MicroRNAs (miRNAs) are small endogenous RNAs that regulate gene expression through post-transcriptional repression of their target messenger RNAs. A study of changes in expression of certain miRNAs in the kidney has supplied evidence on their pathogenic role and therapeutic potential in nephrology. This review proposes a nanotechnology approach based on the binding of analogs or inhibitors of miRNAs formed by peptide nucleic acids (PNAs) to peptides with a transmembrane structure sensitive to a low pH, called pHLIPs (pH [low] insertion peptides). The review draws on the concept that an acidic pH in the microenvironment of the renal tubule may facilitate concentration and distribution of the pHLIP-PNA complex in this organ. In this context, we have demonstrated for the first time that targeted administration of miR-33 inhibitors with the pHLIP system effectively prevents the development of renal fibrosis, thus opening up this technology to new strategies for diagnosis and treatment of kidney diseases.
Los microRNA (miRNA) son ARN endógenos de pequeño tamaño que regulan la expresión génica a través de la represión postranscripcional de sus ARN mensajeros diana. El estudio de los cambios en la expresión de ciertos miRNA en el riñón ha proporcionado evidencias sobre su papel patogénico y potencial terapéutico en nefrología. En esta revisión proponemos un abordaje de nanotecnología basado en la unión de análogos o inhibidores de miRNA formados por ácidos peptidonucleicos (PNA) a péptidos con una estructura transmembrana que es sensible a pH bajo, denominada pHLIP (del inglés pH [low] insertion peptide), apoyándonos en el concepto de que el pH acídico del microambiente tubular renal puede facilitar la concentración y la distribución del complejo pHLIP-PNA en este órgano. En este contexto hemos demostrado por primera vez que la administración dirigida de inhibidores de miR-33 con el sistema pHLIP previene eficazmente del desarrollo de fibrosis renal, abriendo las puertas de esta tecnología a nuevas estrategias de diagnóstico y tratamiento de enfermedades renales.
The microRNA (miRNA) are a family of small noncoding RNAs of 20–25 nucleotides in length that repress translation and/or induce degradation of messenger RNAs (mRNA) target by binding to complementary sequences in the region 3’UTR.1 In 1993, it was described the first miRNA, Lin-4, from Caenorhabditis elegans, and it was not until 2000 that the first miRNA from mammal, let-7, was discovered. Currently, more than 1982 miRNAs have been described in the entire human genome. The ability of a single miRNA to regulate hundreds of mRNAs gives them the ability to modulate the expression of more than 60% of the protein-coding genes (miRBase), intervening in a wide variety of cellular and physiological processes, such as cell cycle, proliferation, apoptosis and metabolism.2
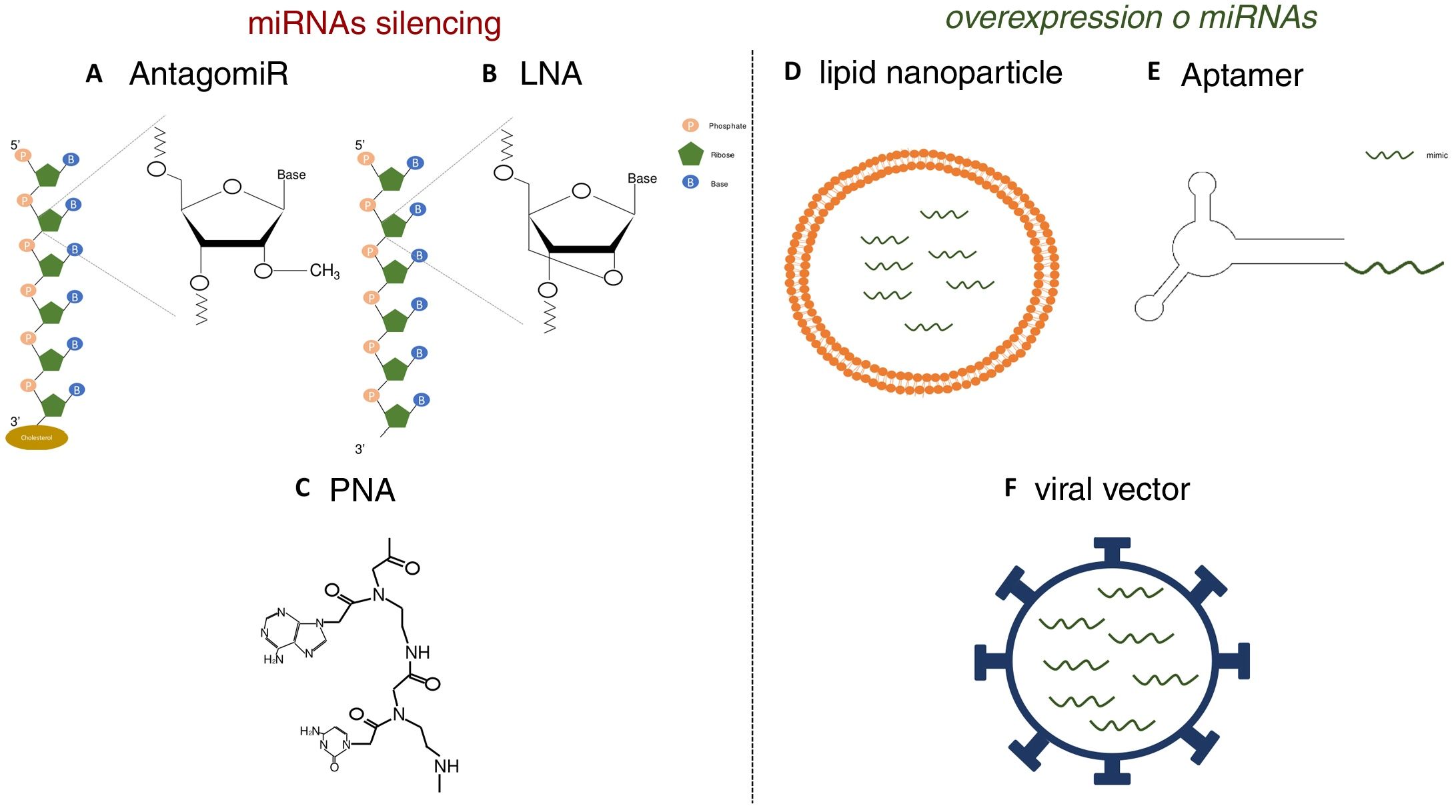
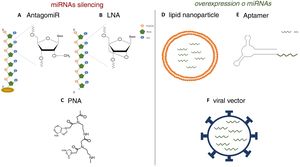
