The reduction of renal mass after radical nephrectomy (RN) for renal neoplasm, could be associated with compensatory hypertrophy of the contralateral kidney. The capacity of compensation will determine the renal function (RF) evolution. Measuring of total renal volume (TRV) of the remaining kidney pre and post RN can help assess the RF evolution.
ObjectivesTo determine the correlation between TRV pre and post nephrectomy (a year of follow-up) with RF.
Materials and methodsA retrospective cohort study was carried out in 47 patients who had undergone RN from 2014 to 2018, due to renal cell carcinoma (confirmed by histopathology).
The TRV was calculated, pre and post (a year of follow-up) RN, using ellipsoid formula equation, which were compared with clinical and analytical data. The results were analyzed by multivariate linear logistic models.
ResultsThe median age at the time of RN was 70 years old (range, 40–88 years). Most of them were men, 66%. The estimated glomerular filtration rate (eGFR) pre and post nephrectomy was 78 (40–100) and 53.3ml/min/m2 (30–90) respectively (p=0.01). The TRV pre and post-nephrectomy was 168.2ml (100.4–257.2) and 187.8ml (115.5–273.1) respectively (p=0.001).
The pre-nephrectomy eGFR (β=0.62; p=0.034) and the TRV (β=1.08; p<0.0001) were positively correlated with the post-nephrectomy TRV, while the eGFR at year of follow-up was correlated negatively (β=−1.18; p=0.047).
ConclusionsThe measurement of pre and post nephrectomy TRV can help to predict renal function evolution at a year of follow-up.
La reducción de la masa renal tras la nefrectomía radical en pacientes con neoplasias renales puede producir la hipertrofia compensadora del riñón contralateral. La capacidad de compensación determinará la evolución de la función renal. La medición del volumen renal total (VRT) del riñón remanente antes y después de la nefrectomía puede ayudar a evaluar la evolución de la función renal.
ObjetivosDeterminar la correlación entre el VRT pre y posnefrectomía con la función renal al año de seguimiento.
Materiales y métodosEstudio retrospectivo de observación en 47 pacientes adultos con neoplasias renales que fueron sometidos a nefrectomía radical. El VRT pre y posnefrectomía (al año de seguimiento) fue calculado mediante la ecuación de la elipsoide (TAC y/o RNM), que fueron comparados con datos clínicos y analíticos. Los resultados fueron analizados mediante regresión lineal uni y multivariante.
ResultadosLa mediana de edad al momento de la nefrectomía fue de 70 años (44-88). La mayoría fueron hombres (66%). El filtrado glomerular estimado (FGe) pre y posnefrectomía fue de 78 (40-100) y 53,3ml/min/m2 (20-90) respectivamente (p=0,01). El VRT pre y posnefrectomía fue de 168,2ml (100,4-257,2) y 187,8ml (115,5-273,1) respectivamente (p=0,001).
El FGe prenefrectomía (β=0,62; p=0,034) y el VRT pre (β=1,08; p<0,0001) se correlacionaron positivamente con el VRT posnefrectomía. Sin embargo, el FGe al año se correlacionó negativamente (β=–1,18; p=0,047).
ConclusionesEn pacientes con neoplasias renales tratados con nefrectomía radical la medición del VRT pre y posnefrectomía pueden ayudar a predecir la evolución de la función renal al año de seguimiento.
In recent years there has been an increase in the incidence of kidney neoplasms. Clear cell carcinoma is one of the most frequent, with a prevalence close to 3% of adult malignancies.1 In clinical practice this represents an important cause of acute renal failure and/or development of chronic kidney disease,2–4 therefore the nephrologist has a relevant and decisive role in the care, management and follow-up of the evolution of kidney function.
After radical nephrectomy it has been reported that a 33% of this patients develop of acute kidney failure (AKF) and have a 4 times greater risk of developing chronic kidney disease (CKD),5–7 the risk is lower in patients undergoing partial nephrectomies.8–11
Immediately after radical nephrectomy, different compensatory mechanisms are initiated on the remaining kidney to minimize the decrease in glomerular filtration rate.
