We report a case of a 48-year-old patient with multiple sclerosis (MS) since she was 18, receiving treatment with beta-interferon 1-a three times a week for the past 9 years and with no other medical treatment or relevant family history.
The patient referred symptoms of upper respiratory infection for the last 15 days for which she had been receiving symptomatic treatment. She presented high blood pressure, decreased urine output (with no macroscopic changes), and impaired renal function. Significant findings of the physical examination included blood pressure values of 190/93mmHg and edema of the lower limbs up to the knees. In addition, crossings and cotton wool spots were observed in the ocular fundus.
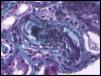
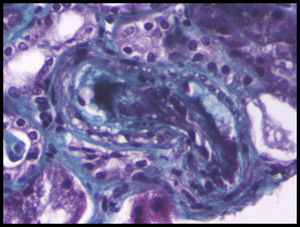
Blood tests showed hemoglobin levels of 9.8g/dL with an MCV of 93.5fl, platelet values of 142mil/uL, and LDH of 588IU/L, total bilirubin of 1.2mg/dL, creatinine of 1.9mg/dL, and proteinuria of 0.8g/24h; in the urine sediment: cell counts were 4–6 RBCs/field, 25–30 leukocytes/field, and common bacteria. A peripheral blood smear was performed, with 2% of schistocytes. The renal Doppler ultrasound and echocardiogram were normal as was the immunological study, HCV, HBV and HIV serology values. Haptoglobin was undectectable, and creatinine was increased to 2.5mg/dL. In the absence of gastrointestinal symptoms, shigatoxin was not tested for. The ADAMTS 13 test was normal and the direct Coombs test was negative. A renal biopsy was performed, which was compatible with thrombotic microangiopathy (Fig. 1).
Suspecting that beta interferon could have been the cause of thrombotic microangiopathy and/or accelerated hypertension, the drug was discontinued. After five months, the patient presented improved renal function with creatinine levels of 1.6mg/dL and with a urine protein/creatinine ratio of 0.13, along with acceptable good blood pressure in the absence of antihypertensive medication (Table 1).
Ubara et al. described one case of haemolytic uremic syndrome (HUS) in a patient undergoing treatment with INFB for 44 days for chronic hepatitis C.1
Subsequently, Herrera et al. presented two cases of patients with thrombotic thrombocytopenic purpura (TTP), both of whom were being treated with INFB for periods of 2 and 4 weeks.2 In our case, TTP was ruled out due to the absence of current neurological symptoms and to the fact that the ADAMTS13 activity and platelets were normal and thrombotic microangiopathy within accelerated hypertension.
Broughton et al.3 and Olea et al.4 reported cases of TMA in patients undergoing treatment with INFB for MS, both of which were similar to the case reported here. Interferon treatment was discontinued and treatment with renin angiotensin system inhibitors was initiated.
The onset of hypertension or poorly controlled blood pressure has been previously described in patients undergoing INFB treatment, with the FDA describing 19 cases of hypertension out of 12,700 patients treated with INFB.
Modrego et al. reported a patient who had been treated for MS with INFB for many years and who was referred with poorly controlled hypertension and a renal biopsy compatible with kidney damage secondary to high blood pressure. The patient continued with treatment for hypertension after discontinuing INFB.5
It is clear that there are many questions in relation to these associations, presently unresolved with the current literature. The pathogenesis of thrombotic microangiopathy in accelerated hypertension is not exactly known but is considered to play an important role in activating the renin–angiotensin–aldosterone system. In the case of our patient, is seems like arterial hypertension, kidney damage and thrombotic microangiopathy might be justified by treatment with interferon. However we cannot exclude that thrombotic microangiopathy is the cause by accelerated hypertension or the result of this one. Being difficult to assess which is the cause and which is the effect.