Various functional studies performed during the last two decades have shown that alterations in miRNA expression may be involved in the development of pathologies such as metabolic diseases, fibrosis and cancer.3 Therefore, it is interesting to develop strategies that allow the use of miRNA as therapeutic targets or biomarkers, since they can be secreted into body fluids. Manipulating of the levels of miRNA is one of the hottest issues in biomedical research today. The most widely used strategies is the use of agonists (mimics), synthetic double-stranded miRNAs with chemical modifications that favor their stability and cell uptake with to achieve overexpression, as well as the use of antagonists (anti-miR), consisting of modified oligonucleotides (short nucleotide sequences) that bind to specific miRNA causing its inhibition.4 In initial studies, in which the administration of these molecules was attempted systemically or locally, it was observed that they were degraded in the bloodstream, obtaining a poor distribution in the different tissues.5 Therefore, various strategies have been developed to improve the stability of synthetic miRNAs and inhibitors and facilitate their transport to cells using different vectors, mainly viral, liposomal or based on nanoparticles.4 The use of chemically modified oligonucleotides is one of these strategies. Thus, the antagomiRor anti-miR, molecules designed for the silencing of miRNA, are synthetic oligonucleotides that have a sterol (cholesterol) group which allows the entry into cells and methylations of the 2’-hydroxyl group of riboses; this chemical modification is commonly found in small RNA from plants and has a protective effect against degradation.6 Krutzfeldt et al.7 achieved complete inhibition of mir-122 in the liver after three intravenous administrations of an antagomiR. Other synthetic molecules used are locked-nucleic-acid-modifiedoligonucleotides (LNA), synthetic molecules made up of modified RNA nucleotides with an extra bridge that connects the 2’ oxygen of ribose with the 4’ carbon, and the peptidonucleic acids (peptide nucleic acids [PNA]), synthetic polymers in which the monosaccharides of nucleic acids have been replaced by a pseudopeptide structure. These molecules have a high affinity to the target miRNA, and a high stability and distribution in a wide variety of tissues of mice. However, they lack the ability to cross the blood- brain barrier and do not have tissue specificity (Fig. 1).8,9 Unlike miRNA inhibitors, chemical modifications cannot be applied to oligonucleotides that are miRNA agonist, since they could modify the target genes.10 For this reason, the most widely used strategies for the delivery of mimetic miRNAs involve the use of lipid nanoparticles, viral vectors or aptamers, the latter being single-stranded oligonucleotides that can interact with receptors present on the cells surface, allowing the entry of miRNA mimic to which it is anchored.11,12 These strategies produce high stability, but the tissue distribution is limited; lungs, liver, endothelial cells and various tumors are the tissues showing a higher expression of the miRNA mimics administered.