Measurement of total renal volume (TRV) using the ellipsoid equation is tool widely used in polycystic kidney disease, being the main predictor of the evolution and prognosis of renal function (RF).12,13
The measurement of the TRV of the remnant kidney before and after radical nephrectomy (after one year of follow-up) in patients with renal neoplasms has not been fully investigated, and certainly it could help to evaluate the evolution of renal function and identity the factors that may affect the change in renal function.
The objective of this study was to determine the pre and post-nephrectomy TRV in the remaining kidney and its correlation with the estimated glomerular filtration rate (eGFR) at one year of follow-up.
Materials and methodsObservational, retrospective study in a court of adult patients (≥18 years) seen in the nephrology consultations of the Rey Juan Carlos de Móstoles Hospital, during the period between January 2014 and December 2018.
The study population included all patients identified in our center's database who underwent radical nephrectomy due to renal neoplasm, clear cell carcinoma confirmed by histological analysis. All patients were reevaluated one year after nephrectomy.
Exclusion criteria included: radical nephrectomies for other causes and partial nephrectomies. Those who did not have computerized axial tomography (CT) and/or magnetic resonance imaging (MRI) during follow-up were also excluded.
Demographic, clinical and biochemical parameters were collected in all cases. This information was obtained from the electronic medical records of our institution which included the comorbidities at the time of the nephrectomy (diabetes, peripheral vascular disease, chronic kidney disease, Charlson comorbidity index).14
Serum creatinine (Cr), eGFR and quantification of proteinuria were recorded, pre-nephrectomy (up to 2 months before nephrectomy), at hospital discharge and at one year of follow-up.
Renal function was assessed by calculating the eGFR using the abbreviated formula MDRD-4.15 The laboratory measurements were carried out in the same hospital by conventional methods.
Total renal volume (TRV) was calculated by using the ellipsoid formula in kidney image obtained by CT and/or MRI. The TRV was obtained before nephrectomy (up to 3 months prenephrectomy) and at one year of follow-up.
Calculation of the TRV (ellipsoid equation)=π/6×length×width×thickness.
To avoid information and selection bias The TRV measurement was carried out by one of the authors who did not have access to clinical or analytical data of the patients. The results of the TRV were compared with the variables of the pre-nephrectomy study and during follow-up.
The study was done in accordance with the principles of the Declaration of Helsinki. Given the minimal risk nature of the study, we were granted a waiver of informed consent.
DefinitionsARF was defined according to the Kidney Disease Improving Global Outcomes (KDIGO)16 classification. An increase in serum Cr concentration greater than 0.3mg/dl from baseline produced in less than 48h, or a 50% increase in baseline serum Cr during the first 7 days after nephrectomy.
CKD was defined as an eGFR less than 60ml/min/1.73m2 for at least 3 months. The eGFR was calculated using the abbreviated formula MDRD-4.15
Study design and statistical methodsData are presented as mean and standard deviation (±SD), or as median and interquartile ranges (IQR). To compare discrete variables we use the Chi-square test (or Fisher's exact). Student's “t” test for independent samples, or the non-parametric Mann–Whitney test was used to compare 2 continuous variables.
The analysis of the variables that are best associated with TRV and renal function at one year of follow-up, we performed univariate and multivariate linear regression models, adjusting the relevant covariates. A p<0.05 was considered statistically significant. Statistical analysis was performed with the IBM SPSS version 21 program (IBM Corp. Armonk, USA).
