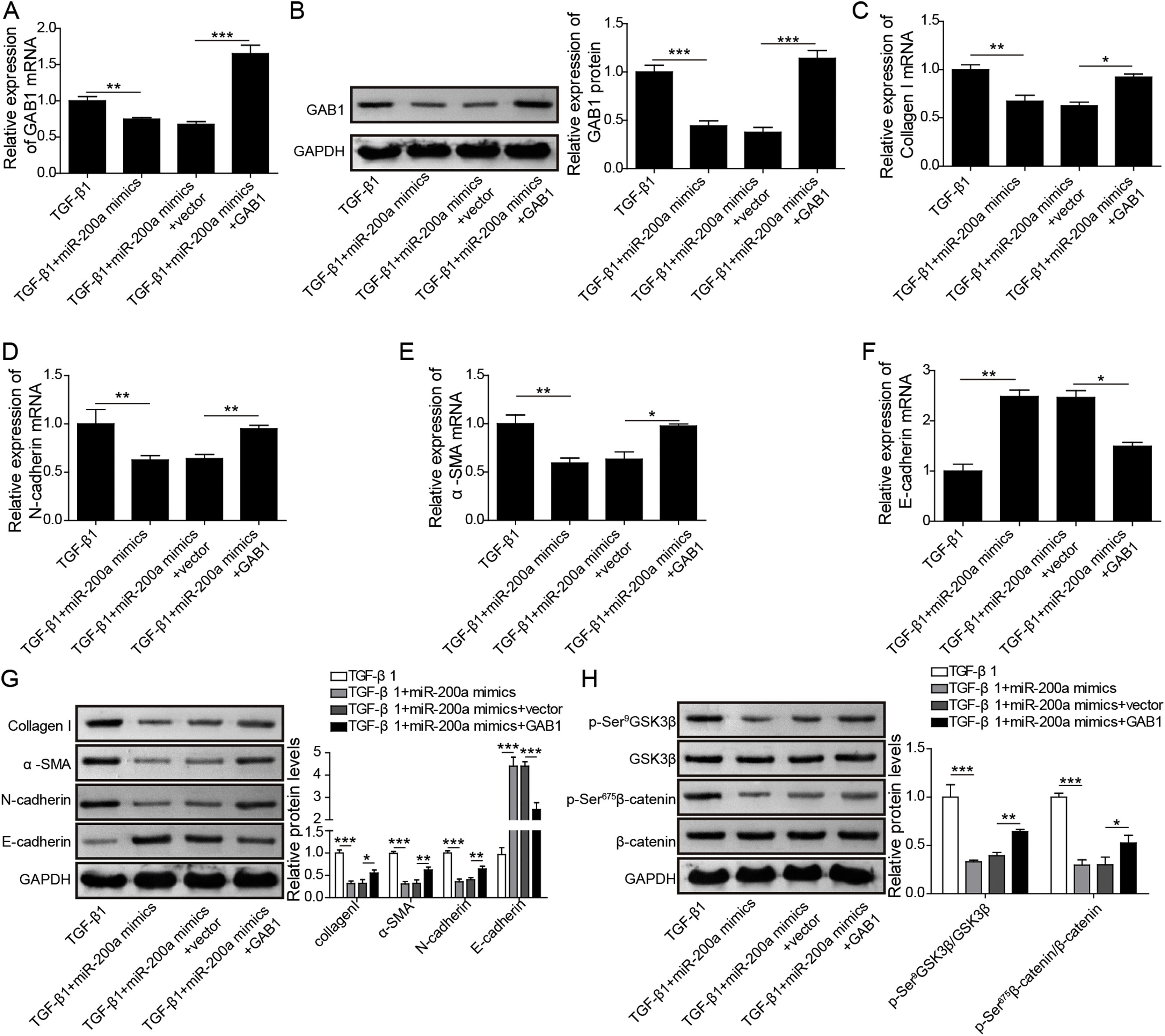
GAB1 increasing reversed the inhibition of miR-200a to TGF-β1-induced EMT and ECM deposition in HK-2 cellsFurthermore, to probe whether miR-200a regulates TGF-β1-induced EMT and ECM deposition via targeting GAB1, we increased GAB1 in the HK-2 cells treated with TGF-β1 following miR-200a overexpression. The results revealed that miR-200a notably suppressed the gene and protein expression of GAB1 in the TGF-β1-induced HK-2 cell, which was recused by GAB1 overexpression (Fig. 5A and B). Importantly, the inhibition of miR-200a overexpression to Collagen I, N-cadherin, and α-SMA gene expression, and the promotion to E-cadherin gene expression were partly reversed by GAB1 increasing (Fig. 5C–F). The western blotting assay results also indicated that the suppression of miR-200a overexpression to Collagen I, α-SMA, and N-cadherin protein expression, and the promotion to E-cadherin protein expression were recused by GAB1 increasing in the HK-2 cells treated with TGF-β1 (Fig. 5G). Meantime, miR-200a overexpression obviously limited the phosphorylation of GSK3β and β-catenin in TGF-β1-induced HK-2 cells, which was reversed following GAB1 increasing (Fig. 5H). Overall, these data indicated that the inhibition of miR-200a to TGF-β1-induced EMT, ECM accumulation and activation of Wnt/β-catenin signaling in HK-2 cells was reversed by GAB1 increasing.