Strategies for the in vivo administration of miRNA antagonists or agonists. AC) Chemical structure of nucleic acid analogs: antagomiR (A), LNA (B) and PNA (C), used as in vivo miRNA silencing strategies. DF) Schematic models of lipid nanoparticles (D), aptamers (E) and viral vectors (F) for in vivo mimetic delivery.
In the kidney, the altered expression of various miRNAs has been linked to the development of various pathologies. Examples of this are miR-214 and miR-21, whose expression increases in models of kidney damage.13,14 In contrast, a decreased expression of miR-17 and miR-200 is related to polycystic kidney disease.15,16 The miR-29 expression is suppressed by TGF-β,17 which promotes the development of renal fibrosis. In recent studies, miRNA expression was inhibited in kidneys of mice with diabetic nephropathy by systemic administration of antagomirs or LNA.18–20 In the case of miR-21, a miRNA involved in the fibrogenesis of different organs, therapeutic inhibitors of miR-21 based on chemically modified oligonucleotides are being studied, in phase II clinical studies, for the treatment of Alport's disease.21 Also, in other studies, reduced microRNA levels were restored in the kidney. It was possible to increase the renal expression of miR-9-5p in mouse models by retroorbital delivery of viral vectors and miR-146a using nanoparticles.22,23 However, the main limitation of these strategies was the low distribution of oligonucleotides in kidney tissue. Furthermore, since miRNAs regulate hundreds of mRNAs involved in a wide variety of processes present in different cell and tissue types, the systemic, non-tissue-specific administration of a miRNA or an antagonist could have significant undesirable side effects.5 Therefore, it is important the develop strategies that allow an increased distribution of antagomiR or miRNA mimetics in an specific organ such as the kidney. This would make possible new treatment routes in renal pathology.
Synthesis and applications of PNAsPNAs are compounds of synthetic origin that contain a nucleobase sequence fixed on a poly-N- (2-aminoethyl) glycine chain.24 The complementarity of the bases of the PNA with those of a DNA or RNA allows the formation of stable dimers, with wide applications in the regulation of the functionality of nucleic acids (gene expression, antisense therapies) or as diagnostic tools (detection of nucleic acids).25
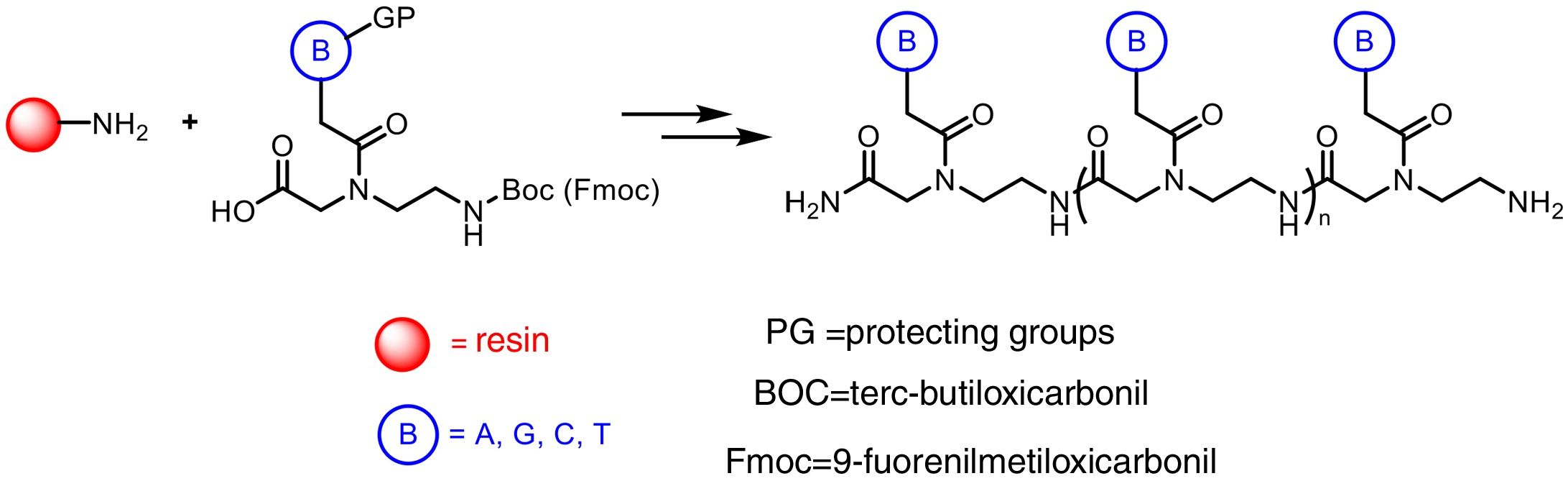
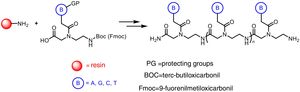