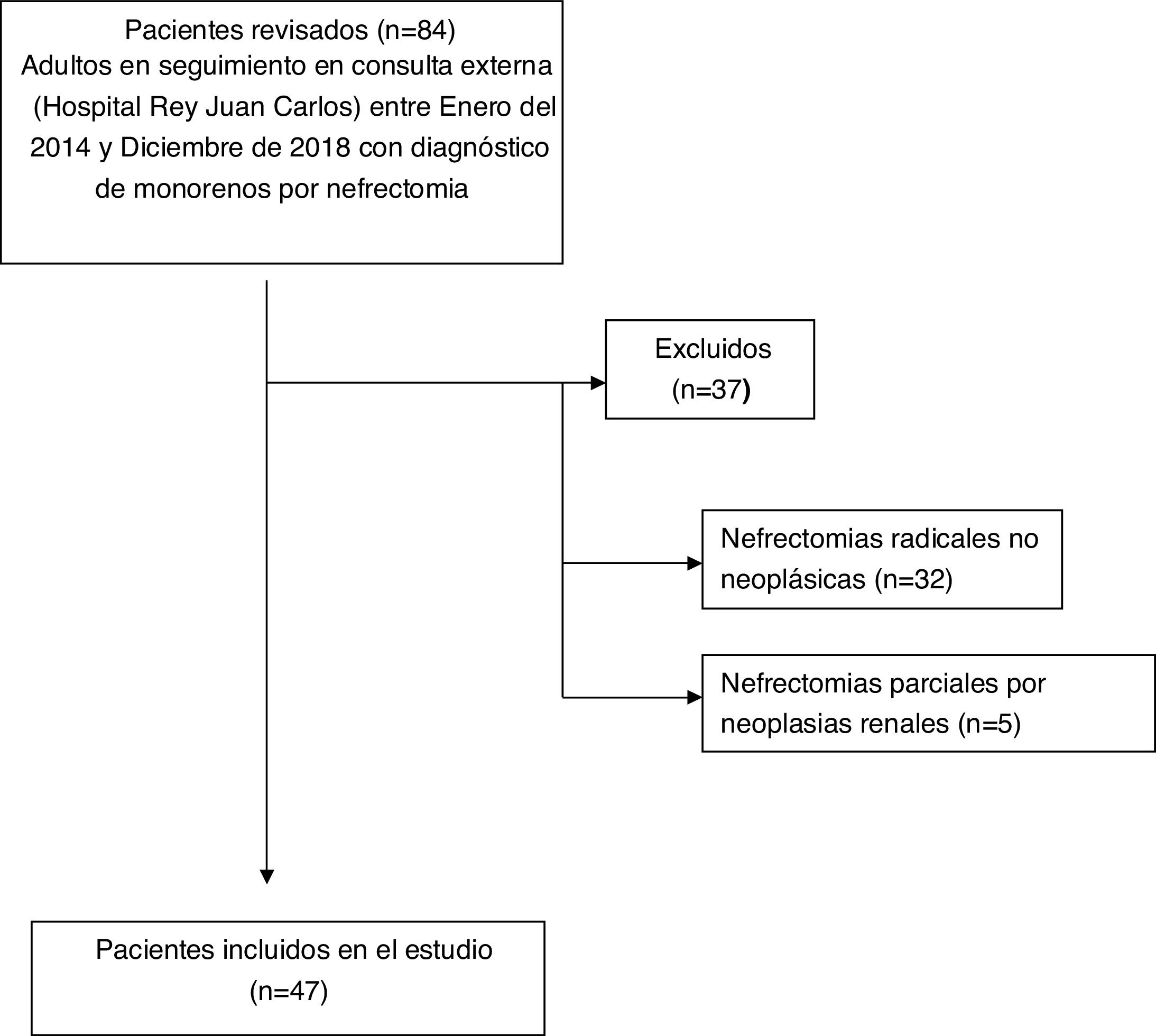
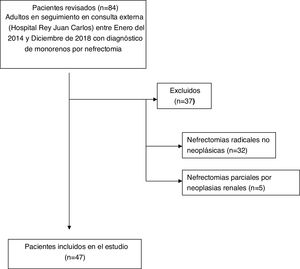
ResultsThe study group included 47 patients according to the flow chart shown in Fig. 1.