The regulation mechanism of miR-200a to TGF-β1-induced EMT and ECM deposition in HK-2 cells. TGF-β1-induced HK-2 cells were co-transfected miR-200a mimics with or without GAB1 overexpression vector. At 48h after transfection, (A) GAB1 gene expression was analyzed utilizing qRT-PCR. (B) Western blot was accomplished to ensure the expression level of GAB1 protein. (C–F) qRT-PCR was implemented to ensure the expression levels of Collagen I, N-cadherin, α-SMA, and E-cadherin genes in the cells. (G) The ECM-related proteins, Collagen and α-SMA, and the EMT-related markers, N-cadherin and E-cadherin, expression were confirmed utilizing Western blot. (H) The activation of Wnt/β-catenin signaling pathway was analyzed through examining the phosphorylation levels of GSK3β and β-catenin by Western blot.
In our present study, the data showed that miR-200a played an anti-fibrosis effect in the rats experienced UUO and in the HK-2 cells treated with TGF-β1 by regulating EMT and ECM deposition via GAB1 and Wnt/β-catenin signaling. Long-term ureteral obstruction could lead to renal interstitial inflammation and fibrosis. Besides, TGF-β1 is an accepted pro-fibrotic factor and facilitates EMT, ECM deposition and cell apoptosis. Hence, UUO surgery and TGF-β1 induction are usually utilized to establish renal fibrosis model in vivo and in vitro, respectively.10,20
miR-200a is a member of miR-200 family and is multifunctional. It was reported that miR-200a participates in the development of multiple human disorders, such as ischemic heart diseases, nonalcoholic fatty liver disease, cerebral ischemic stroke, renal carcinoma and diabetic nephropathy.21–25 In recent years, some studies indicated that miR-200a may display anti-fibrotic effect. In the TGF-β1-induced human aortic endothelial cells, one of the cardiac interstitial fibrosis model, increasing of miR-200a effectively suppresses α-SMA and fibroblast-specific protein-1 expression and the process of endothelial-mesenchymal transition.26 In bleomycin-induced pulmonary fibrosis model, miR-200a was proved to mediate the anti-fibrotic effect of tadalafil in the disease through regulating Sonic Hedgehog, TGF-β, α-SMA and other fibrosis-related signaling pathways. Besides, miR-200a also was confirmed to attenuate the renal fibrosis in ApoE knockout mice and suppress TGF-β1- and TGF-β2-induced EMT in rat proximal tubular epithelial cells, while the regulatory mechanism of it remain unclear.27 In this study, our data also indicated that miR-200a level was declined in the rats experienced UUO and in the HK-2 cells treated with TGF-β1. Overexpression of miR-200a could notably attenuate the renal fibrosis in UUO rats. Meantime, miR-200a increasing suppressed Collagen I, α-SMA and N-cadherin expression, but encouraged E-cadherin expression in the renal tissues of the rats experienced UUO and the HK-2 cells treated with TGF-β1. Overexpression of miR-200a inhibited EMT and ECM accumulation and improved renal fibrosis.
Furthermore, we analyzed the targets of miR-200a utilizing TargetScan Human, starBase and miRDB databases, the results from above three databases exhibited consistently that GAB1 may be a downstream target of miR-200a. GAB1 is an adaptor protein, and it shows a crucial role in the receptor tyrosine kinases-mediated intracellular signal transduction.28 GAB1 is one of the members of GAB family which also includes GAB2 and GAB3. During tissue development, GAB are considered as signal “amplifiers” in the transduction of many signaling pathways.29 A previous study reported that GAB1 shows pro-fibrotic effect in systemic sclerosis.14 However, in the bile duct ligation mice, GAB1 knockout was reported to exacerbate liver fibrosis and inflammation, indicating that GAB1 maybe play an anti-fibrotic effect.30 Hence, we inspected the mRNA and protein levels of GAB1 in the renal tissues of rats and HK-2 cells. Our results revealed that GAB1 was increased in the rats went through UUO and in the HK-2 cells treated with TGF-β1. Overexpression of miR-200a significantly suppressed GAB1 expression as well as the Wnt/β-catenin signaling pathway. The combination of miR-200a and GAB1 3′-UTR was ensured by luciferase reporter assay. Importantly, the inhibition of miR-200a to TGF-β1-induced EMT, ECM accumulation and activation of Wnt/β-catenin signaling pathway was recused following GAB1 overexpression. These data suggested that miR-200a attenuated renal fibrosis through targeting GAB1 and the Wnt/β-catenin signaling pathway.
ConclusionIn conclusion, these findings made known that miR-200a overexpression could notably abate ureteral obstruction-caused renal fibrosis in vivo, and suppress EMT and ECM deposition in TGF-β1-induced HK-2 cells in vitro through inhibiting Wnt/β-catenin signaling pathway by sponging GAB1. Our work indicated that miR-200a may be a very promising therapeutic target for renal fibrosis and abnormal renal function.
Ethics approval and consent to participateAll animal procedures were approved by the Committee of Animal Use and Care of Zhuzhou Central Hospital. All animal experiments were carried out strictly in accordance with the Guideline for Animal Research.
Consent for publicationThe informed consent obtained from study participants
Availability of data and materialAll data generated or analyzed during this study are included in this published article.
FundingThis work was supported by Hunan Natural Science Foundation (2021JJ50068).
Conflict of interestThe authors declare that they have no conflict of interest.
We would like to give our sincere gratitude to the reviewers for their constructive comments.