The preparation of PNA is usually performed using solid phase synthesis techniques through the formation of peptide bonds, in a similar manner to the conventional peptides and even by means of automated procedures. The monomers required for the synthesis are commercially available, each containing the four canonical bases and appropriate protecting groups such as tert-butyloxycarbonyl (Boc) or 9-fluorenylmethyloxycarbonyl (Fmoc). These protective groups of amines present at the N-terminal end and in the side chain of the PNA monomers are necessary for the solid phase synthesis of their polymers, based on the incorporation of PNA monomers at the N-terminal end of the emerging polymer anchored to a support, through cycles of alternate deprotection and coupling reactions. Also, these protecting groups are necessary to avoid unwanted side reactions with the various amino acid side chains. The N-terminal Boc protection system is removed with trifluoroacetic acid (TFA), forming a positively charged amino group, which is neutralized and coupled to the incoming activated amino acid. Final removal of the peptide from the solid support occurs simultaneously with the deprotection of the side chain using anhydrous hydrogen fluoride. This is the approach chosen to synthesize peptides containing base-sensitive residues, since in the case of the Fmoc system the deprotection uses a base, typically 20–50% piperidine in N, N-dimethylformamide, while the final acid cleavage occurs under milder acidic conditions (TFA).
The appropriate choice of resins, spacers (linkers) and solvents, as well as the use of techniques such as microwave irradiation, allow access to PNA chains containing 15–20 monomers, generally with good yields and high purities (Fig. 2).
The main advantage of the PNA chains compared to the corresponding oligonucleotides is their high metabolic stability against the action of both nucleases and proteases. However, PNAs exhibit poor permeability due to their essentially lipophilic character, which is also due to by their conformational flexibility. To overcome this limitation, different modifications have been described in the structure of PNAs, both in the polypeptide chain (introduction of additional substituents and/or chiral elements) and in the nitrogenous bases themselves, in order to decrease the conformational mobility and improve affinity in the formation of hybrid structures with RNA or DNA. Recently, it has also been proposed the selective introduction of interposed amino acids into a PNA backbone, so that the information they encode is used as a potential structural self-assembly element. In this way, the incorporation of hydrophobic or hydrophilic amino acid chains would allow modulating the secondary structure of the PNA skeleton and the formation of micellar amphipathic aggregates, controlling the solubility and cellular permeability of these PNA chains. Likewise, the specific binding of DNA or RNA molecules with a sequence complementary to that encoded by the PNA chains could allow a controlled disassembly of these structures.26
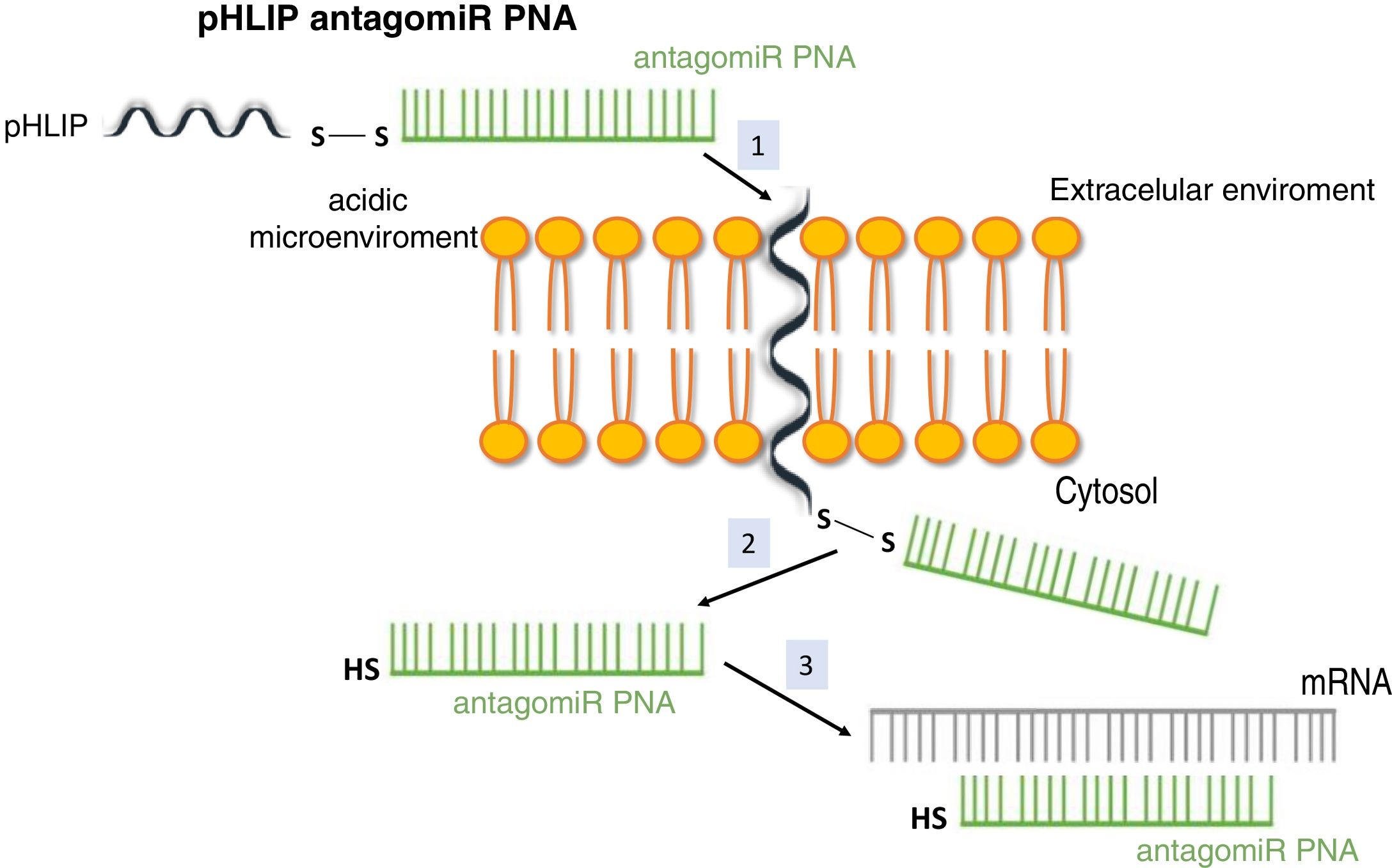
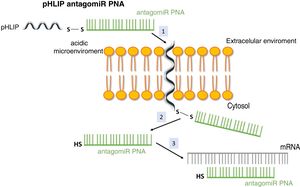