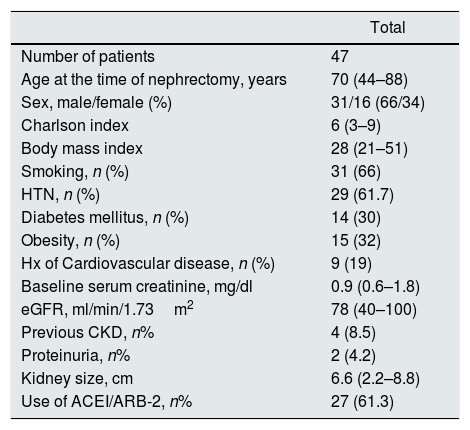
Table 1 shows the main characteristics of the patients included in the study.
characteristics of the study group (pre-nephrectomy).
| Total | |
|---|---|
| Number of patients | 47 |
| Age at the time of nephrectomy, years | 70 (44–88) |
| Sex, male/female (%) | 31/16 (66/34) |
| Charlson index | 6 (3–9) |
| Body mass index | 28 (21–51) |
| Smoking, n (%) | 31 (66) |
| HTN, n (%) | 29 (61.7) |
| Diabetes mellitus, n (%) | 14 (30) |
| Obesity, n (%) | 15 (32) |
| Hx of Cardiovascular disease, n (%) | 9 (19) |
| Baseline serum creatinine, mg/dl | 0.9 (0.6–1.8) |
| eGFR, ml/min/1.73m2 | 78 (40–100) |
| Previous CKD, n% | 4 (8.5) |
| Proteinuria, n% | 2 (4.2) |
| Kidney size, cm | 6.6 (2.2–8.8) |
| Use of ACEI/ARB-2, n% | 27 (61.3) |
ARA2: angiotensin 2 receptor antagonists; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; ACEI: angiotensin converting enzyme inhibitors; IQR: interquartile range.
Data are presented as median (interquartile range), unless otherwise specified.
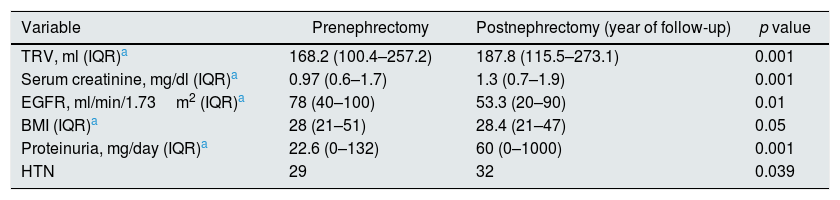
A 66% of the patients were men (31/16), with a median age of 70 years (range: 44–88). The diameter of the tumor was 6.6cm (range: 2.2–8.8). Left nephrectomy was performed in 51% of patients (24/23). Prenephrectomy Crs and eGFR were 0.9mg/dl (0.6–1.8) and 78ml/min/1.73m2 (40–100) respectively. The pre-nephrectomy TRV was 168.2ml (100.4–257.2) (Table 2).
Quantitative variables at the time of nephrectomy and at one year of follow-up.
| Variable | Prenephrectomy | Postnephrectomy (year of follow-up) | p value |
|---|---|---|---|
| TRV, ml (IQR)a | 168.2 (100.4–257.2) | 187.8 (115.5–273.1) | 0.001 |
| Serum creatinine, mg/dl (IQR)a | 0.97 (0.6–1.7) | 1.3 (0.7–1.9) | 0.001 |
| EGFR, ml/min/1.73m2 (IQR)a | 78 (40–100) | 53.3 (20–90) | 0.01 |
| BMI (IQR)a | 28 (21–51) | 28.4 (21–47) | 0.05 |
| Proteinuria, mg/day (IQR)a | 22.6 (0–132) | 60 (0–1000) | 0.001 |
| HTN | 29 | 32 | 0.039 |
eGFR: estimated glomerular filtration rate; BMI: body mass index; IQR: interquartile range; TRV: total renal volume.