System pH (low) peptide insertion (pHLIP)To date, the most efficient way to achieve good cell permeability in a PNA involved the formation of conjugates. In this context, certain peptide compounds called cell penetrating peptides (CPP) have the ability to cross cell membranes using procedures such as direct translocation or endocytosis, that have been used successfully in the transport of PNA.27 Other possibilities evaluated have been the use of complexes of PNA with liposomes or using a vehicle supported on graphene oxide.28 CPPs are peptides of 6–30 amino acids, mostly positively charged and amphipathic in nature, which allow cellular internalization of macromolecules, polypeptides and oligonucleotides with high efficiency and low toxicity. Some CPPs are derived from natural biomolecules (e.g., Tat, derived from an HIV-1 protein), while others (e.g., polyarginine) are obtained by synthetic methods. The bonds between CPPs and transported molecules can be covalent in nature (conjugation with sulfosuccinimidyl sulfate, carbodiimide and thiol-amine) or non-covalent (biotin- streptavidin, electrostatic and metal affinity interactions).29 Directly related to CPPs is the nanotechnology based on peptides with a transmembrane structure that is sensitive to acidic pH, called pHLIP (pH [low] insertion peptide). These peptides contain a sequence of 35–40 amino acids, and do not usually present a defined secondary structure in a neutral or slightly basic medium. At a more acid pH, the pHLIP protonatable residues and the negatively charged become neutral, increasing the global hydrophobicity of the pHLIP and, therefore, its affinity for the plasma membrane. In addition, the presence of hydrophobic residues in the central region of the sequence enables the formation of a helicoidal structure at this pH, which is capable of intercalating in the cell membrane in a unidirectional manner. Therefore, the conjugation of certain molecules, among which are antagomiR, to the C-terminal end of these peptides in a cysteine or lysine residue, through a disulfide bridge (stable outside the cell, but labile in the cytoplasm due to the reducing environment), that allows its selective transport through cell membranes in a non-endocytic manner, based on small changes in extracellular acidity. The kinetics of membrane insertion can be modulated according to the amino acid sequence of the peptide. After the release of the molecule, when the residue at the C-terminal end of the pHLIP is exposed to the normal pH of the cell, it is deprotonated, causing the peptide detachment from the membrane (Fig. 3).30
Vehiculization of PNA using a cell penetrating peptide (pHLIP). 1. Insertion of the pHLIP into the cell membrane, leaving the C-terminal end in the cytosol. 2. Breaking of the disulfide bond due to the reducing environment of the cell interior. 3. Hybridization of the PNA with the target mRNA.
The pHLIP system was developed for anti-tumor purposes, exploring the property of the acidic environment of certain tumors to release PNA molecules inside the cell.31 In this work, we describe how was achieved an efficient targeted transport of anti-miR-155 to a murine lymphatic tumor, this is an important milestone in miRNA-based antitumor therapy. It was observed, that this complex was accumulated the same degree as in tumors in tissues undergoing inflammation and in kidneys. The latter has a high rate of catabolism for low molecular weight proteins and certain acidic regions in the cortical tubular interstitium.32,33 In addition, acid-base homeostasis in higher mammals requires the formation of acidic urine (pH∼5). This is achieved through the reabsorption of bicarbonate in the proximal tubule and the secretion of protons (H+) along the nephron, particularly in the regions of the proximal and distal tubule and the collecting tubule. This is achieved by transporters and exchangers with cell specificity which generates acidic tubular fluid and surrounding acidic tubulo-interstitial tissue in some regions of the kidney.34 The presumed biological advantage provided by the acidic microenvironment of certain kidney structures led us to propose a nanotechnology approach based on the binding of antagomiR to pHLIP peptides to transport them selectively to kidney tissue in the treatment of chronic kidney disease (CKD). In this way, the elimination of these molecules via the liver would be reduced, facilitating their entry into cells through a non-endocytic or receptor-mediated route. In addition, the chronic inflammation is intimately related to the development and subsequent progression of