Thirty-five patients (70.6%) met criteria of ARF after nephrectomy, the majority AKIN 1 (77% [27/35]). Of the 4 patients who had chronic kidney disease at the time of nephrectomy, only one developed ARF.
During the follow-up period, 6 patients (13%) received adjuvant therapy (antiangiogenic drugs: pazopanib [3], axitinib [1]; immunotherapy with nivolumab [2]).
Table 2 shows the evolution of pre-nephrectomy quantitative variables and the values at one year of follow up. The eGFR decreased significantly from 78 (range 40–100) to 53.3ml/min/1.73m2 (range, 20–90) (p=0.01), this was associated with an increase in serum Cr from 0.97 (0.6–1.7) to 1.3mg/dl (0.7–1.9) (p=0.001). The pre-nephrectomy TRV increased significantly after one year of follow-up, from 168.2ml (100.4–257.2) to 187.8ml (115.5–273.1) (p=0.001).
No significant differences were observed in the body mass index after one year of follow-up.
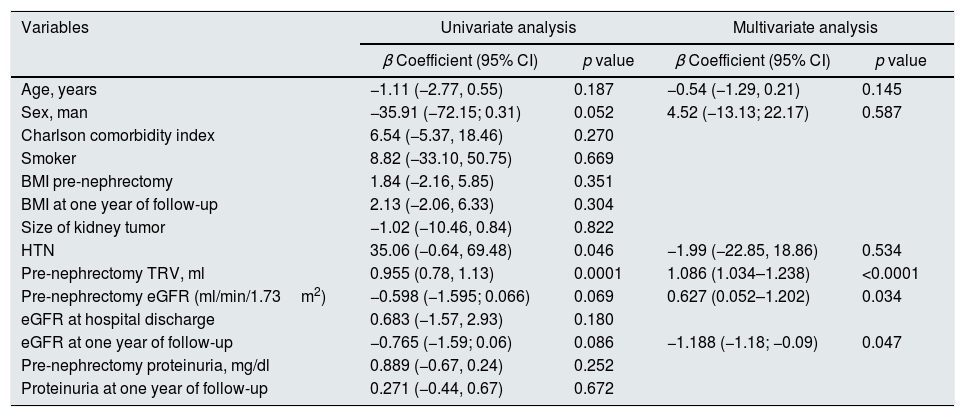
Table 3 shows the results of univariate and multivariate linear regression. The results are the best equation that determine the total renal volume after one year of follow-up. It was found a positive correlation with eGFR pre-nephrectomy (β=0.62; p=0.034) and TRV pre-nephrectomy (β=1.08; p<0.0001), while with eGFR at one year the correlation was negative (β=−1.18; p=0.047).
Variables that influenced the total renal volume at one year of follow-up (univariate and multivariate linear regression analysis).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| β Coefficient (95% CI) | p value | β Coefficient (95% CI) | p value | |
| Age, years | −1.11 (−2.77, 0.55) | 0.187 | −0.54 (−1.29, 0.21) | 0.145 |
| Sex, man | −35.91 (−72.15; 0.31) | 0.052 | 4.52 (−13.13; 22.17) | 0.587 |
| Charlson comorbidity index | 6.54 (−5.37, 18.46) | 0.270 | ||
| Smoker | 8.82 (−33.10, 50.75) | 0.669 | ||
| BMI pre-nephrectomy | 1.84 (−2.16, 5.85) | 0.351 | ||
| BMI at one year of follow-up | 2.13 (−2.06, 6.33) | 0.304 | ||
| Size of kidney tumor | −1.02 (−10.46, 0.84) | 0.822 | ||
| HTN | 35.06 (−0.64, 69.48) | 0.046 | −1.99 (−22.85, 18.86) | 0.534 |
| Pre-nephrectomy TRV, ml | 0.955 (0.78, 1.13) | 0.0001 | 1.086 (1.034–1.238) | <0.0001 |
| Pre-nephrectomy eGFR (ml/min/1.73m2) | −0.598 (−1.595; 0.066) | 0.069 | 0.627 (0.052–1.202) | 0.034 |
| eGFR at hospital discharge | 0.683 (−1.57, 2.93) | 0.180 | ||
| eGFR at one year of follow-up | −0.765 (−1.59; 0.06) | 0.086 | −1.188 (−1.18; −0.09) | 0.047 |
| Pre-nephrectomy proteinuria, mg/dl | 0.889 (−0.67, 0.24) | 0.252 | ||
| Proteinuria at one year of follow-up | 0.271 (−0.44, 0.67) | 0.672 | ||
R2 (corrected): 0.865.