renal fibrosis, as well as to the associated metabolic changes that renal fibroblasts undergo to obtain energy through a glycolytic process instead of through the oxidation of fatty acids. This situation would confer an added advantage to the use of the pHLIP system.35–37
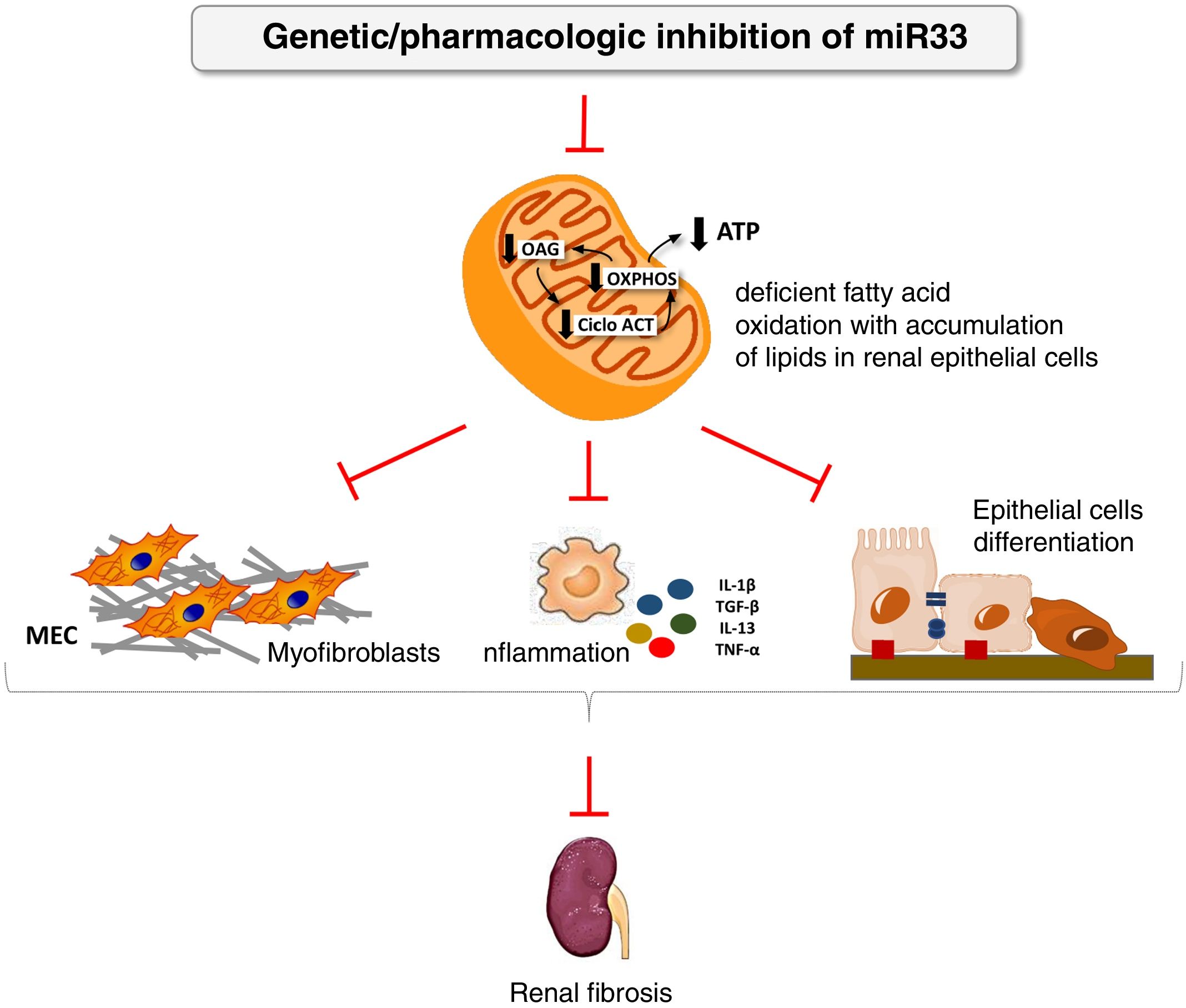
We have applied for the first time the pHLIP system for the kidney-directed administration of miR-33 inhibitors to determine whether inhibition of this microRNA, key to lipid metabolism, could be an effective therapeutic strategy to prevent damage renal.38 In this work, we demonstrate a renal accumulation and uptake of fluorescent pHLIP constructs in primary tubular cells inducing the expression of miR-33 target genes involved in fatty acid oxidation. Similar to the observed in miR-33-/-animals, treatment with anti-miR-33 pHLIP constructs prevented the induction of genes involved in kidney fibrosis and inflammation after treatment in the models of kidney damage induced by unilateral obstruction by ureter ligation and folic acid-induced nephropathy, as well as impaired kidney function. This proof of concept supports the development of therapeutic strategies aimed at reducing the potential pro-fibrogenic role of miRNAs in kidney disease (Fig. 4).
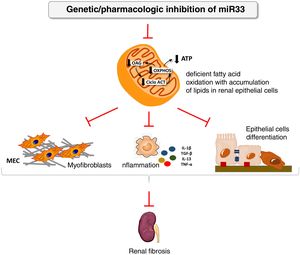
miR-33 as a new regulator of lipid metabolism during renal fibrosis. The loss of miR-33 improves fatty acid oxidation, prevents dedifferentiation of renal epithelial cells and accumulation of extracellular matrix, attenuating renal fibrosis.
ATP: adenosine triphosphate; ACT cycle: tricarboxylic acid cycle; ECM: extracellular matrix; OAG: fatty acid oxidation; OXPHOS: oxidative phosphorylation.
PNAs are the stable structural analogs of ribonucleotides most frequently used as non-coding RNAs therapeutic tools.24,39 The delivery of PNA molecules with the pHLIP system in vivo could represent an efficient and safe method. This method overcomes the main limitations of gene regulation strategies based on the use of miRNAs, such as organ specificity and bioavailability of the products.31 The pHLIP system has been studied to efficiently dispense PNAs of up to −7kDa in a pH-dependent manner; this limitation has been reduced with more hydrophobic molecules.40 Its accumulation in the kidney makes it as a powerful and novel therapeutic tool for the prevention or treatment of multiple pathologies of this organ.38 However, some limitations should be noted. In animal studies, it has been observed that modifying the pH of drinking water regulates the presence of the peptide in the kidney; the administration of bicarbonate reduces its accumulation in the kidney, which would limit the modulation of kidney accumulation by pH.32,41 Furthermore, the fact that this peptide is made up of D-amino acids instead of L-amino acids makes it have the same affinity for the lipid bilayer but reduces its presence in the kidney, possibly due to its greater stability in the blood.32 Likewise, a recent study suggests that a larger size of the constructs reduces its passage through the glomerular filtration barrier. A reduced velocity of membrane insertion kinetics also decreases the likelihood of being concentrated in kidney tissue and excreted in the urine.40
Although this methodology has been optimized for antagomiR, no obstacle has been described, a priori, to develop its use with miRNA, since the distribution of the PNA complex by pHLIP does not seem to be affected by the nucleotide sequence. In addition, this system has a series of properties that makes it adaptable. Allows anchoring to fluorescent molecules to monitor peptide distribution,42 while the variation of its sequence allows the adjustment of the pharmacokinetic properties and the target cells. The translocation of the peptide is rapid (seconds to minutes) and depends on the pH and concentration, which is reduced with the use of these conjugates as compared to the use of free antagomir.7,43 In the studies performed, it has been used an intravenous administration of the anti-miR-33 pHLIP constructs at a concentration of 1mg/kg of body weight in 4 doses for the treatment of renal fibrosis38 and in 2 doses for the treatment of lymphoma.31 Other studies focused on diagnosis show that pHLIP peptides bound to a fluorescent molecule at concentrations of 1μM given intravenously were used for the detection of ischemic regions in the myocardium44 and given intraperitoneal at 100μM in 3 doses in cases of pulmonary infection.45 Toxicity studies have been performed using concentrations of up to 4mg/kg of body weight of pHLIP constructions; monitorization of animals for 2 months revealed no physiological or behavioral changes.32 As compared to peptides that enter the cells, the pHLIP remains in the cell membrane after insertion, translocating one end to the cytoplasm and leaving the other end in the extracellular space. Therefore, the peptide pHLIP possesses dual delivery capabilities: it can not only inject and release molecules into the cytoplasm, but also allow the transport to the cell membrane of hydrophobic molecules attached to the N-terminal end of the pHLIP.46
Although promising, the use of the pHLIP system as a tool in the prognosis, diagnosis and treatment of kidney disease is still a challenge. More experimental studies on the delivery of PNA in specific models of kidney disease are necessary, as well as more detailed study on pharmacokinetics and pharmacodynamics, and its introduction in clinical trials with application to the most explored miRNAs. In the development of this application, the most relevant limitations are related to the specificity and sensitivity of the system such as the accumulation not directed in tissues with an acidic microenvironment induced by situations of ischemia, inflammation or infection.44,45 Since small changes in pH may lead to a significant biological effect through the alteration of cell functions and survival, it is necessary a more precise adjustment of the chemical properties of this transport system to improve its sensitivity.47 Another aspect that need to be consider is the fact that the pH is often lower in the cell surfaces than in the extracellular space48; also important is the different level of acidification in the various regions of the kidney and the dynamics of pH change in the transition from healthy to damaged kidney tissue, in each type of injury.49,50 Thus, hypoxic microenvironments or changes in cellular metabolism in damaged tissues affects the acidification of the extracellular environment, so that acidosis could also be a useful as marker for the imaging of different stages of kidney disease, using the pHLIP system coupled to easily detectable molecules.33
FinancingThis work has been financed by projects of the Ministry of Economy and Competitiveness (MINECO), SAF2012-31388 (SL), SAF2015-66107-R (SL), PI17/01513 (DRP) and CTQ2017-85263-R (JJV), co-financed by the European Regional Development Funds, Instituto de Salud Carlos III REDinRENRD12/0021/0009 (SL), RD16/0009/0016 (DRP) and RD16/0009/0015 (JJV), Community of Madrid «NOVELREN» B2017/BMD-3751 (SL, DRP and JJV), Spanish Society of Nephrology (Fundación Senefro 2017) (SL) and Fundación Renal “Iñigo Alvarez de Toledo” (SL), all from Spain. The CBMSO receives institutional support from the «Ramón Areces» Foundation. This work was also supported by grants from the NIH (R35HL135820), the American Heart Association (16EIA27550005), and the Foundation Leducq Transatlantic Network of Excellence in Cardiovascular Research MicroRNA-based Therapeutic Strategies in Vascular Disease (CFH).
Conflict of interestsThe authors declare that they have no conflict of interest related to the contents of this article.
Please cite this article as: Miguel V., Rey C, Aceña JL, Maqueda F, Fernández-Hernando C, Rodríguez-Puyol D, et al. El sistema pHLIP como vehículo de microRNA en el riñón. Nefrologia. 2020;40:491–498.