eGFR: estimated glomerular filtration rate; CI: confidence interval; BMI: body mass index; TRV: total renal volume.
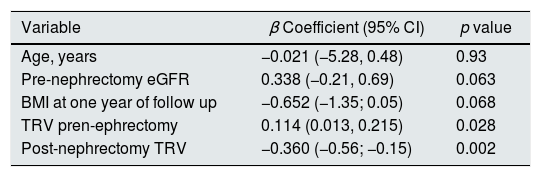
Table 4 shows the multivariate linear regression analysis of variables that influenced the eGFR at one year of follow-up. A positive correlation was observed with respect to the pre-nephrectomy TRV (β=0.114; p=0.028) and negative with the TRV after one year of follow up (β=−360; p=0.002).
Independent factors associated with estimated glomerular filtration rate at one year of follow-up (multivariate linear regression model).
| Variable | β Coefficient (95% CI) | p value |
|---|---|---|
| Age, years | −0.021 (−5.28, 0.48) | 0.93 |
| Pre-nephrectomy eGFR | 0.338 (−0.21, 0.69) | 0.063 |
| BMI at one year of follow up | −0.652 (−1.35; 0.05) | 0.068 |
| TRV pren-ephrectomy | 0.114 (0.013, 0.215) | 0.028 |
| Post-nephrectomy TRV | −0.360 (−0.56; −0.15) | 0.002 |
Variables that did not enter in the best prediction equation: Charlson comorbidity index (p<0.10), albumin, proteinuria at one year of follow-up, tumor size, ACEI-ARA-2 (p=0.2).
R2 corrected: 0.687.
eGFR: estimated glomerular filtration rate; BMI: body mass index; TRV: total renal volume.
At one year of follow-up, 60% of the patients (28/47) had developed CKD; the most frequent was CKD stage 3A (70% [20/28]). No patient required renal replacement therapy in the postoperative period or at one year of follow-up. Regarding the use of ACE inhibitors and ARB-2 after nephrectomy, treatment was temporarily suspended in 26% of the patients (9/35) who developed ARF, at the end of the study and a 92% of these patients continued on treatment with ACEI/ARA-2.
DiscussionThe results of this study show that there is a positive correlation between pre and post-nephrectomy TRV and the pre-nephrectomy eGFR. However, surprisingly, the eGFR and TRV at one year of follow-up had a negative correlation. This finding contrasts with our current knowledge based on previous studies, which have shown that the loss of 50% of the renal mass after radical nephrectomy initiates compensatory mechanisms early in an attempt to alleviate the decrease in glomerular filtration rate. However, these mechanisms can be modified depending on age, tobacco consumption, obesity, hypoalbuminemia, the development of post-nephrectomy acute renal failure, which will ultimately determine the evolution of renal function and the risk of progression to CKD.17–19
Whether the compensation mechanisms after nephrectomy in the renal oncological context are different from those observed in other clinical situations remains to be elucidated. Thus, some studies have found a negative correlation between renal function and size of the tumor, especially in tumors larger than 5cm.20,21 In our cohort, the median tumor diameter was 6cm, which may have influenced on the negative correlation of post-nephrectomy renal function.
The evolution of renal function after the loss of renal mass in the nephrectomized population behaves differently, depending on the etiology that determines the removal. In living kidney transplant donor patients, it appears that there is no significant loss of kidney function during follow-up,22 whereas in nephrectomized patients for reasons other than donation, the results are variable. Nephrectomized patients for non-neoplastic causes maintain renal function many years after nephrectomy, especially in a young population without major comorbidities. In contrast, other published series show worse results with progressive deterioration of renal function and development of proteinuria and progression to CKD.23
The TRV measured by the ellipsoid equation is an easy-to-use tool thanks to the availability of imaging tests (CT and/or MRI), being the most important biomarker that determine the evolution and prognosis of the function in patients with polycystic kidney disease.12,13 Here, an inverse correlation is also observed between TRV and renal function over time. Although in these cases of polycystic kidney disease there is a clear explanation, due to the increase in cysts in number and volume with compression of the rest of the nephrons (decrease in renal mass) and interstitial fibrosis.
In this study we have tried to relate the volume of the remaining kidney before and after nephrectomy with renal function at one year of follow-up in a population with renal neoplasia.
Given the fact that serum Cr and eGFR may remain normal even with 50% kidney damage,24 it is necessary to implement and search for new more sensitive markers capable to detect early which patients after radical nephrectomy for renal neoplasms are more susceptible to a poor evolution of kidney function and progression to CKD. Our results suggest that the measurement of TRV before and after nephrectomy in the remaining kidney could identify early those patients at risk of developing CKD, being probably a more significant parameter to consider when renal damage is subclinical, being useful as a possible biomarker that provides relevant information on the evolution of renal function in this group of patients.
This study has potential limitations:
- 1.
First, due to the short follow-up, the long-term evolution of renal function cannot be determined, so a longer follow-up would be necessary to evaluate the natural course of renal function in this group of patients.
- 2.
Second, the measurement of total renal volume with CT and/or renal MRI in a non-protocolized manner could have altered the results, associated with the measurement by a single observer that may cause an information bias.
- 3.
Third, 24-h creatinine clearance was not available, which would have helped and provided more information with a better assessment and evolution of eGFR in this group of patients since the different formulas for measuring eGFR in this group of patients are not fully validated. The disadvantage of obtaining creatinine clearance is the difficulty in obtaining urine samples and the great variability of creatinine metabolism in various clinical settings.
Although this study has drawbacks and limitations, and only included a relatively small number of patients, it is a homogeneous series from a single center in a disease that is increasing, which helps to understand that the measurement of total renal volume before and after nephrectomy sing the ellipsoid equation can represent a sensitive method to determine the early evolution of renal function and the compensatory capacity of the remaining kidney after radical nephrectomy for renal neoplasms.
ConclusionsThe conclusions generated from this study may be limited by its retrospective nature, however, they serve to demonstrate that the measurement of pre and post-nephrectomy TRV in the remaining kidney (year of follow-up) using the ellipsoid equation in nephrectomized patients due to renal neoplasms is strongly correlated with the evolution of the eGFR at one year of follow-up. These determinants may be useful to assess and monitor renal function and the compensatory capacity of the remaining kidney. Additional studies are necessary to determine the compensation mechanisms and prognostic factors of the population nephrectomized due to renal neoplasms.
Key concepts- •
Nephrectomies for renal neoplasms are a frequent cause of acute renal failure and chronic kidney disease, on the rise in recent years.
- •
The evolution and behavior of renal function in nephrectomized patients for renal neoplasms may be different from other non-neoplastic causes.
- •
Measurement of TRV in the remaining kidney in nephrectomized patients for renal neoplasms may be useful to assess the evolution of renal function.
The authors declare that they have no conflict of interest related to the publication of this article.
Please cite this article as: Pampa-Saico S, Alexandru S, Pizarro-Sánchez MS, López-Picasso M, Puente-Suárez LG, Barba R, et al. Volumen renal total y función renal en pacientes nefrectomizados por neoplasias renales. Nefrologia. 2021;41:446–452.